Abstract
Mass mortality and low growth highly decrease the production efficiency and sustainable aquaculture development of the sea cucumber Apostichopus japonicus in summer. Sea urchin feces was proposed to address the summer problems. A laboratory study was conducted for ~ 5 weeks to investigate survival, food consumption, growth and resistance ability of A. japonicus cultured with the feces of sea urchins fed kelp (KF feces, group KF), the feces of sea urchins fed prepared feed (FF feces, group FF), and the prepared sea cucumber feed (group S) at high temperature (25 °C). The sea cucumbers of group KF had better survival (100%) than those of the group FF (~ 84%), higher CTmax (35.9 °C) than those of the group S (34.5 °C), and the lowest skin ulceration proportion (0%) when they were exposed to an infectious solution among the three groups. These results suggest that the feces of sea urchins fed kelp is a promising diet for improving the survival and enhancing the resistance in A. japonicus aquaculture in summer. Sea cucumbers fed significantly less FF feces after 24 h of ageing than the fresh FF feces, suggesting this kind of feces became unsuitable for A. japonicus in a short time (within 48 h). However, the 24 h of ageing at 25 °C for the high fiber feces of sea urchins fed kelp had no significant effects on the fecal consumption of sea cucumbers. In the present study, both fecal diets provide better individual growth to sea cucumbers than the prepared feed. Yet, the feces of sea urchins fed kelp provided the highest weight gain rate (WGR) to sea cucumbers. Therefore, the feces of sea urchins fed kelp is a promising food to reduce the mortality, to address the problems of summer, and to achieve higher efficiency in A. japonicus aquaculture in summer.
Similar content being viewed by others
Introduction
The sea cucumber Apostichopus japonicus is the most commercially important echinoderm in China, Japan, and Russia1,2,3. However, high temperature aggravates a number of other severe problems, including bacterial infectious diseases, decay of food, and mortality of sea cucumbers, causing considerable economic losses, and resulting in a serious decline in resources4,5,6. Besides, poor growth rate further restricts the development of the aquaculture industry of sea cucumbers in summer7,8,9. Therefore, it is essential to reduce the mortality rate and improve the growth of cultured A. japonicus in summer.
The feces of sea urchins, which is rich in active bacteria, digestive enzymes, protozoa and other nutritious organic materials, is a common food for A. japonicus10,11,12,13. Ingesting feces that contains these active substances14,15, enhances survival and disease resistance for sea cucumbers15,16,17,18. Further, sea cucumbers fed feces of sea urchins achieve good growth in the integrated multi-trophic aquaculture (IMTA) system, which had no additional food18,19,20. Apostichopus japonicus fed feces had better growth than those fed prepared feed at the optimum temperatures and in winter20. Therefore, the feces of sea urchins, as a food with high nutritional value activity organic matter11,21, has a great potential to improve the growth of A. japonicus in summer. It is thus imperative to determine whether fecal diet can solve the problems of sea cucumber aquaculture in summer.
Feces of sea urchins fed kelp is a standard fecal category, because kelp is a common food for sea urchins22. Further, the feces of sea urchins fed prepared feed is a good source of food for sea cucumbers at the optimum temperature and in winter20. Due to the active substances in the feces, these two kinds of feces have different and specific characteristics after excretion, in which the nutritive value of feces changes complexly over time11. The nutritive value of feces usually increases, then decreases, and eventually loses its nutritional value11. Considering this process would be affected by the different fecal composition12 and sped up by high water temperature in summer, it is important to examine the influence of fecal composition on the usability at high temperature.
The present study investigated that whether a diet of sea urchin feces improves the survival, growth, and resistance of A. japonicus in summer. We further explored the effect of different fecal diets on feeding behavior of A. japonicus at high temperature, to determine the usability of fecal diets and how nutritional composition affects the usability of fecal diets in summer.
Results
Survival rate
Diets significantly affected survival rate of sea cucumbers (Kruskal–Wallis = 12.620, P = 0.002). The sea cucumbers of group KF (100 ± 0.00%) had significantly higher survival rate than those of group FF (84.38 ± 8.84%, P < 0.001). The survival rate of sea cucumbers of group KF was not significantly different from that of group S (91.06 ± 9.11%, P = 0.098). There was no significant difference in survival rate between the groups FF and S (P = 0.058, Fig. 1).
Food consumption
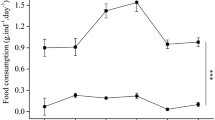
Food consumption showed no significant difference between sea cucumbers fed fresh KF feces (3.29 ± 0.42 g) and fed KF feces aged for 24 h (3.57 ± 0.41 g) (Mann–Whitney U = 45.500, P = 0.161). Sea cucumbers consumed significantly less FF feces aged for 24 h (2.53 ± 0.44 g) than fresh FF feces (3.94 ± 0.20 g) (Mann–Whitney U = 0.000, P < 0.001). Food consumption showed no significant difference between the sea cucumbers fed fresh sea cucumber feed (2.75 ± 0.26 g) and aged sea cucumber feed (2.80 ± 0.25 g) (F = 0.033, P = 0.707, Fig. 2).
Food consumption of Apostichopus japonicus fed fresh food and food aged for 24 h for the three groups (mean ± SD, N = 8). KF, FF, S refer to group KF (consume the feces of sea urchins fed kelp), group FF (consume the feces of sea urchins fed prepared feed) and group S (consume the sea cucumber feed). The asterisk ***means P < 0.001.
Growth
Growth of individual sea cucumbers in groups KF (27.43 ± 5.60%) and FF (30.46 ± 5.50%) were significantly higher than that in group S (15.09 ± 2.56%, P < 0.001 for group KF and group FF, Fig. 3, left).
Individual growth rates of Apostichopus japonicus in the three groups (mean ± SD, N = 8, left). Weight gain rate (WGR) of Apostichopus japonicus in the three groups (mean ± SD, N = 8, right). KF, FF, S refer to group KF, group FF and group S. The asterisks ** and *** mean P < 0.01 and P < 0.001, respectively.
Weight gain rate (WGR) of individual sea cucumbers of group KF (28.40 ± 6.75%) was significantly higher than that of the other two groups (P = 0.009 for group FF, P < 0.001 for group S). No significant difference was found between groups FF (10.02 ± 11.12%) and S (7.83 ± 6.55%, P = 0.962; Fig. 3, right).
Skin ulceration proportion
Skin ulceration proportion (per sea cucumber) was significantly lower in group KF (0.00 ± 0.00%) than in groups FF (27.14 ± 42.77%; df = 19, Z = 10.950, P = 0.009) and S (24.90 ± 39.24%; df = 19, Z = 11.550, P = 0.006). There was no significant difference in skin ulceration proportion between groups FF and S (df = 19, Z = 0.600, P = 0.886; Fig. 4, left).
Skin ulceration proportion of Apostichopus japonicus covered with ulcers after the exposure to infectious solution in the three groups (mean ± SD, N = 20, left). CTmax of A. japonicus in the three groups (mean ± SD, N = 12, right). KF, FF, S refer to group KF, group FF and group S. The asterisk ** means P < 0.01.
The critical thermal maximum (CTmax)
CTmax of sea cucumbers fed KF feces (35.90 ± 0.38 °C) was significantly higher than in those fed sea cucumber feed (34.52 ± 0.87 °C, F = 10.966, P = 0.001). There was no significant difference in CTmax between groups KF and FF (35.33 ± 0.74 °C, P = 0.109), and between groups FF and S (P = 0.081; Fig. 4, right).
Crude protein, fat and fiber concentration of the three diets
The concentration of crude protein of FF feces (14.31 ± 1.00% dry weight) was significantly higher than the other two diets (P < 0.001 for KF feces, P < 0.001 for sea cucumber feed). Sea cucumber feed (3.43 ± 0.17% dry weight) and the KF feces (2.42 ± 0.37% dry weight) had no significant difference in concentration of crude protein (P = 0.157). The sea cucumber feed has the significantly higher fat concentration (1.40 ± 0.03% dry weight) than the KF feces (P = 0.009). KF feces (0.62 ± 0.11% dry weight) and FF feces (0.80 ± 0.02%) dry weight, FF feces and sea cucumber feed had no significant difference in fat concentration (P = 0.229, P = 0.153). The feces of sea urchins fed kelp (KF) has the highest fiber concentration among the three diets (21.31 ± 0.98% dry weight, P < 0.001). The feces of sea urchins fed prepared feed (10.02 ± 0.32% dry weight) had significantly higher fiber concentration than sea cucumber feed (2.15 ± 0.15% dry weight, P < 0.001, Table 1).
Discussion
In recent years, mass mortality of cultured sea cucumbers has occurred frequently in summer, causing on average about 30% of the lost income of A. japonicus aquaculture4,5. Therefore, improving the survival rate in summer is an important consideration for sea cucumber aquaculture5. In the present study, A. japonicus fed KF feces had significantly higher survival rate than those of the feed group, suggesting the feces of sea urchins fed kelp is a promising food for sea cucumber aquaculture in summer. Further, sea cucumbers in groups S and FF had no significant difference in survival rate. Therefore, although the feces of sea urchins fed prepared feed could be used as a suitable food source for improving the survival for A. japonicus at the optimum temperature and in winter20, the prepared feed feces has no advantage in improving survival in summer.
Although A. japonicus normally survive over a large temperature range of 30 °C4,23,24, rapid changes in water temperature could trigger stress on sea cucumbers and even cause death25,26,27,28. CTmax is the temperature at which an organism loses muscle coordination in response to external stimulation6,29. Apostichopus japonicus fed KF fecal diet showed significantly higher CTmax than those fed sea cucumber feed, suggesting the KF fecal diet enhances the resistance of sea cucumbers to high temperature. Besides, water temperature extends beyond a direct effect on sea cucumbers9,30. It leads to bacterial infectious diseases31. High temperature increases the activity of Vibrio, and thus causes the high incidence of skin ulceration syndrome (one of the most serious bacterial diseases of A. japonicus) in summer32,33. Our results showed that the sea cucumbers fed the KF feces had no skin ulceration, which was significantly lower than the incidence of skin ulceration of sea cucumbers in the other two groups when being exposed to the same infectious solution (made by using sea cucumbers that had died of the skin ulceration syndrome). Ingesting feces of sea urchins fed kelp, which is rich in bacteria and digestive enzymes, probably enhances the resistance, and immune ability of A. japonicus34,37. Further, the pathogens usually attack individuals with low immunity, and are hardly pathogenic to healthy individuals5. Better resistance to high temperature (an increase in CTmax) and pathogen attack provided by the feces of sea urchin fed kelp further support the present result of the highest survival, suggesting that this kind of feces is an effective food to solve the summer problem and prevent mass mortality in summer.
Food consumption is another important factor that is greatly related to aquaculture efficiency of A. japonicus in summer7. Sea cucumbers of the FF group had the highest food consumption on fresh FF feces in all food consumption tests, indicating fresh FF feces of sea urchins is an appropriate food in summer. Fresh FF feces of sea urchins may be more nutritious and/or meet more nutritional requirements of sea cucumbers22,35,36. However, this kind of feces became less suitable for sea cucumbers after 24–48 h of ageing, since sea cucumbers of group FF had significant less fecal consumption when consumed feces aged 24 h at 25 °C, compared to the fresh feces of sea urchins fed prepared feed. This large reduction in fecal consumption implies that the feces of sea urchins fed prepared feed decays in a short time (within 48 h). This kind of feces appears to decay in 48–72 h of ageing time at 15 °C and over 72 h ageing at 5 °C37, but decays within 48 h at 25 °C. These results indicate that high temperature accelerated the deterioration of feces. Further, sea cucumbers of KF group had similar amount of food consumption on fresh KF feces and aged KF feces. The 24 h of ageing at 25 °C for the KF feces had no significant effects on fecal consumption, suggesting that this kind of feces does not decay in a short time (48 h). Different ageing processes and decay time are the results of the action of microorganisms and enzymes in different feces11,12. Considering that nutrient composition of feces would affect this process, we measured the nutritional concentration of the three foods, and found the highest concentration of protein (~ 15% dry weight), a low fat (~ 1% dry weight), high ash (~ 74% dry weight) and low fiber concentration (~ 10% dry weight) in the feces of sea urchins fed the prepared feed. The high protein concentration could explain why this feces deteriorated in 24–48 h, because proteins deteriorate more quickly than fiber at high temperatures38. The spoiled proteins would trigger harmful bacteria, and thus cause deterioration of water quality and massive mortalities38, which explains the low survival of sea cucumbers. The concentration of crude fiber is the highest (~ 22% dry weight) in feces of sea urchins fed kelp among the three diets. As feces ages, feces may become more nutritional, palatable and absorbable owing to pre-processing function of microorganisms on fibers11. The nutrient composition, which is high in fiber, low in fat (~ 0.6% dry weight) and protein concentration (~ 2.5% dry weight), may be responsible for the non-decay in a short time (48 h) for the KF feces. Food consumption on the fresh sea cucumber feed has no significant difference with that of feed aged for 24 h. This can be explained by the absence of active substances in the fresh dry sea cucumber feed.
We further evaluated the growth of sea cucumbers over a 5-week culture period for the great relation between feeding, the nutrition value of food, and growth39. Sea cucumbers fed fecal diets (~ 30% of group FF, ~ 28% of group KF) had a higher individual growth rate than those fed prepared feed (~ 15%), suggesting the fecal diets have a great potential in improving individual growth in summer. The protein-rich feces of sea urchins fed prepare feed can meet the nutrients needed to grow sea cucumbers, according to individual growth rate and earlier experimental results of the good growth of sea cucumbers in winter and at 15 °C20. Yet the productivity needs to take into account the overall efficiency, including survival and growth in summer. Due to the 100% survival, weight gain of sea cucumbers fed the KF feces of sea urchins is the highest among three groups. The feces of sea urchins fed kelp is more suitable for reducing mortality and addressing the problems and achieving higher efficiency in the A. japonicus aquaculture in summer.
Methods
Animals
Apostichopus japonicus were transported from Dalian Xinyulong Ecological Seedling Industry Co., Ltd. to the Key Laboratory of Mariculture & Stock Enhancement in the North China’ s Sea. They were temporarily cultured in a tank (length × width × height: 75 × 45 × 40 cm) with a temperature-controlled system (Huixin Co., Dalian, China). They were fed sea cucumbers commercial feed (An yuan Industry Co., Ltd.) with sea mud (1: 6) until the experiment started on May 10, 2021.
The sea urchin Strongylocentrotus intermedius (test diameter = 26.74 ± 5.41 mm) from the Key Laboratory of Mariculture & Stock Enhancement in the North China’ s Sea, Ministry of Agriculture and Rural Affairs, Dalian Ocean University were maintained in two fiberglass tanks (length × width × height: 75 × 45 × 40 cm; HXSWT-101, Huixin Co., Dalian, China) with temperature-controlled systems at 25 °C without feeding for five days. The sea urchins from each tank were randomly separated and cultured in 6 cylindric cages (20 cm in diameter, 0.5 cm mesh size) at 25 °C and fed the fresh brown alga Saccharina japonica ad libitum and prepared sea urchin feed ad libitum in two tanks, respectively. The plastic grate at the bottom of the cages kept the food and sea urchins in the cages, but allowed the feces to pass through. The feces of sea urchins fed kelp (KF feces), and feces of sea urchins fed feed (FF feces) from the two tanks were removed by siphoning and collected or cleaned daily at 9:00 am. There was no major variation in water temperature (25.0 ± 1.0 °C) and salinity (30.68 ± 0.36‰) of the three tanks during the acclimation period, according to the daily measurement with an YSI probe (YSI Incorporated, OH, USA). The photoperiod was 12D: 12L. The seawater was renewed daily.
Experimental design
Three diets were set as the experimental factor: KF feces (group KF), FF feces (group FF) and commercial feed of sea cucumbers (Anyuan Industry Co., Ltd.) with sea mud (1: 6) (group S) for the three group of A. japonicus. Each of 64 sea cucumbers (body weight = 2.79 ± 0.66 g) were randomly put into eight experimental plastic devices at 25 °C (length × width × height: 20 × 20 × 15 cm, with fixed shelters on the bottom) (Fig. 5A) for one group. The five weeks of culturing was conducted from 10 May 2021 to 14 June 2021.
The conceptual diagrams showing the containers for culture (A), feeding consumption experiment (B), disease challenge assay (C), and thermal tolerance (D). KF, FF, S refer to KF feces (the feces of sea urchins consumed kelp), FF feces (the feces of sea urchins consumed feed) and the sea cucumber feed (commercial feed mix with sea mud = 1: 6), respectively.
Water temperature was 25 ± 0.5 °C, controlled by a temperature-controlled systems (Huixin Co., Dalian, China). The salinity was 30.9 ± 0.72‰ and dissolved oxygen was 6.7 ± 0.3 g/mL (mean ± SD), according to daily measurement with a YSI probe (YSI Incorporated, OH, USA). The oxygenated seawater was renewed every two days.
The number of dead A. japonicus was recorded after the five-week culturing period. Survival rate was calculated as the number of survived individuals divided by the number of all involved sea cucumbers. Then we measured the food consumption, growth performance, and subsequently preformed disease challenge and thermal tolerance tests, to investigate whether the fecal diets provide benefits to sea cucumbers.
Food consumption
Food consumption was measured after the five-week experiment. Five sea cucumbers were placed in one plastic device (length × width × height: 20 × 20 × 15 cm, Fig. 5B). First, we fed them with corresponding food (5 g wet KF feces, 5 g wet FF feces, and 5 g sea cucumber feed) and cultured for 24 h at 25 °C using the method of water bath in three temperature-controlled tanks (length width height: 75 × 45 × 35 cm, HXSWT-101, Huixin Co., Dalian, China). The remained feces and remained sea cucumber feed were collected, dried and weighted after 24 h. We subsequently renewed the water and continued the experiment with 5 g wet KF feces aged for 24 h, 5 g wet FF feces aged for 24 h, and 5 g sea cucumber feed aged for 24 h in sea water. The remained feces and remained sea cucumber feed were collected, dried and weighted after 24 h (48 h after the experiment began). The whole experiment had eight replicates using different sea cucumbers for each group (N = 8). Sea cucumbers were put back for the following experiments after the harmless experiment of food consumption.
Food consumption was measured as follow40:
Food consumption = average food consumption per 5 sea cucumbers (g/24 h), A0 = weight of provided food (g dried weight), A1 = weight of uneaten food (g dried weight). To avoid inactivate the active substances on feces before food composition, we respectively drying another three samples of KF feces and FF feces (wet weight = 5 g) as the A0 for the groups KF and FF.
Growth
Sea cucumbers were drained in a net for one minute and then weighted using an electric balance (G & G Co., USA) for all the groups at the end of the experiment (N = 8). Individual growth rate was calculated according to the following formula41:
Weight gain rate was calculated according to the following formula42:
Wt is terminal wet weight of sea cucumbers (g); W0 is initial wet weight of sea cucumbers (g).
Disease challenge assay
Skin ulcerative syndrome is a highly infectious disease caused by bacterial infection in A. japonicus43. To observe the resistance of sea cucumbers fed different diets to the skin ulceration syndrome, we collected the carcasses of 20 sea cucumbers that died of the skin ulceration syndrome and mixed with 600 mL of seawater for 24 h as an infectious solution. Twenty healthy sea cucumbers were individually placed into 4 containers (length × width × height: 20 × 20 × 15 cm, each containing 4 L seawater; Fig. 5C) for each group (N = 20, Fig. 5C). Thirty mL of infectious solution was then injected into each box. We recorded the condition of sea cucumbers 48 h later using a digital camera (Legria HF20; mage Canon, Tokyo, Japan) and used IamgeJ (version 1.51n) to measure the area of ulcers or white spots on the skin of each sea cucumber. The area of ulcers per sea cucumber was calculated as follows:
St = the area of ulcers or white spots of each sea cucumbers (cm2), S = the area of each sea cucumbers (cm2).
Thermal tolerance
Twelve healthy sea cucumbers were randomly selected and placed in one plastic tank (length × width × height: 75 × 45 × 35 cm, HXSWT-101, Huixin Co., Dalian, China, N = 12, Fig. 5D) for each group. Water temperature increased from 25 °C to the lethal temperature by 1 °C hour-1. The temperature at which sea cucumbers showed any sign as ulcers, white spots, no response to external stimulation or evisceration was recorded as the CTmax.
Nutritional composition of diets
Samples of fresh KF feces and FF feces and sea cucumbers feed were collected to measure their organic composition (crude protein, crude fiber and crude fat) (N = 3, 50 g dry weight for each sample). Semi-micro Kjeldahl nitrogen method was used to measure the crude protein concentration of the two kinds of dried feces and feed. Digestion, distillation, absorption and titration were used during the included procedures44. The dried samples of each diet were boiled with a mixed solution (1.25% dilute acid and dilute alkali) for 30 min to measure their crude fiber concentration45. Soxhlet method was performed to assess the crude fat concentration of the three diets. About 10 g of samples were ashed at 550 °C for 48 h to measure the ash concentration of three diets45.
Statistical analysis
Normal distribution and homogeneity of variance were assessed using the Kolmogorov–Smirnov test and Levene's test, respectively. Kruskal–Wallis test was used to compare the survival rate between the three groups. Mann–Whitney U test was preformed to compare the food consumption within the groups FF and KF, and the independent T-test was used to compare the food consumption within the group S and the fiber proportions among three diets. One-way ANOVA were used to compare the WGR, individual growth rate, CTmax and the crude protein among the three groups. Kruskal–Wallis test was performed to compare the differences of skin ulceration proportion and the crude fat among the three groups, because the data were non-normal and/or lacked homogeneity in the variance. All data analyses were performed using SPSS 19.0 statistical software. A probability level of P < 0.05 was considered being significant.
Data availability
All data generated or analyzed during this study are included in Supplementary Information file.
References
Sloan, N. A. Echinoderm fisheries of the world: a review. Echinodermata. In: Proceedings of the 5th International Echinoderm Conference. A. Balkema, Rotterdam, pp 109–124 (1984)
Jiang, S. H., Ren, Y. C., Tang, B. P., Li, C. F. & Jiang, C. B. Development status and countermeasures of Apostichopus japonicus culture industry in China. J. Agric. Sci. Tech-Iran 019(009), 15–23 (2017).
Sun, J.M., Zhang, L.B., Pan, Y., Lin, C.G. & Yang, H.S. Effect of water temperature on diel feeding, locomotion behavior and digestive physiology in the sea cucumber Apostichopus japonicus. J. Exp. Biol. 221(9), jeb177451 (2018)
Yu, Z. H., Zhou, Y., Yang, H. S. & Hu, C. Q. Bottom culture of the sea cucum, ber Apostichopus japonicus Selenka (Echinodermata: Holothuroidea) in a fish farm, southern China. Aqua. Res. 45(9), 1434–1441 (2014).
Huo, D., Liu, S. L. & Yang, H. S. Analysis of causes and corresponding strategies for summer massive mortalities of sea cucumber. Studia Marina Sinic 52, 47–58 (2017) (in Chinese with an English abstract).
Wang, Q. L. Effects of temperature acclimation on growth and thermotolerance of sea cucumber, Apostichopus japonicus. Doctoral thesis. Ocean University of China (2012) (in Chinese with an English abstract).
Yang, H. S. et al. Effects of body size and water temperature on food consumption and growth in the sea cucumber Apostichopus japonicus (Selenka) with special reference to aestivation. Aquacult. Res. 36, 1085–1092 (2005).
Sun, J.M., Zhang, L.B., Pan, Y., Lin, C.G. & Yang, H.S. Effect of water temperature on diel feeding, locomotion behaviour and digestive physiology in the sea cucumber Apostichopus japonicus. J. Exp. Biol. 221(9), jeb177451 (2018).
Sun, Z. L. et al. Seasonal changes in food uptake by the sea cucumber Apostichopus japonicus in a farm pond: evidence from C and N stable isotopes. J. Ocean U. China 12(1), 160–168 (2013).
Mamelona, J. & Pelletier, É. Green urchin as a significant source of fecal particulate organic matter within nearshore benthic ecosystems. J. Exp. Mar. Biol. Ecol. 314(2), 163–174 (2005).
Sauchyn, L. K. & Scheibling, R. E. Degradation of sea urchin feces in a rocky subtidal ecosystem: implications for nutrient cycling and energy flow. Aquat. Biol. 6, 99–108 (2009).
Dethier, M. N. et al. Feces as food: The nutritional value of urchin feces and implications for benthic food webs. J. Exp. Mar. Biol. Ecol. 514, 95–102 (2019).
Harris, J. M. The presence, nature, and role of gut microflora in aquatic invertebrates: A synthesis. Microb. Ecol. 25(3), 195–231 (1993).
Li, J., Tan, B. & Mai, K. Dietary probiotic bacillus OJ and isomaltooligosaccharides influence the intestine microbial populations, immune responses and resistance to white spot syndrome virus in shrimp (Litopenaeus vannamei). Aquaculture 291, 35–40 (2009).
Thompson, J., Gregory, S., Plummer, S., Shields, R. J. & Rowley, A. F. An in vitro and in vivo assessment of the potential of Vibrio spp. as probiotics for the Pacific white shrimp Litopenaeus vannamei. J. Appl. Microbiol. 109(4), 1177–1187 (2010).
Zhao, Y. et al. Effects of potential probiotic Bacillus subtilis T13 on growth, immunity and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 32, 750–755 (2012).
Yang, Z. P., Sun, J. M., Xu, Z., Zhang, C. C. & Zhou, Q. Beneficial effects of Metschnikowia sp. C14 on growth and intestinal digestive enzymes of juvenile sea cucumber Apostichopus japonicus. Anim. Feed Sci. Technol. 197, 142–147 (2014).
Grosso, L. et al. Integrated multi-trophic aquaculture (IMTA) system combining the sea urchin Paracentrotus lividus, as primary species, and the sea cucumber Holothuria tubulosa as extractive species. Aquaculture 534, 736268 (2021).
Sonnenholzner-Varas, J. I., Moreira, J. A. & Orrala, M. M. P. Growth performance and survival of Holothuria theeli (holothurian) fed with feces of Tripneustes depressus (echinoid): A multi-trophic aquaculture approach. Aquaculture 512, 734345 (2014).
Yu, Y. S., Qiao, H.Y., Ding, P., Tian, R.H., Sun, J.N., Hu, F.Y., Wu, G., Chang, Y.Q. & Zhao, C. Effects of sea urchin feces on behaviors, digestion ability, growth, and resistance of the sea cucumber Apostichopus japonicus. Front. Mar. Sci. 1514 (2022)
Wotton, R. S. & Malmqvist, B. Feces in Aquatic Ecosystems: Feeding animals transform organic matter into fecal pellets, which sink or are transported horizontally by currents; these fluxes relocate organic matter in aquatic ecosystems. Bioscience 51, 537–543 (2001).
Lyons, D. A. & Scheibling, R. E. Effect of dietary history and algal traits on feeding rate and food preference in the green sea urchin Strongylocentrotus droebachiensis. J. Exp. Mar. Biol. Ecol. 349, 194–204 (2007).
Dong, Y. & Dong, S. Growth and oxygen consumption of the juvenile sea cucumber Apostichopus japonicus (Selenka) at constant and fluctuating water temperatures. Aquac. Res. 37, 1327–1333 (2016).
Li, L. & Li, Q. Effects of stocking density, temperature, and salinity on larval survival and growth of the red race of the sea cucumber Apostichopus japonicus (Selenka). Aquacult. Int. 18(3), 447–460 (2018).
Tang, Y. et al. Identification of bacterial community composition in freshwater aquaculture system farming of Litopenaeus vannamei reveals distinct temperature-driven patterns. Int. J. Mol. Sci. 15(8), 13663–13680 (2014).
Huo, D. et al. Global-warming caused changes of temperature and oxygen alter the proteomic profile of sea cucumber Apostichopus japonicus. J. Proteomics 193, 27–43 (2018).
Xu, H. C. et al. Microbial communities in sea cucumber (Apostichopus japonicus) culture pond and the effects of environmental factors. Aquac. Res. 50(4), 1257–1268 (2019).
Zhang, Z. et al. Environmental factors promote pathogen-induced skin ulceration syndrome outbreak by readjusting the hindgut microbiome of Apostichopus japonicas. Aquaculture 507, 155–163 (2019).
Kilgour, D. M. & McCauley, R. W. Reconciling the two methods of measuring upper lethal temperatures in fishes. Environ. Biol. Fish. 17(4), 281–290 (1986).
Wang, L., Wei, C., Chang, Y. Q., & Ding, J. Response of bacterial community in sea cucumber Apostichopus japonicus intestine, surrounding water and sediment subjected to high-temperature stress. Aquaculture 535 (2021)
Zhang, H. et al. Dynamic changes of microbial communities in Litopenaeus vannamei cultures and the effects of environmental factors. Aquaculture 455, 97–108 (2016).
Schreier, H. J., Mirzoyan, N. & Saito, K. Microbial diversity of biological filters in recirculating aquaculture systems. Curr. Opin. Biotech. 21(3), 318–325 (2010).
Zhang, C., Liang, W., Zhang, W. & Li, C. Characterization of a metalloprotease involved in Vibrio splendidus infection in the sea cucumber Apostichopus japonicus. Microb. Pathogenesis 101, 96–103 (2016).
Kuhn, D. D., Boardman, G. D., Lawrence, A. L., Marsh, L. & Flick, J. F. Jr. Microbial floc meal as a replacement ingredient for fish meal and soybean protein in shrimp feed. Aquaculture 296, 51–57 (2009).
Goff, S. A. & Klee, H. J. Plant volatile compounds: sensory cues for health and nutritional value?. Science 311, 815–819 (2016).
Xia, S. et al. Feeding preferences of the sea cucumber Apostichopus japonicus (Selenka) on various seaweed diets. Aquaculture 344, 205–209 (2012).
Yu, Y. S., Sun, J. N., Zhao, Z. H., Ding, P., Yang, M. F., Hu, F. Y., Qiao, Y. H., Wang, L., Chang, Y. Q. & Zhao, C. Effects of water temperature, age of feces, light intensity and shelter on the consumption of sea urchin feces by the sea cucumber Apostichopus japonicus. Aquaculture 554 (2022)
Cai, M. L. et al. Dietary Haematococcus pluvialis promotes growth of red swamp crayfish Procambarus larkia (Girard, 1852) via positive regulation of the gut microbial co-occurrence network. Aquaculture 551, 737900 (2020).
Yang, H.S., Yuan, X.T., Zhou, Y., Mao, Y.Z., Zhang, T., & Liu, Y. Effects of body size and water temperature on food consumption and growth in the sea cucumber Apostichopus japonicus (Selenka) with special reference to aestivation. Aquacult. Res. 36(1), 085–1092 (2005)
Hu, F. Y. et al. An effective facility decreases disease transmission and promotes resistance ability of small sea urchins Strongylocentrotus intermedius: A potential application in the long line culture. Aquaculture 547, 737542 (2022).
Wang, H., Zhang, X.C., Liu, J.B., Sun, Y. & Leng, X.F. Model and benefit analysis of seaweed, sea urchins and sea cucumbers in shallow sea. Hebei Yuye 4(12), 27–30 (2020) (in Chinese)
Wang, J. H. et al. Growth performance and digestion improvement of juvenile sea cucumber Apostichopus japonicus fed by solid-state fermentation diet. Aquacul. Nutr. 23, 1312–1318 (2017).
Zhang, C.Y., Wang, Y.G. & Rong, X.J. Isolation and identification of causative pathogen for skin ulcerative syndrome in Apostichopus japonicus. J. Fish. China. 30(1), 118–123 (2016) (in Chinese with English abstract)
Chen, Z., Shi, M., Wang, Q. & Zhang, X. Protein content measurement of food using the method of Kjeldahl determination. Anim. Husbandry XJ China 5, 22–24 (2018) (in Chinese).
Cheng, J. Improvement of determination method of feed conventional analysis. Master’s Thesis. Northeast Agr. Univ. China (2016). (in Chinese with an English abstract).
Acknowledgements
This study is funded by a research project for marine economy development in Liaoning province (for Jun Ding), High-level talent support grant for innovation in Dalian (2020RD03), National Key Research and Development Program of Dalian (2022YF16SN066), National Natural Science Foundation of China (41506177), and Liaoning Province “Xingliao Talents Plan” project (XLYC2002107). We appreciate Prof. John Lawrence for his editorial suggestions.
Author information
Authors and Affiliations
Contributions
C.Z. and Y.Y. did the investigation, conceived and designed the experiment. YY, P.D., Y.Q., X.W., Y.L., T.Z. performed the experiment. Y.Y., P.D., Y.Q. performed statistical analysis and visualization. Y.Y., C.Z. wrote the first draft of the manuscript. C.Z. reviewed and edited the manuscript. C.Z., Y.C. and J.D. provided resources for this study. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, Y., Ding, P., Qiao, Y. et al. The feces of sea urchins as food improves survival, growth, and resistance of small sea cucumbers Apostichopus japonicus in summer. Sci Rep 13, 5361 (2023). https://doi.org/10.1038/s41598-023-32226-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32226-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.