Abstract
Patients with coronavirus disease 2019 (COVID-19) were shown to have reduced serum testosterone levels compared to healthy individuals. Low testosterone levels are linked with the development of erectile dysfunction (ED). In this case-controlled study, 20 healthy controls and 39 patients with ED 3 months after recovering from mild-to-moderate COVID-19 pneumonia were studied. The patients ranged in age from 31 to 47 years. To identify early and late COVID-19 infections, real-time polymerase-chain reaction (RT-PCR) and COVID-19 antibody testing were done. The levels of luteinizing hormone (LH), follicular stimulating hormone (FSH), total testosterone (TT), free testosterone (FT), free androgenic index (FAI), and sex hormone-binding globulin (SHBG) were measured. The sexual health inventory for patients (SHIM) score was used to measure the erectile function of the patients and controls. When compared to the controls, the TT serum level in long COVID-19 (LC) patients with ED was low (p = 0.01). In contrast to controls, FT and FAI were both lower in LC patients with ED. (p = 0.001). FSH serum levels did not significantly differ (p = 0.07), but in ED patients, LH serum levels were elevated. SHIM scores were associated with low TT (p = 0.30), FT (p = 0.09), and high LH (p = 0.76) in LC patients with ED. Male patients with decreased serum levels of LH and testosterone may have hypothalamic-pituitary–gonadal axis dysfunction, which could lead to the development of LC-induced ED. Therefore, an in-depth research is necessary to confirm the causal link between COVID-19 and ED in LC patients.
Similar content being viewed by others
Introduction
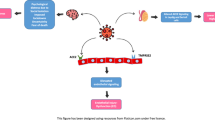
The world was affected by the most recent coronavirus pandemic in 2019 (COVID-19), which was brought on by the brand-new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 is the largest member of the betacoronaviridae family and is an enveloped positive-sense RNA virus1. Angiotensin-converting enzyme 2 (ACE2), which is expressed in renal tubular cells, endothelial cells, lung alveolar cell type 2, cardiomyocytes, and testis, serves as both its receptor and entry point in the target cells2. Cellular transmembrane protease serine 2 (TMPRSS2) trims and activates the SARS-CoV-2 spike proteins to bind ACE2, which facilitates the binding of SARS-CoV-2 to ACE22. Prostate, testis, and endothelium cells all have elevated levels of TMPRSS2 expression3. The renin-angiotensin system (RAS) is subsequently disrupted as a result of the interaction between the SARS-CoV-2 viruses and ACE23. Angiotensin II (Ang II), a vasoconstrictor, is converted into the vasodilators Ang-(1–9) and Ang-(1–7) by the enzyme ACE24. As a result, elevated levels of circulating Ang II cause endothelial dysfunction and enhanced pro-inflammatory cytokines, which are connected to ACE2 downregulation5 (Fig. 1).
Most COVID-19 patients recover with low mortality; however, some patients experience long-term symptoms, described as Long COVID (LC)6. LC was originally recognized as COVID-19 patients who had persistent symptoms for weeks after acute SARS-CoV-2 infection. These symptoms included dyspnea, fatigue, myalgia, insomnia, and cognitive and olfactory disorders7. However, these symptoms may last for months in some patients and negatively impact their daily activities8. LC can be classified as post-acute COVID-19 with symptoms that last longer than 3 weeks after the onset of the illness, or as chronic COVID-19 with symptoms that last more than 12 weeks9. However, previous studies have reported that patients with LC had persistent symptoms in varying frequencies and durations among COVID-19 survivor10,11.
Endocrine disruption in LC or testicular damage may be caused by hypothalamic-pituitary–gonadal (HPG) axis dysfunction brought on by SARS-CoV-2 infection12. A case–control study by Okcelik, 2021 found that compared to healthy controls, COVID-19 patients have lower testosterone serum levels13. Other research has shown how testosterone regulates human erectile function and male sexual responsiveness14. Additionally, erectile dysfunction (ED) has been connected to low testosterone levels14. A systematic review and meta-analysis of 204 published articles comprising 2092 COVID-19 patients and 1138 controls showed that SARS-CoV-2 infection is associated with a reduction of total testosterone (TT), and impaired sperm production15.
As an end, endothelial dysfunction, low serum TT levels, and SARS-CoV-2 infection and subsequent hypercytokinemia interact to cause ED in patients with LC. A SARS-CoV-2 infection may also result in long-term complications such cardiovascular problems, endothelial dysfunction, and endocrine dysfunction9,12. Therefore, early hyper-inflammatory status and associated multi-systemic injuries associated with acute SARS-CoV-2 infection may extend into COVID-19 survivors, causing LC and subsequently ED8. The present study hypothesizes that COVID-19 patients may experience long-term cardio metabolic, endocrine and neurological complications which affect the normal physiology of erectile function could result in ED16. Different studies revealed that LC patients were associated with development of ED12,17. However, the underlying mechanism for development of ED in LC patients was not fully elucidated. Therefore, this study aimed to elucidate the link between ED and testosterone in patients with LC.
Materials and methods
The present case-controlled study involved 39 patients, aged 31–47 years, three months after overcoming mild-to-moderate COVID-19 pneumonia with the ED. They were recruited randomly from Al-Shiffa Medical Center, Baghdad, Iraq from June to October 2020, and were compared with matched 20 healthy controls that were recruited among relatives of the patients and outpatient visitors. The selection of patients with ED was performed according to the specific diagnostic criteria reported by Segraves, 201018. The sample data were selected and drawn from a normally distributed population, and the sample size calculation was determined using baseline incidence, population variance, and treatment effect size with alpha = 0.05 and beta as power = 1 − β.
The Scientific Jury and Ethical Committee of Mustansiriyah University in Iraq granted permission and gave their seal of approval for the current study (ethical approval number 45QTR/2020) in accordance with the Helsinki Declaration. All participants provided knowledgeable consent for their participation in the study. Following detailed medical histories were collected and physical examinations, the control participants and selected patients were divided up into the following categories. Group A: LC patients with ED (n = 39), Group B: healthy controls (n = 20). The selection of patients with LC was accomplished according to established diagnostic criteria for LC19.
Inclusion and exclusion criteria
Inclusion criteria
Patients who were male and had healed from COVID-19 but still had at least one lasting symptom, who were also diagnosed with ED (3 months after acute COVID-19 recovery), and were participating in this research and ranged in age from 31 to 47 years without previous history of ED prior COVID-19.
Exclusion criteria
The following patients were excluded from the study: those with a history of severe COVID-19, diabetes mellitus, hypothyroidism, congenital hypogonadism, chronic liver diseases, acute and chronic kidney diseases, acute and chronic bacterial infections, psychological and mental disorders, history of pelvic trauma, and those receiving treatment with diuretics or β-blockers.
Anthropometric parameters
All participants had their systolic (SBP) and diastolic (DBP) blood pressures taken, and a specific equation was used to compute the pulse pressure (PP) and mean arterial pressure (MAP). PP = SBP-DBP, MAP = DBP + 1/3 PP20. Additionally, the explicit formula BMI = body weight (kg)/height (m2) was used to calculate body mass index (BMI)21.
Biochemical parameters
All selected patients and controls had an overnight fast before having 10 mL of blood taken. The blood samples were centrifuged for 5 min at 3000 rpm that stored till time of analysis. Early and late SARS-CoV-2 infections were identified using real-time polymerase-chain reaction (RT-PCR) and COVID-19 antibody testing. For both IgG and IgM, the cut-off level was 0.00–0.04 mLU/mL, respectively.
Regarding hormonal assay, in this study, sex hormone-binding globulin (SHBG) and TT were evaluated using ELISA methods (Human testosterone and SHBG, ELISA Kit; MGC126834, Elabscience, Wuhan, China). Free testosterone (FT) was measured according to a previous study22. Free androgenic index (FAI) was calculated using the equation FAI = 100 × [TT/SHBG]23. Additionally, ELISA techniques were used to measure luteinizing hormone (LH) and follicular stimulating hormone (FSH).
Erectile function assessment
LC patients and controls’ erectile functioning were calculated using Sexual Health Inventory for Men (SHIM) score24. SHIM which is a patient self-administrated questionnaire for assessment of male sexual dysfunction, scoring from 1 to 21 scores, with 98% sensitivity and 88% specificity24. The observed SHIM scores in the LC patients ranged from 1 to 21, with the following characterizations: normal = > 21, mild ED is 17–21, moderate ED is 8–16, and severe ED is 1–7. The only observed scores in the LC/ED group ranged from 1 to 21. However, baseline psychiatric evaluations of the recruited patients were not performed.
Statistical analysis
The mean ± standard deviation (SD), number (n), and percentages (%) used in this study were used to express and present the data. Data analysis was carried out utilizing SPSS, version 24. (IBM Corp., Armonk, NY, USA). To determine the significance of the difference between the LC patients and controls, an unpaired Student’s t-test was used. p values < 0.05 were regarded as significant.
Ethics approval
The Scientific Jury and Ethical Committee of Mustansiriyah University in Iraq granted permission and gave their seal of approval for the current study (Ethical Approval Number 45QTR/2020) in accordance with the Helsinki Declaration. All individuals gave their informed consent to participate in the study.
Consent to participate
All individuals taking part in the study gave their informed consent.
Results
A total of 65 eligible patients and healthy controls were enrolled in the current study. Six patients were excluded due to diabetes mellitus (n = 3), severe hypertension (n = 2), and unidentified reasons (n = 1). Therefore, a total of n = 59 were recruited and continued the study, with n = 39 post-COVID-19 patients, and n = 20 healthy controls (Fig. 2). In the current investigation, LC patients with ED had blood pressure profiles that were higher than those of the controls (p < 0.01), with the exception of pulse pressure (PP) where the difference was not statistically significant (p = 0.06). Patients with LC have anti-SARS-CoV-2 IgG antibodies, and were associated with other comorbidities, including hypertension (23.07%) and asthma (7.69%). Regarding the patients’ response histories to phosphodiesterase inhibitors (PDEIs), most of the patients were responders, n = 30 (76.92%), compared to the non-responders (p = 0.04). Additional distinctive features are illustrated in Table 1.
Hormonal changes in LC patients with ED showed that TT serum levels were lower compared with the controls (18.52 ± 6.95 nmol/L vs. 22.95 ± 5.91 nmol/L, (p = 0.01). A similar trend was observed for FT and FAI (p = 0.001). FSH serum levels did not significantly differ (p = 0.07), although the LH serum levels were increased in LC patients with ED (Table 2). Regarding SHIM scores, LC patients with ED had lower SHIM scores compared with the controls (18.56 ± 2.75 vs. 23.98 ± 1.64, p < 0.0001) (Fig. 3). Moreover, SHIM scores were associated with low TT (OR, 2.18, 95% CI 0.48–9.87, p = 0.30), FT (OR 4.62, 95% CI 0.76–27.86, p = 0.09), and high LH (OR 1.33, 95% CI 0.20–8.70, p = 0.76) in LC patients with ED (Table 3).
Discussion
Acute COVID-19 can certainly cause ED due to cytokine storm, endothelial dysfunction, cardiovascular instability, psychological impact, and hypoxia, as well as due to fear of sexual contact25,26. This novel study demonstrated the potential link between COVID-19 and ED in individuals who had recovered from mild-to-moderate COVID-19. Here, LC patients with ED were observed to have higher blood pressure profiles compared with healthy controls, with 23% patients being hypertensive and regular users of antihypertensive medications. Nonetheless, and based on previous studies, long-term cardiovascular complications are also linked to SARS-CoV-2 infection27,28. Additionally, LC may exacerbate underlying hypertension and other cardiovascular complications due to immunological and inflammatory instability29,30. It is noteworthy that the effects of LC appear to be very similar to other systemic diseases, such as diabetes mellitus, which cause microvascular dysfunction and autonomic neuropathy31,32.
In the present study, patients with ED have low TT and FT serum levels, and high LH serum levels compared with controls. Okcelik, 2021 confirmed that COVID-19 was associated with low TT serum levels due to suspected testicular injury induced by SARS-CoV-213. It has been shown that Leydig cells and seminiferous tubule damage due to severe SARS-CoV-2 infection may impede spermatogenesis33. Therefore, the net effect of testicular injury in COVID-19 may adversely impact the androgenic profile and sex drive in patients with LC. It has been reported that testosterone is the primary regulator of sex desire, motivation, and penile erection in both humans and animals, thus, a reduction in testosterone may lead to the development of ED34.
Of note, thrombosis, cytokine storm, and hyperinflammation, SARS-CoV-2 infection may impair the HPG axis35,36. This may extend for a period and cause ED and male infertility. Due to a decrease in the production of LH and FSH from the anterior pituitary gland and gonadotropin-releasing factor (GnRF) from the hypothalamus, these pathological alterations may potentially result in central hypogonadism37. In the current investigation, LC patients' LH serum levels were lower than those of the controls, indicating dysfunction of the HPG axis. However, FSH serum level was insignificantly higher compared with the controls, which could be due to Leydig and Sertoli cell dysfunction38. Tabar et al. shown how the ACE2-mediated pathway may be used by SARS-CoV-2 infection to harm Sertoli cells and trigger the onset of male infertility39. Moreover, other endocrinopathies in LC patients, such as thyroid dysfunction and insulin resistance, may cause functional hypogonadotropic hypogonadism12,40. These findings suggest that dysfunction of the HPG axis in LC patients could be the possible mechanism for the development of ED.
Notably, down-regulation of ACE2 receptors in the testis and penile cavernous tissue leads to a reduction in testosterone secretion from Leydig cells41, and induction of AngII dependent-cavernous vasoconstriction due to reduction of Ang1-7/Mas bioactivity42. This is because testicular Ang1-7/Mas complex, which is expressed in Leydig and Sertoli cells, are distorted by high local or circulating AngII43. Thus, low testosterone-dependent ED could be caused by RAS dysregulation in COVID-1944.
Since high testosterone serum levels increase expressions of the angiotensin-converting enzyme-2/serine transmembrane protease serine 2 (ACE2/TRMPSS2) axes, which is essential for the entry and pathogenesis of SARS-CoV-2, it has been demonstrated that a reduction in testosterone levels in COVID-19 and LC patients may be a counter-balancing protective mechanism against SARS-CoV-2 propagation and induced complications45. Additionally, high testosterone serum levels increase the permissive effect of AngII through the expression of angiotensin type 1 receptors46. Similar to this, experimental study investigation from Mishra et al., 2016 showed that testosterone injection decreases the expression of the vasodilator angiotensin type 2 receptors (AT2R) in rats47. According to past and present experiences, testosterone therapy for the treatment of hypogonadism and ED in LC patients should be avoided because hyperandrogenic status is linked to the severity of COVID-1948. Of note, animal and human studies have reported that hypogonadism is linked with pro-inflammatory cytokines which may adversely affect erectile function mainly in aging men because testosterone blocks a variety of cytokines, including TNF-α, IL-1β, and IL-649. Therefore, down-regulation of ACE2 by pro-inflammatory cytokines in hypogonadism may augment the seriousness of COVID-19 in men because of the negative correlation between COVID-19 mortality and the expression of ACE250. However, testosterone exerts dimorphic roles in COVID-19 infections. At optimal levels, it is protective against the severe form for males but otherwise for females. It has been reported that testosterone serum level is reduced by aging and cardio-metabolic diseases including T2DM, obesity, dyslipidemia, heart failure, and atherosclerosis, which are common risk factors for the development of COVID-19 severity51. In addition, Pro-inflammatory cytokines, mainly IL-6, are involved in the pathogenesis of acute lung injury (ALI), acute respiratory distress syndrome (ARDS), and cytokine storm-induced multi-organ damage in COVID-1952. Of note, testosterone inhibits the synthesis and release of IL-6 and downregulates the expression of IL-6 receptors51. Therefore, IL-6 serum level is augmented in hypogonadism patients that increase their susceptibility for COVID-19 severity53. Therefore, COVID-19-induced reduction in circulating testosterone may induce ALI due to increase of pro-inflammatory.
Lower SHIM scores were also observed in patients with ED in comparison with the controls. These scores were associated with low TT, FT, FAI, and high LH, as reported by Cappelleri and Rosen, 200554. Therefore, ED in LC patients has a complicated etiology and not simply attributable to low testosterone serum levels, as endothelial dysfunction, oxidative stress, pro-inflammatory cytokine hyper-activation, and high AngII serum levels are also interrelated in the onset of ED55,56. Furthermore, the response to the effect of phosphodiesterase type 5 inhibitors during sexual excitation could be a possible diagnostic tool for the assessment of ED57.
Notably, the autonomic nervous system is crucial for the regulation of male erection and ejaculation58, and autonomic dysfunction is associated with the development of ED59. Interestingly, different studies have revealed that LC patients experience dysautonomic features because auto-antibodies against muscarinic receptors and β adrenoceptors have developed60,61. Unfortunately, in the present study, autonomic functions were not assessed precisely. However, the blood pressure of LC patients was higher compared to the controls that might due to dysregulation of RAS by SARS-CoV-2 infection with increasing of pro-inflammatory and vasoconstrictor AngII, and reduction of anti-inflammatory and vasodilator Ang1-73,62,63. Chen et al.64 from a cohort study confirmed that dysregulation of RAS in acute SARS-CoV-2 infection with increasing AngII could continue for long time and predispose for development of hypertension in LC patients. These verdicts proposed development of autonomic dysfunction in LC patients.
There underlying mechanism of LC-induced ED could be related to the development long-lasting endothelial dysfunction, autonomic neuropathy, endocrine dysfunction, testicular injury, and neuropsychiatric disorders12,65. However, the precise cause of ED in LC patients was not fully elucidated that needs further studies.
The present case-controlled study has a variety of limitations, including a limited sample size and a case-controlled study design that may affect the identification of causality and outcomes. Additionally, biomarkers of endothelial and oxidative stress and the ACE2/TRMPSS2 axis were not evaluated. Evaluation of anxiety, depression, stress levels, and psychological profile of the LC patients were also not conducted, although these factors may all interfere with erectile function. Furthermore, the overnight erection test, injection test, penile colored Doppler test, and ultrasound for ED that is more specific were not performed in the present study. Inflammatory biomarkers were also not estimated although they may reflect inflammatory status in LC patients. Given that ED, regardless of covid-19, increases with age, particularly after the age of 40, though we not have considered stratifying by age. In particular, LC patients with ED were not compared with LC patients without ED that is an integral point to show effect of LC on the hormonal changes and their relation in the development of ED. Nonetheless, despite of these limitations, strength of this case-controlled study it was regarded as an initial step toward authenticating the underlying causes of ED in LC patients. The present study confirmed that LC patients may experience a rate of ED due to reduction of testosterone levels.
Conclusions
LC-induced ED may develop due to dysfunction of the HPA axis in male patients, as evidenced by reduced testosterone and LH serum levels. Therefore, in-depth research is required to confirm the causal link between COVID-19 and ED in LC patients.
Clinical significance
The COVID-19 pandemic is brought on by SARS-CoV-2. There have been claims that COVID-19 patients have lower testosterone serum levels when compared with healthy controls. Additionally, low testosterone levels are linked with ED, so testosterone therapy may improve sexual function and prevent or reduce ED.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Al-Kuraishy, H. M., Al-Gareeb, A. I., Alkazmi, L., Alexiou, A. & Batiha, G. E. Levamisole therapy in COVID-19. Viral Immunol. 34, 722–725. https://doi.org/10.1089/vim.2021.0042 (2021).
Al-Kuraishy, H. M. et al. Arginine vasopressin and pathophysiology of COVID-19: An innovative perspective. Biomed. Pharmacother. 143, 112193. https://doi.org/10.1016/j.biopha.2021.112193 (2021).
Al-Kuraishy, H. M., Al-Gareeb, A. I. & El-Saber Batiha, G. E. The possible role of ursolic acid in Covid-19: A real game changer. Clin. Nutr. ESPEN 47, 414–417. https://doi.org/10.1016/j.clnesp.2021.12.030 (2022).
Al-Kuraishy, H. M. et al. COVID-19 and phosphodiesterase enzyme type 5 inhibitors. J. Microsc. Ultrastruct. 8, 141–145. https://doi.org/10.4103/JMAU.JMAU_63_20 (2020).
Lugnier, C., Al-Kuraishy, H. M. & Rousseau, E. PDE4 inhibition as a therapeutic strategy for improvement of pulmonary dysfunctions in Covid-19 and cigarette smoking. Biochem. Pharmacol. 185, 114431. https://doi.org/10.1016/j.bcp.2021.114431 (2021).
Al-Kuraishy, H. M. et al. Covid-19-induced dysautonomia: A menace of sympathetic storm. ASN Neuro 13, 17590914211057636. https://doi.org/10.1177/17590914211057635 (2021).
Alkazmi, L. et al. Dantrolene and ryanodine receptors in COVID-19: The daunting taskand neglected warden. Clin. Exp. Pharmacol. Physiol. https://doi.org/10.1111/1440-1681.13756 (2023).
Batiha, G. E., Al-Kuraishy, H. M., Al-Gareeb, A. I. & Welson, N. N. Pathophysiology of post-COVID syndromes: A new perspective. Virol. J. 19, 158. https://doi.org/10.1186/s12985-022-01891-2 (2022).
Greenhalgh, T., Knight, M., A’Court, C., Buxton, M. & Husain, L. Management of post-acute Covid-19 in primary care. BMJ 370, m3026. https://doi.org/10.1136/bmj.m3026 (2020).
Datta, S. D., Talwar, A. & Lee, J. T. A proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 infection: Illness beyond acute infection and public health implications. JAMA 324, 2251–2252. https://doi.org/10.1001/jama.2020.22717 (2020).
Amenta, E. M. et al. Postacute COVID-19: An overview and approach to classification. Open Forum Infect. Dis. 7, 509. https://doi.org/10.1093/ofid/ofaa509 (2020).
Sansone, A. et al. The sexual long COVID (SLC): Erectile dysfunction as a biomarker of systemic complications for COVID-19 long haulers. Sex. Med. Rev. 10, 271–285. https://doi.org/10.1016/j.sxmr.2021.11.001 (2022).
Okçelik, S. COVID-19 pneumonia causes lower testosterone levels. Andrologia 53, e13909. https://doi.org/10.1111/and.13909 (2021).
Rastrelli, G., Corona, G. & Maggi, M. Testosterone and sexual function in men. Maturitas 112, 46–52. https://doi.org/10.1016/j.maturitas.2018.04.004 (2018).
Corona, G. et al. Andrological effects of SARS-Cov-2 infection: A systematic review and meta-analysis. J. Endocrinol. Investig. 45, 2207–2219. https://doi.org/10.1007/s40618-022-01801-x (2022).
Hayden, M. R. An immediate and long-term complication of COVID-19 may be type 2 diabetes mellitus: The central role of β-cell dysfunction, apoptosis and exploration of possible mechanisms. Cells 9, 2475. https://doi.org/10.3390/cells9112475 (2020).
Sevim, M., Alkis, O., Kartal, I. G., Telli, S. & Aras, B. A factor not to be ignored in post-COVID-19 erectile dysfunction; psychological effect, a prospective study. Andrologia 54, e14443. https://doi.org/10.1111/and.14443 (2022).
Segraves, R. T. Considerations for diagnostic criteria for erectile dysfunction in DSM v. J.. J. Sex. Med. 7, 654–660. https://doi.org/10.1111/j.1743-6109.2009.01684.x (2010).
Raveendran, A. V. Long COVID-19: Challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metab. Syndr. 15, 145–146. https://doi.org/10.1016/j.dsx.2020.12.025 (2021).
Al-Kuraishy, H. M., Hussien, N. R., Al-Naimi, M. S., Al-Gareeb, A. I. & Lugnier, C. Statins therapy improves acute ischemic stroke in patients with cardio-metabolic disorders measured by lipoprotein-associated phospholipase A2 (Lp-PLA2): New focal point. Neurol. India 69, 1637–1644. https://doi.org/10.4103/0028-3886.333482 (2021).
Al-Kuraishy, H. M. & Al-Gareeb, A. I. Effect of orlistat alone or in combination with Garcinia Cambogia on visceral adiposity index in obese patients. J. Intercult. Ethnopharmacol. 5, 408–414. https://doi.org/10.5455/jice.20160815080732 (2016).
Ozata, M., Oktenli, C., Bingol, N. & Ozdemir, I. C. The effects of metformin and diet on plasma testosterone and leptin levels in obese men. Obes. Res. 9, 662–667. https://doi.org/10.1038/oby.2001.90 (2001).
Al-Kuraishy, H. M. & Al-Gareeb, A. I. Erectile dysfunction and low sex drive in men with type 2 DM: The potential role of diabetic pharmacotherapy. J. Clin. Diagn. Res. 10, 21–26. https://doi.org/10.7860/JCDR/2016/19971.8996 (2016).
Baradaran, N. et al. The association of bicycle-related genital numbness and Sexual Health Inventory for Men (SHIM) score: Results from a large, multinational, cross-sectional study. BJU Int. 124, 336–341. https://doi.org/10.1111/bju.14396 (2019).
Ibarra, F. P. et al. Impact of the COVID-19 pandemic on the sexual behavior of the population. The vision of the east and the west. Int. Braz. J. Urol. 46(Suppl 1), 104–112. https://doi.org/10.1590/S1677-5538.IBJU.2020.S116 (2020).
Al-Kuraishy, H. M. et al. Effects of β-blockers on the sympathetic and cytokines storms in Covid-19. Front. Immunol. 12, 749291. https://doi.org/10.3389/fimmu.2021.749291 (2021).
Mechanick, J. I. et al. Coronavirus and cardiometabolic syndrome: JACC focus seminar. J. Am. Coll. Cardiol. 76, 2024–2035. https://doi.org/10.1016/j.jacc.2020.07.069,Pubmed:33092738 (2020).
El-Saber Batiha, G., Al-Gareeb, A. I., Saad, H. M. & Al-Kuraishy, H. M. COVID-19 and corticosteroids: A narrative review. Inflammopharmacology 30, 1189–1205. https://doi.org/10.1007/s10787-022-00987-z (2022).
Acanfora, D. et al. The cross-talk between thrombosis and inflammatory storm in acute and long-COVID-19: Therapeutic targets and clinical cases. Viruses 13, 1904. https://doi.org/10.3390/v13101904 (2021).
Babalghith, A. O. et al. The potential role of growth differentiation factor 15 in COVID-19: A corollary subjective effect or not? Diagnostics 12, 2051. https://doi.org/10.3390/diagnostics12092051 (2022).
Defeudis, G. et al. Erectile dysfunction and diabetes: A melting pot of circumstances and treatments. Diabetes Metab. Res. Rev. 38, e3494. https://doi.org/10.1002/dmrr.3494 (2022).
Al-Kuraishy, H. M., Al-Gareeb, A. I., Waheed, H. J. & Al-Maiahy, T. J. Differential effect of metformin and/or glyburide on apelin serum levels in patients with type 2 diabetes mellitus: Concepts and clinical practice. J. Adv. Pharm. Technol. Res. 9, 80–86. https://doi.org/10.4103/japtr.JAPTR_273_18 (2018).
Li, H. et al. Impaired spermatogenesis in COVID-19 patients. EClinicalmedicine 28, 100604. https://doi.org/10.1016/j.eclinm.2020.100604 (2020).
Corona, G. et al. Meta-analysis of results of testosterone therapy on sexual function based on international index of erectile function scores. Eur. Urol. 72, 1000–1011. https://doi.org/10.1016/j.eururo.2017.03.032 (2017).
Selvaraj, K. et al. Testicular atrophy and hypothalamic pathology in COVID-19: Possibility of the incidence of male infertility and HPG axis abnormalities. Reprod. Sci. 28, 2735–2742. https://doi.org/10.1007/s43032-020-00441-x (2021).
Al-Kuraishy, H. M., Al-Gareeb, A. I., Qusty, N., Alexiou, A. & Batiha, G. E. Impact of sitagliptin on non-diabetic Covid-19 patients. Curr. Mol. Pharmacol. 15, 683–692. https://doi.org/10.2174/1874467214666210902115650 (2022).
Grinspon, R. P. et al. Diagnosis of male central hypogonadism during childhood. J. Endocr. Soc. 5, 145. https://doi.org/10.1210/jendso/bvab145 (2021).
Winters, S. J., Moore, J. P. Jr. & Clark, B. J. Leydig cell insufficiency in hypospermatogenesis: A paracrine effect of activin–inhibin signaling? Andrology 6, 262–271. https://doi.org/10.1111/andr.12459 (2018).
Tabar, A. N., Sojoudi, K., Henduei, H. & Azizi, H. Review of Sertoli cell dysfunction caused by COVID-19 that could affect male fertility. Zygote 30, 17–24. https://doi.org/10.1017/S0967199421000320 (2022).
Al-Kuraishy, H. M. et al. The prospective effect of allopurinol on the oxidative stress index and endothelial dysfunction in Covid-19. Inflammation 45, 1651–1667. https://doi.org/10.1007/s10753-022-01648-7 (2022).
Douglas, G. C. et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology 145, 4703–4711. https://doi.org/10.1210/en.2004-0443 (2004).
Bragina, M. E. et al. Characterization of the renin-angiotensin system in aged cavernosal tissue and its role in penile fibrosis. J. Sex. Med. 17, 2129–2140. https://doi.org/10.1016/j.jsxm.2020.08.008 (2020).
Reis, A. B. et al. Angiotensin (1–7) and its receptor Mas are expressed in the human testis: Implications for male infertility. J. Mol. Histol. 41, 75–80. https://doi.org/10.1007/s10735-010-9264-8 (2010).
Ojeda, N. B. et al. Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1421–R1427. https://doi.org/10.1152/ajpregu.00096.2010 (2010).
Salah, H. M. & Mehta, J. L. Hypothesis: Sex-related differences in ACE2 activity may contribute to higher mortality in men versus women with COVID-19. J. Cardiovasc. Pharmacol. Ther. 26, 114–118. https://doi.org/10.1177/1074248420967792 (2021).
Mishra, J. S., More, A. S., Gopalakrishnan, K. & Kumar, S. Testosterone plays a permissive role in angiotensin II-induced hypertension and cardiac hypertrophy in male rats. Biol. Reprod. 100, 139–148. https://doi.org/10.1093/biolre/ioy179 (2019).
Mishra, J. S., Hankins, G. D. & Kumar, S. Testosterone downregulates angiotensin II type-2 receptor via androgen receptor-mediated ERK1/2 MAP kinase pathway in rat aorta. J. Renin Angiotensin Aldosterone Syst. 17, 4875. https://doi.org/10.1177/1470320316674875 (2016).
Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720. https://doi.org/10.1056/NEJMoa2002032 (2020).
Mohamad, N. V. et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 22, 129–140. https://doi.org/10.1080/13685538.2018.1482487 (2019).
Chen, J. et al. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 19, e13168. https://doi.org/10.1111/acel.13168 (2020).
Al-Kuraishy, H. M., Al-Gareeb, A. I., Faidah, H., Alexiou, A. & Batiha, G. E. Testosterone in COVID-19: An adversary bane or comrade boon. Front. Cell. Infect. Microbiol. 11, 666987. https://doi.org/10.3389/fcimb.2021.666987 (2021).
Batiha, G. E. et al. Hypertension and its management in COVID-19 patients: The assorted view. Int. J. Cardiol. Cardiovasc. Risk Prev. 11, 200121. https://doi.org/10.1016/j.ijcrp.2021.200121 (2021).
Papadopoulos, V., Li, L. & Samplaski, M. Why does COVID-19 kill more elderly men than women? Is there a role for testosterone? Andrology 9, 65–72. https://doi.org/10.1111/andr.12868 (2021).
Cappelleri, J. C. & Rosen, R. C. The Sexual Health Inventory for Men (SHIM): A 5-year review of research and clinical experience. Int. J. Impot. Res. 17, 307–319. https://doi.org/10.1038/sj.ijir.3901327 (2005).
Kalyanaraman, B. Do free radical NETwork and oxidative stress disparities in African Americans enhance their vulnerability to SARS-CoV-2 infection and COVID-19 severity? Redox Biol. 37, 101721. https://doi.org/10.1016/j.redox.2020.101721 (2020).
Marchetti, M. COVID-19-driven endothelial damage: Complement, HIF-1, and ABL2 are potential pathways of damage and targets for cure. Ann. Hematol. 99, 1701–1707. https://doi.org/10.1007/s00277-020-04138-8 (2020).
Isidori, A. M., Giammusso, B., Corona, G. & Verze, P. Diagnostic and therapeutic workup of erectile dysfunction: Results from a Delphi consensus of andrology experts. Sex. Med. 7, 292–302. https://doi.org/10.1016/j.esxm.2019.04.001 (2019).
Clement, P. & Giuliano, F. Physiology and pharmacology of ejaculation. Basic Clin. Pharmacol. Toxicol. 119(Suppl 3), 18–25. https://doi.org/10.1111/bcpt.12546 (2016).
Karupasamy, G. & Karthick, K. Autonomic dysfunction in cardiovascular system of type 2 diabetic mellitus—A bedside evaluation. Int. Arch. Integr. Med. 5, 30–33 (2018).
Barizien, N. et al. Clinical characterization of dysautonomia in long COVID-19 patients. Sci. Rep. 11, 14042. https://doi.org/10.1038/s41598-021-93546-5 (2021).
Johansson, M. et al. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: The Swedish experience. JACC Case Rep. 3, 573–580. https://doi.org/10.1016/j.jaccas.2021.01.009 (2021).
Al-Kuraishy, H. M., Al-Gareeb, A. I., Alkazmi, L., Habotta, O. A. & Batiha, G. E. High-mobility group box 1 (HMGB1) in COVID-19: Extrapolation of dangerous liaisons. Inflammopharmacology 30, 811–820. https://doi.org/10.1007/s10787-022-00988-y (2022).
Al-Kuraishy, H. M. & Al-Gareeb, A. I. From SARS-CoV to nCoV-2019: Ruction and argument. Arch. Clin. Infect. Dis. 15, 102624. https://doi.org/10.5812/archcid.102624 (2020).
Chen, G. et al. Hypertension as a sequela in patients of SARS-CoV-2 infection. PLoS ONE 16, e0250815. https://doi.org/10.1371/journal.pone.0250815 (2021).
Kaynar, M., Gomes, A. L. Q., Sokolakis, I. & Gül, M. Tip of the iceberg: Erectile dysfunction and COVID-19. Int. J. Impot. Res. 34, 152–157. https://doi.org/10.1038/s41443-022-00540-0 (2022).
Acknowledgements
The authors greatly appreciate the support of Princess Nourah bint Abdulrahman University in funding this research through: Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2023R167), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization: H.M.A., and A.I.A.-G. Data curation: H.M.A., A.I.A.-G., M.M.E.-B., and G.E.-S.B. Formal analysis: H.M.A., A.I.A.-G., M.M.E.-B., S.J.A., R.K.A.-A., T.A.E.-M. and G.E.B. Methodology: H.M.A., and A.I.A.-G. Investigation: M.M.E.-B., H.F., S.J.A., R.K.A.-A., T.A.E.-M., J.-M.S., M.D.W., and G.E.B. Visualization: H.M.A., A.I.A.-G., M.M.E.-B., T.A.E.-M. and G.E.B. Writing—original draft: H.M.A., M.M.E.-B., S.J.A., R.K.A.-A., G.E.B., H.F., J.-M.S., M.D.W., and A.I.A.-G. Writing—review & editing: H.M.A., A.I.A.-G., M.M.E.-B., and G.E.-S.B. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-kuraishy, H.M., Al-Gareeb, A.I., Alarfaj, S.J. et al. Long COVID and risk of erectile dysfunction in recovered patients from mild to moderate COVID-19. Sci Rep 13, 5977 (2023). https://doi.org/10.1038/s41598-023-32211-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32211-5
This article is cited by
-
SARS-CoV-2 infection and dysregulation of nuclear factor erythroid-2-related factor 2 (Nrf2) pathway
Cell Stress and Chaperones (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.