Abstract
Around one-third of patients diagnosed with COVID-19 develop a severe illness that requires admission to the Intensive Care Unit (ICU). In clinical practice, clinicians have learned that patients admitted to the ICU due to severe COVID-19 frequently develop ventilator-associated lower respiratory tract infections (VA-LRTI). This study aims to describe the clinical characteristics, the factors associated with VA-LRTI, and its impact on clinical outcomes in patients with severe COVID-19. This was a multicentre, observational cohort study conducted in ten countries in Latin America and Europe. We included patients with confirmed rtPCR for SARS-CoV-2 requiring ICU admission and endotracheal intubation. Only patients with a microbiological and clinical diagnosis of VA-LRTI were included. Multivariate Logistic regression analyses and Random Forest were conducted to determine the risk factors for VA-LRTI and its clinical impact in patients with severe COVID-19. In our study cohort of 3287 patients, VA-LRTI was diagnosed in 28.8% [948/3287]. The cumulative incidence of ventilator-associated pneumonia (VAP) was 18.6% [610/3287], followed by ventilator-associated tracheobronchitis (VAT) 10.3% [338/3287]. A total of 1252 bacteria species were isolated. The most frequently isolated pathogens were Pseudomonas aeruginosa (21.2% [266/1252]), followed by Klebsiella pneumoniae (19.1% [239/1252]) and Staphylococcus aureus (15.5% [194/1,252]). The factors independently associated with the development of VA-LRTI were prolonged stay under invasive mechanical ventilation, AKI during ICU stay, and the number of comorbidities. Regarding the clinical impact of VA-LRTI, patients with VAP had an increased risk of hospital mortality (OR [95% CI] of 1.81 [1.40–2.34]), while VAT was not associated with increased hospital mortality (OR [95% CI] of 1.34 [0.98–1.83]). VA-LRTI, often with difficult-to-treat bacteria, is frequent in patients admitted to the ICU due to severe COVID-19 and is associated with worse clinical outcomes, including higher mortality. Identifying risk factors for VA-LRTI might allow the early patient diagnosis to improve clinical outcomes.
Trial registration: This is a prospective observational study; therefore, no health care interventions were applied to participants, and trial registration is not applicable.
Similar content being viewed by others
Introduction
The Severe Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic has affected over 500 million people worldwide, and almost 6 million people have died of this infection and its complications (https://covid19.who.int)1,2. SARS-CoV-2 causes coronavirus disease-19 (COVID-19), a multi-system illness with high morbidity and mortality related to progressive life-threatening pneumonia3,4. Patients frequently need ventilatory support due to the advanced disease severity5,6,7. Even though ventilatory supports are life-saving treatments, these are commonly associated with infectious and non-infectious complications8,9,10. Patients treated with ventilator support that develop secondary infections have more extended ICU and hospital length of stay, worsening clinical outcomes11,12,13,14,15.
Up to 50.5% of COVID-19 patients develop ventilator-associated lower respiratory tract infection (VA-LRTI), compared to 30.3% in patients with other severe pulmonary infections, such as influenza16,17,18. VA-LRTI is frequently categorised as ventilator-associated pneumonia (VAP) or ventilator-associated tracheobronchitis (VAT) in clinical practice and by international treatment guidelines19. Early reports highlighted that potentially difficult-to-treat bacteria (i.e., Pseudomonas aeruginosa, Staphylococcus aureus, and Klebsiella pneumoniae, among others) are common in VA-LRTI associated with COVID-19 in small studies mainly in high-income countries20. However, the underlying microbiology, the associated risk factors, and the impact on clinical outcomes for VA-LRTI in COVID-19 patients remain uncertain, especially in lower-income countries. Understanding risk factors related to VA-LRTI will suggest targeted prevention strategies, particularly within strained healthcare systems.
In this context, we conducted a multicentre, multinational, prospective observational cohort study in Latin America and Europe to characterise the cumulative incidence of VA-LRTI in COVID-19 patients, its underlying microbiology, associated risk factors, and its association with clinical outcomes.
Materials and methods
This was a retrospective analysis of two prospective observational cohort studies of patients admitted to 84 ICUs due to severe SARS-CoV-2 infection in ten countries (i.e., Spain, Ireland, Andorra, Colombia, Chile, Ecuador, Mexico, Argentina, Uruguay, and Brazil) between March 2020 and January 2021. The patients were included in a voluntary registry created by the Latin American Intensive Care Network (Red LIVEN—https://www.redliven.org/web/)21 and the Spanish Society of Intensive Care Medicine (SEMICYUC)21,22. Data were collected prospectively by the attending physicians by reviewing medical records, laboratory data, and radiological records. All consecutive cases admitted who met the entry criteria were included in the registry. Both the Ethics Committee of the Clínica Universidad de La Sabana (IRB#2020AN28) and Hospital Joan XXIII (IRB#CEIM/066/2020) approved the study. All data were anonymized, allowing the requirement for informed consent to be waived. The ICU admission criteria and treatment decisions were left to the discretion of the attending physicians and were not provided in the study’s protocol.
The cohort includes patients older than 18 years hospitalised in the ICU due to severe SARS-CoV-2 infection and receiving invasive mechanical ventilation (IMV). Confirmed SARS-CoV-2 infection was determined by reverse transcription-polymerase chain reaction (rt-PCR) in a respiratory sample at each hospital based on local protocols. All COVID-19 patients admitted to the ICU with the clinical diagnosis of VAP or VAT were included in the VA-LRTI group. No patient received antibiotic treatment before the initiation of IMV. We excluded patients without pathogen isolated at the moment of the clinical diagnosis of interest (i.e., VAP or VAT). Patients that received IMV for less than 72 h were excluded. Finally, patients with a coinfection documented within the first 48 h of admission were excluded. All other patients were included and analysed in this study. The attending physician determined each patient's treatments and microbiological identification strategy.
The following variables were recorded during ICU admission: demographic data, comorbidities, symptoms, physiological variables collected during the first 24 h of ICU admission, systemic complications, and treatments used during the admission. For comparisons, patients admitted between February 22 and July 1, 2020, were classified in the subgroup of the “first wave,” while those admitted to the ICU until February 28, 2021 were considered a “second wave”. Variables with more than 30% missing data were discarded.
VA-LRTI was defined as a clinical syndrome of VAP or VAT in ventilated patients, as defined by the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and Asociación Latinoamericana del Tórax (ALAT) guidelines19. VAP was defined as pneumonia that arises more than 48 h after endotracheal intubation; VAT as fever with no other recognizable cause, with new or increased sputum production, positive endotracheal aspirates (ETA) culture (> 106 CFU/mL) yielding a new bacterium and no radiographic evidence of nosocomial pneumonia. Additionally, only patients with the isolation of at least one respiratory pathogen known to be capable of causing pneumonia in a respiratory sample (e.g., tracheal aspirate, bronchoalveolar lavage, or pleural fluid) after the first 48 h of ICU admission were considered to have VA-LRTI.
The study aimed to determine the cumulative incidence, clinical characteristics, and outcomes of ventilated COVID-19 patients diagnosed with VA-LRTI. Moreover, to identify the risk factors associated with the development of VA-LRTI, the risk of hospital mortality among these patients, and describe the etiological microbiology.
Statistical analysis
Categorical variables are presented in counts (percentages) and were evaluated through the Chi-square test. Continuous variables were expressed as median (interquartile ranges) or mean (standard deviation). T-Student test or Wilcoxon–Mann–Whitney test was performed according to data distribution.
A random forest (RF) model was used to predict the probability of hospital mortality. A total of 500 estimators were used in this model to calculate the area under the model’s receiver operating curve (AUROC), cross-validation was performed. A recursive elimination feature was carried out to select the smallest possible subset of variables that generate a model with adequate performance based on its AUROC. According to the Gini importance, the elimination is done by removing the variable with the least important from the dataset. To interpret the contribution of the optimal subset of variables in the model, the Python Treeinterpreter library was used. A multivariate logistic regression model was carried out with sociodemographic, admission data, and clinical outcomes variables identified as relevant in the final RF model (independent variables) to determine the Odds ratios (ORs) related to hospital mortality.
Another logistic regression model was developed to quantify the adjusted risk of VA-LRTI with all the sociodemographic, admission data, and clinical outcomes variables. A significance level of 0.05 and a confidence level of 95% were chosen. All statistical analysis was carried out in R studio 1.3.1056, Python 3.9.5–3.7.9, and IBM SPSS 28 for MAC. A more detailed description of the models and statistical analyses are presented in the online supplement.
Results
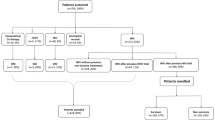
A total of 3287 patients were included in this study (Fig. 1, Table 1). The median [IQR] age was 63.0 years [54.5–71.0], and 70.5% [2317/3287] were male. Common comorbidities were hypertension 46.6% [1532/3287], obesity 36.4% [1193/3287], and diabetes mellitus 24.9% [819/3287].
Cumulative incidence of VA-LRTI by pandemic waves and by geographic region
A total of 28.8% [948/3287] patients were diagnosed with VA-LRTI. VAP cumulative incidence was 18.6% [610/3287], and VAT cumulative incidence was 10.3% [338/3287]. Most of the patients in our study were enrolled during the first pandemic wave (76.5% [2516/3287]), with a higher VAP cumulative incidence observed during the second wave (14.7% [371/2516] vs. 31.0% [239/771], p < 0.001). Also, VA-LRTI proportion was higher in Latin America than Europe (36.7% [368/1004] vs. 25.4% [580/2283], p < 0.001).
Microbiology
A total of 1252 bacteria species were isolated. The most frequently identified pathogens were Pseudomonas aeruginosa (21.2% [266/1253]), followed by Klebsiella pneumoniae (19.1% [239/1253]), Staphylococcus aureus (15.5% [194/1253]), and Citrobacter freundii (6.4% [80/1253]). For VAT, the most frequently identified pathogens were K. pneumoniae (29.7% [127/427]), followed by P. aeruginosa (15% [64/427]), and for VAP most frequent pathogens were P. aeruginosa (24.5% [202/826]) followed by S. aureus (17.8% [147/826]). Notably, most identified pathogens were Gram-negative roots and potentially difficult-to-treat bacteria (Fig. 2). We did not collect information on antibiotic resistance patterns in these pathogens.
Aetiological pathogens. Here is presented the frequency (in counts) of the etiological isolations in the whole cohort of patients with VA-LRTI. (A) Shows microorganisms isolated in the general cohort. (B) Represents the VAP cohort and Panel C depicts the VAT cohort. Within the “Other” group are microorganisms with less than five isolates (Achromobacter xylosoxidans, Acinetobacter lwoffii, Citrobacter koseri, Hafnia alvei, Moraxella catharralis, Neisseria cinerea, Providencia rettgeri, Raoultella planticola, Raoultella ornithinolytica, Streptococcus anginosus, and Streptococcus pyogenes).
Several differences were found between Latin America’s and Europe’s microbiology. Regarding the most frequently identified bacterial pathogens in Latin America were Klebsiella pneumoniae (33.5% [164/489), followed by Pseudomonas aeruginosa (12.5% [61/489]), and Staphylococcus aureus (10.6% [52/489). While in Europe, Pseudomonas aeruginosa (26.8% [205/764]), Staphylococcus aureus (18.6% [142/764]), and Citrobacter freundii (10.1% [77/764]) were the most frequently identified bacteria. Notably, Acinetobacter baumannii was Latin America's fourth most prevalent bacteria (10.6% [52/489]). In contrast, this bacteria was not as predominant in Europe (2.1% [16/764]) (Table 2).
VA-LRTI clinical characteristics and outcomes
Patients who developed VAP and VAT had similar age (median [IQR] VAP: 65.0 years [57.0–72.0]; VAT: 62.0 years [54.0–69.8]), and were most often male (VAP: 72.5% [442/610]; VAT: 69.8% [236/338]), and had similar laboratory results (Table 1). The most prevalent comorbidities were hypertension, obesity, and diabetes in both groups (Table 1).
Regarding treatments during hospital admission, those who developed VAP more often received corticosteroid treatment (VAP: 74.9% [457/610]; VAT: 70.4% [238/338]), while those with VAT more frequently received lopinavir/ritonavir (VAP: 1.9% [50/610]; VAT: 0.9% [25/338]). The antimicrobial usage was similar among groups. Patients who develop VAP had longer hospital LOS when compared to VAT (median [IQR] VAP: 40 days [27.0–57.0]; VAT: 24.5 days [16.0–44.0]) and more days under IMV (median [IQR] VAP: 25.9 days [16.0–38.0]; VAT: 15.0 days [11.0–25.0]). Finally, the prevalence of acute kidney injury (AKI) during ICU stay was similar among both groups (VAP: 42.0% [256/610]; VAT: 41.4% [140/338]) as well the mortality rates (VAP: 43.9% [268/610]; VAT: 47.3% [160/338]) (Table 3).
Impact of VA-LRTI on hospital mortality
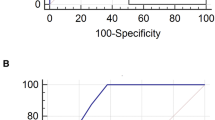
The Random Forest (RF) analyses identified AKI, VAP development, prone position ventilation, number of comorbidities, and days under IMV as risk factors associated with higher hospital mortality. Immunomodulant and antiviral treatments showed less than 1% importance according to the Gini feature importance; thus, these were unrelated to the addressed outcome. The ROC curve of the constructed model using the selected variables in the RF had a mean [SD] of 0.87 [± 0.05] to predict hospital mortality (Fig. 3). Moreover, in the multivariate logistic regression analysis, we found an OR [95% CI] of 2.79 [2.31–3.38] for AKI during ICU stay and 1.81 [1.40–2.34] for VAP. However, the association between VAT and hospital mortality was not statistically significant, despite the higher point estimates (1.34 [0.98–1.83]) (Fig. 4).
An automatized model to determine the impact of ventilator-associated lower respiratory tract infection on hospital mortality. (A) Presents variables more strongly associated with hospital mortality according to the Gini importance. (B) Represents the contribution of the variables to the output; the red values indicate a high-value contribution of the variable, and the blue values are a low-value contribution. The positive values in the plot indicate a high probability of hospital mortality, and negative values indicate a low likelihood of hospital mortality. (C) Presents each cross-validation trial’s receiver operative curve (ROC) for the subset of the selected variables. The blue curve represents the average of the ROC curves of each test, and the average area under de ROC is also presented. The most significant variables associated with hospital mortality according to their Gini importance and contribution to the output were Hospital-LOS, higher days under IMV, old age, higher creatinine levels on admission, and lower PaO2/FiO2 on ICU admission. Also, the development of AKI during ICU stays and VA-LRTI were risk factors associated with hospital mortality.
Logistic regression models to identify factors associated with ventilator-associated lower respiratory tract infection (VA-LRTI) (A) and the impact of VA-LRTI on hospital mortality (B). Logistic regression was performed with the optimal subset of variables obtained with the random forest model for the two outcomes (VA-LRTI and hospital mortality). The odds ratios (OR) are graphically represented in the Forest plot for better medical interpretability. (A) Presents the odd proportions of the risk for VA-LRTI, and (B) is shown the odds ratios for the hospital mortality.
Risk factors associated with the development of VA-LRTI
In the RF analysis and the logistic regression analysis carried out to identify the variables associated with the development of VA-LRTI, we found that the development of AKI during ICU stay (OR, 95% CI 1.32 [1.11–1.57]), along with days under IMV (OR, 95% CL 1.06 [1.05–1.07]) and high CRP serum concentration on ICU admission (OR% CL: 1.01 [1.01–1.01]) were significantly associated with the development of this VA-LRTI (Fig. 4). Notably, prior history of Diabetes Mellitus, hypertension, and obesity were not associated with the development of VA-LRTI.
Ethics approval and consent to participate
Both the Ethics Committee of the Clínica Universidad de La Sabana (IRB#2020AN28) and Hospital Joan XXIII (IRB#CEIM/066/2020) approved the study. All patients signed informed consent to participate in the study. Local guidelines and international regulations were taken into account, and the Declarations of Helsinki and Colombian Resolution No 008430 of 1993.
Discussion
We found that VA-LRTI occurs in more than a quarter of patients with severe COVID-19 admitted to the ICU, often with potentially difficult-to-treat pathogens, and regional differences in incidence (higher incidence in Latin America). The most frequently isolated pathogen was Pseudomonas aeruginosa. AKI during ICU stay, longer duration under IMV, and higher CRP levels at ICU admission were risk factors independently associated with the development of VA-LRTI. Notably, severe COVID-19 patients who developed VAP had higher mortality, while the association between VAT and hospital mortality was not statistically significant.
Our study found a cumulative incidence of 28.8% for VA-LRTI in patients with severe COVID-19 that required IMV and admission to the ICU, which is consistent with the systematic reviews that highlight an incidence varying between 21 to 64%23,24. Pulmonary superinfections are reported to be more frequent in COVID-19 patients compared to other viral aetiologies18,25,26. Of note, the cumulative incidence of VAT is estimated to be 14% in the European population18. Importantly, we found that the cumulative incidence of VA-LRTI (i.e., VAP and VAT) was higher in patients enrolled in Latin America when compared to the incidence in European Countries during the same study period, which is a novel finding. To the best of our knowledge, these differences have not been described in the literature. Notably, these differences build into the argument that each country and hospital should have local protocols and guidelines; and that one size does not fit all when discussing infection prevention.
We found that the most frequent etiological microorganisms of VA-LRTI isolated in the respiratory samples were Pseudomonas aeruginosa, Klebsiella pneumoniae, and Staphylococcus aureus, which are considered potentially challenging to treat pathogens as they tend to either generate new or have resistance to carbapenems, and beta-lactams, amongst other antibiotics used in this context27,28,29,30,31. Although a similar prevalence of difficult-to-treat pathogens has been reported previously18,32, these were smaller cohorts, restricted to European countries. Moreover, the microbiology observed in Latin America and Europe was notably different. Klebsiella pneumoniae was the principal bacteria in Latin America. This data is concerning since this microorganism may become frequently resistant and constitutes a challenging pathogen to treat, especially in countries with limited resources and no new antibiotics being widely available. It is important to highlight that Klebsiella pneumoniae has been highly reported in patients with COVID-19 in Latin America33,34. Interestingly, the prevalence of Acinetobacter baumannii was particularly higher in Latin America, which is also concerning. Rangel et al. found one of the highest Acinetobacter baumannii resistance rates in Latin America. This infection is increasing in COVID-19 patients due to disease severity and has been associated with prolonged hospitalization and associated immune dysfunction35. Thus, our study adds to this key literature by highlighting geographic differences and including patients in limited-resource settings such as South America.
We found that patients who developed VAP but not VAT were independently associated with increased hospital mortality, which is consistent with previous studies23,36. Interestingly, the higher risk of mortality observed in patients ventilated in the prone position was unexpected, given the potential value of the prone position in severe ARDS. However, this study was not intended to explore the effect of this therapy on clinical outcomes. We hypothesise that the prone position might facilitate the dissemination of microorganisms to the lower respiratory tract37,38,39, leading to a greater risk for VAP development. Nonetheless, a 2022 systematic review and network meta-analysis including 20 randomised clinical trials state that the prone position did not increase the VAP incidence40. Thus, more studies are required to understand this finding further. Of note, factors associated with mortality were similar to those variables associated with VA-LTRI, such as AKI during admission, days under mechanical ventilation, and the number of comorbidities. Identifying risk factors for the development of VA-LRTIVAP is pivotal to optimizing preventive strategies and hypothesizing potential underlying mechanisms25,41,42,43.
Since the pandemic’s beginning, several studies have attempted to identify the factors associated with increased mortality in patients with severe COVID-19. The most frequent factors associated with worse clinical outcomes in patients with severe COVID-19 are older age, male gender, several comorbid conditions, dexamethasone treatment, the development of AKI during admission, and more days under mechanical ventilation12,14,22,44,45,46,47. For instance, a study conducted with 9657 patients showed an increased incidence of AKI in COVID-19 patients that was associated with a significant increase in the risk of death (HRs of 3.4 [95% CI 3.0–3.9] for AKI25,41,42. Since COVID-19 patients have a greater risk of AKI development48, it is critical to prevent this complication. In this regard, the 25th Acute Disease Quality Initiative (ADQI) Workgroup recommends healthcare professionals individualise fluid and haemodynamic management based on a dynamic assessment of the cardiovascular status and limit nephrotoxic drug exposure where possible49.
We report the largest cohort of COVID-19 patients that evaluate risk factors and mortality associated with VA-LRTI. Our cohort patients from very different health care settings in 10 countries, from Europe and Latin America, involving different COVID-19 waves, giving external validity. We adopted the international consensus case definitions for our exposure of interest (VA-LRTI), had positive culture to inform LRTI diagnoses, and used an outcome without ascertainment bias (hospital mortality). Although our study did not include standardized protocols to prevent and diagnose VA-LRTI, the participating centres used internationally accepted guidelines to prevent and diagnose VA-LRTI in patients with severe COVID-19, including pneumonia zero protocols. We did not collect time from intubation to the development of VA-LRTI or the resistance patterns of the identified pathogens, which is a limitation and should be taken into account for future research. However, we did include the number of days patients remained under IMV, which may serve as a surrogate. Finally, although our study did not include standardized protocols for the treatment of COVID-19 patients between the different ICUs, the WHO recommendations were broadly followed in the participating centres.
In conclusion, ventilator-associated lower respiratory tract infections in patients with COVID-19 are frequent and associated with higher mortality, especially in those that develop VAP. Studies aimed to identify the mechanisms responsible for these complications and trials of prevention strategies are needed to identify potential treatments that could improve outcomes.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ICU:
-
Intensive care unit
- VA-LRTI:
-
Ventilator-associated lower respiratory tract infection
- COVID-19:
-
Coronavirus disease-19
- HIV/AIDS:
-
Human immunodeficiency virus/acquired immunodeficiency syndrome
- SARS-CoV-2:
-
Severe Respiratory Syndrome Coronavirus 2
- VAP:
-
Ventilator-associated pneumonia
- VAT:
-
Ventilator-associated tracheobronchitis
- rt-PCR:
-
Reverse transcription-polymerase chain reaction
- IMV:
-
Invasive Mechanical Ventilation
- ERS:
-
European Respiratory Society
- ESICM:
-
European Society of Intensive Care Medicine
- ESCMID:
-
European Society of Clinical Microbiology and Infectious Diseases
- ALAT:
-
Asociación Latinoamericana del Tórax
- ETA:
-
Endotracheal aspirates
- LOS:
-
Length of stay
- RF:
-
Random forest
- AUROC:
-
The area under the model’s receiver operating curve
- ORs:
-
Odds ratios
- IQR:
-
Interquartile range
- CRP:
-
C reactive protein
- AKI:
-
Acute renal injury
- HRs:
-
Hazard ratio
References
ISARIC Clinical Characterisation Group. COVID-19 symptoms at hospital admission vary with age and sex: Results from the ISARIC prospective multinational observational study. Infection 49(5), 889–905 (2021).
Chalmers, J. D. et al. Management of hospitalised adults with coronavirus disease 2019 (COVID-19): A European Respiratory Society living guideline. Eur. Respir. J. 57(4), 1 (2021).
Gupta, A. et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 26(7), 1017–1032 (2020).
ISARIC Clinical Characterisation Group. COVID-19 symptoms at hospital admission vary with age and sex: Results from the ISARIC prospective multinational observational study. Infection 49, 889 (2021).
Gundem, T. et al. Ventilatory support for hypoxaemic intensive care patients with COVID-19. Tidsskr Nor Laegeforen 140(11), 1 (2020).
Ospina-Tascon, G. A. et al. Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19: A randomized clinical trial. JAMA 326(21), 2161–2171 (2021).
Reyes, L. F. et al. Clinical characteristics, risk factors and outcomes in patients with severe COVID-19 registered in the International Severe Acute Respiratory and Emerging Infection Consortium WHO clinical characterisation protocol: A prospective, multinational, multicentre, observational study. ERJ Open Res. 8(1), 2021 (2022).
Ferrer, M. & Torres, A. Epidemiology of ICU-acquired pneumonia. Curr. Opin. Crit. Care 24(5), 325–331 (2018).
Higgs, A. et al. Guidelines for the management of tracheal intubation in critically ill adults. Br. J. Anaesth. 120(2), 323–352 (2018).
Westblade, L. F., Simon, M. S. & Satlin, M. J. Bacterial coinfections in coronavirus disease 2019. Trends Microbiol. 29(10), 930–941 (2021).
Goncalves Mendes Neto, A. et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J. Med. Virol. 93(3), 1489–1495 (2021).
Grasselli, G. et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern. Med. 180(10), 1345–1355 (2020).
Lim, Z. J. et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am. J. Respir. Crit. Care Med. 203(1), 54–66 (2021).
Buehler, P. K. et al. Bacterial pulmonary superinfections are associated with longer duration of ventilation in critically ill COVID-19 patients. Cell Rep. Med. 2(4), 100229 (2021).
Falcone, M. et al. Superinfections caused by carbapenem-resistant Enterobacterales in hospitalized patients with COVID-19: A multicentre observational study from Italy (CREVID Study). JAC Antimicrob. Resist. 4(3), 064 (2022).
Shafran, N. et al. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci. Rep. 11(1), 12703 (2021).
Langford, B. J. et al. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 27(4), 520–531 (2021).
Rouze, A. et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: A European multicenter cohort study. Intensive Care Med. 47(2), 188–198 (2021).
Torres, A. et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur. Respir. J. 50(3), 1 (2017).
Paparoupa, M. et al. The prevalence of early- and late-onset bacterial, viral, and fungal respiratory superinfections in invasively ventilated COVID-19 patients. J. Med. Virol. 94, 1 (2021).
Reyes, L. F. et al. Clinical characteristics, systemic complications, and in-hospital outcomes for patients with COVID-19 in Latin America. LIVEN-Covid-19 study: A prospective, multicenter, multinational, cohort study. PLoS ONE 17(3), e0265529 (2022).
Rodriguez, A. et al. Deploying unsupervised clustering analysis to derive clinical phenotypes and risk factors associated with mortality risk in 2022 critically ill patients with COVID-19 in Spain. Crit. Care 25(1), 63 (2021).
Ippolito, M. et al. Ventilator-associated pneumonia in patients with COVID-19: A systematic review and meta-analysis. Antibiotics (Basel) 10(5), 1 (2021).
Fumagalli, J., Panigada, M., Klompas, M. & Berra, L. Ventilator-associated pneumonia among SARS-CoV-2 acute respiratory distress syndrome patients. Curr. Opin. Crit. Care 28(1), 74–82 (2022).
Povoa, P., Martin-Loeches, I. & Nseir, S. Secondary pneumonias in critically ill patients with COVID-19: Risk factors and outcomes. Curr. Opin. Crit. Care 27(5), 468–473 (2021).
Rouze, A. & Nseir, S. Hospital-acquired pneumonia/ventilator-associated pneumonia and ventilator-associated tracheobronchitis in COVID-19. Semin. Respir. Crit. Care Med. 43, 243 (2022).
Russo, A. Spotlight on new antibiotics for the treatment of pneumonia. Clin. Med. Insights Circ. Respir. Pulm. Med. 14, 1179548420982786 (2020).
Metlay, J. P. et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 200(7), e45–e67 (2019).
Aliberti, S. et al. Global initiative for meticillin-resistant Staphylococcus aureus pneumonia (GLIMP): An international, observational cohort study. Lancet. Infect. Dis 16(12), 1364–1376 (2016).
Restrepo, M. I. et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: A multinational point prevalence study of hospitalised patients. Eur. Respir. J. 52(2), 1 (2018).
Clancy, C. J., Schwartz, I. S., Kula, B. & Nguyen, M. H. Bacterial superinfections among persons with coronavirus disease 2019: A comprehensive review of data from postmortem studies. Open Forum Infect. Dis. 8(3), 065 (2021).
Grasselli, G. et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest 160(2), 454–465 (2021).
Thomas, G. R. et al. Increased detection of carbapenemase-producing enterobacterales bacteria in latin America and the Caribbean during the COVID-19 pandemic. Emerg. Infect. Dis. 28(11), 1–8 (2022).
Hedberg, P. et al. Bacterial co-infections in community-acquired pneumonia caused by SARS-CoV-2, influenza virus and respiratory syncytial virus. BMC Infect. Dis. 22(1), 108 (2022).
Rangel, K., Chagas, T. P. G. & De-Simone, S. G. Acinetobacter baumannii infections in times of COVID-19 pandemic. Pathogens 10(8), 1 (2021).
Nseir, S. et al. Relationship between ventilator-associated pneumonia and mortality in COVID-19 patients: A planned ancillary analysis of the coVAPid cohort. Crit. Care 25(1), 177 (2021).
Li Bassi, G. & Torres, A. Ventilator-associated pneumonia: Role of positioning. Curr. Opin. Crit. Care 17(1), 57–63 (2011).
Rabilloud, M. & Guerin, C. Prone position and VAP incidence in the PROSEVA trial: Attention to the causal question when interpreting competing risk analysis-response to comments by Ranzani et al.. Intens. Care Med. 42(12), 2121–2122 (2016).
Ayzac, L. et al. Ventilator-associated pneumonia in ARDS patients: The impact of prone positioning. A secondary analysis of the PROSEVA trial. Intens. Care Med. 42(5), 871–878 (2016).
Pozuelo-Carrascosa, D. P. et al. Body position for preventing ventilator-associated pneumonia for critically ill patients: A systematic review and network meta-analysis. J. Intens. Care 10(1), 9 (2022).
Barbera, L. K. et al. HIV and COVID-19: Review of clinical course and outcomes. HIV Res. Clin. Pract. 22(4), 102–118 (2021).
Luyt, C. E. et al. Pulmonary infections complicating ARDS. Intens. Care Med. 46(12), 2168–2183 (2020).
Giacobbe, D. R. et al. Incidence and prognosis of ventilator-associated pneumonia in critically ill patients with COVID-19: A multicenter study. J. Clin. Med. 10(4), 555 (2021).
Asano, T. et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 6(62), 1 (2021).
Bastard, P. et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci. Immunol. 6(62), 1 (2021).
Falcone, M. et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: A prospective observational study. J. Antimicrob. Chemother. 76(4), 1078–1084 (2021).
Reyes, L. F. et al. Dexamethasone as risk-factor for ICU-acquired respiratory tract infections in severe COVID-19. J. Crit. Care 69, 154014 (2022).
Fisher, M. et al. AKI in hospitalized patients with and without COVID-19: A comparison study. J. Am. Soc. Nephrol. 31(9), 2145–2157 (2020).
Nadim, M. K. et al. COVID-19-associated acute kidney injury: Consensus report of the 25th acute disease quality initiative (ADQI) workgroup. Nat. Rev. Nephrol. 16(12), 747–764 (2020).
Funding
This work was supported by the SEMICYUC (Spanish Society of Intensive Care Medicine and Coronary Units) and the Universidad de La Sabana, Chía, Colombia (LFR). All authors were not precluded from accessing data in the study, and they accept responsibility for submitting it for publication.
Author information
Authors and Affiliations
Consortia
Contributions
L.F.R., A.R., M.S.H., I.M.L. contributions to conception; L.F.R., A.R., M.S.H., and I.M.L. design of work; L.F.R., A.R., M.S.H., I.M.L. acquisition; L.F.R., A.R., Y.V.F., S.D., R.G.G., A.B., C.C.S.M., E.D.I.P., M.S.H., I.M.L. analysis L.F.R., A.R., M.S.H., and I.M.L. interpretation data; L.F.R., A.R., Y.V.F., S.D., E.G.G., A.B., M.S.H., I.M.L. creation of software; L.F.R., A.R., Y.V.F., S.D., E.G.G., A.B., C.S.M., E.D.I.P., G.M., G.O.T., G.H., W.S., A.M.D., M.J., M.V.A., E.D., M.B., J.S.V., R.F., A.A.M., L.S., W.F., J.L.L.V., F.V.V., A.E., A.L.V., R.J.G., I.S., M.S.H., I.M.L. drafted and revised the work. All authors have approved the submitted version and agreed to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reyes, L.F., Rodriguez, A., Fuentes, Y.V. et al. Risk factors for developing ventilator-associated lower respiratory tract infection in patients with severe COVID-19: a multinational, multicentre study, prospective, observational study. Sci Rep 13, 6553 (2023). https://doi.org/10.1038/s41598-023-32265-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32265-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.