Abstract
This study compared the time profile of FEV1 after COPD diagnosis among rapid decliners, slow decliners, and sustainers in the year of COPD diagnosis. COPD subjects were identified from the annual medical checkup records of Hitachi, Ltd., employees in Japan (April 1998-March 2019). Subjects were categorized into 3 groups (rapid decliner [decrease of FEV1 ≥ 63 mL/year], slow decliner [< 63 and ≥ 31 mL/year], and sustainer [< 31 mL/year]) for 5 years. The time profile of FEV1 was compared using mixed-effects model for 5 years after diagnosis; risk factors of rapid decliner were detected using logistic model/gradient boosting decision tree. Of 1294 eligible subjects, 18.6%, 25.7%, and 55.7% were classified as rapid decliners, slow decliners, and sustainers, respectively. The annual rates of FEV1 decline were similar 3 years before and until COPD diagnosis. The mean FEV1 in rapid decliners was 2.82 ± 0.04 L in year 0 and 2.41 ± 0.05 L in year 5, and in sustainers, it was 2.67 ± 0.02 L and 2.72 ± 0.02 L (year 0, p = 0.0004). In conclusion, FEV1 declined yearly before diagnosis and the time profiles of FEV1 were different in the 3 groups after COPD diagnosis. Therefore, appropriate treatment of the 3 groups with regular lung function tests is necessary to follow FEV1 decline after COPD onset.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of death globally and a significant driver of healthcare economic costs1,2,3. Irreversible airway obstruction and progressive lung function decline are representative of the nature of the disease1,2,3,4.
Multiple studies have linked rapid forced expiratory volume in 1 s (FEV1) decline with worse disease outcomes4,5,6,7,8,9. A more rapid rate of FEV1 decline has been related to more frequent exacerbations4. Moreover, rapid FEV1 decline is associated with an increased risk of COPD-related hospitalizations and deaths4,5,6,7,8,9,10. Factors associated with rapid lung function decline include age and sex11. A study that included over 10,000 subjects showed that FEV1 rapid decliners were older and more likely to be male than nonrapid decliners11. In addition, identifying factors associated with rapid FEV1 decline at an early stage of COPD is of particular clinical importance because annual FEV1 decline is greater at an early stage of COPD than at an advanced stage12,13. Several factors are involved in the FEV1 decline in COPD subjects, including cigarette smoking and the frequency of exacerbations4,7. However, information on risk factors in mild-moderate COPD subjects is limited14. Robust real-world evidence is needed to improve our understanding of the nature of rapid FEV1 decline.
In the current study, we aimed to compare the time profile of FEV1 before and after COPD diagnosis among rapid decliners, slow decliners, and sustainers and identify the patient characteristics by leveraging an annual medical checkup database.
Methods
Study design and data source
This was a retrospective cohort study that analyzed characteristics among subjects with COPD. Data were collected from the annual medical checkup of current and retired Hitachi, Ltd., employees as well as their families in Japan from April 1998 to March 2019. This database includes the data for approximately 16,000 employees and their families, with ages ranging from 18 to 75 years. Details of the data source have been described in our previous study15. Subjects with COPD aged 30–75 years who had undergone at least 3 annual medical checkups within 5 years were analyzed for this study. The index date was defined as the date of COPD diagnosis (Fig. 1). The annual medical checkup includes clinical measurements such as routine FEV1 in the absence of suspected COPD and questionnaires to examine the health conditions of employees (see Supplementary Table 1). Information on the equipment used is unavailable, and spirometry was conducted in accordance with the guideline recommended by the Japanese Respiratory Society for lung function testing16. Individual informed consent was obtained using an opt-out model in agreement with the Institutional Review Board at Hitachi, Ltd. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Study population
Subjects with a pre-bronchodilator (pre-BD) FEV1/forced vital capacity (FVC) measurement of < 0.7 during an annual lung function test for 2 consecutive years were defined as having COPD17. Subjects with a pre-BD FEV1/FVC measurement of ≥ 0.7 in at least 3 consecutive annual lung function tests were regarded as non-COPD subjects17. When more than 3 records were available for non-COPD subjects, the 3 most recent records were analyzed. Individuals with ˂ 2 lung function tests were excluded. Those who had lung cancer or asthma were excluded, as most subjects with asthma-COPD overlap had a confirmed asthma diagnosis, were predominantly female, had a longer duration of COPD, used respiratory medications and statins more frequently, and had more comorbidities, which may introduce a bias15,18. In order to compare the time profiles of FEV1 over 5 years after COPD diagnosis, subjects were categorized by their FEV1 measurements based on the criteria used in the Hokkaido COPD Cohort Study: ≥ 63 mL/year as rapid decliners, < 63 and ≥ 31 mL/year as slow decliners, and < 31 mL/year as sustainers19.
Outcomes
The time profile of FEV1 evaluated across COPD diagnosis was the primary outcome of this study. Characteristics of subjects diagnosed with COPD were also compared between the 3 groups. The factors associated with a rapid FEV1 decline were investigated using a machine learning (ML) approach between rapid decliners and nonrapid decliners (slow decliners + sustainers). The answers obtained through a questionnaire at the year of COPD diagnosis were summarized.
Statistical analysis
Lung function time profile after COPD diagnosis (longitudinal analysis)
FEV1 of the 3 groups was calculated for 5 years after COPD diagnosis. The time profiles of the 3 groups were compared using a mixed-effects model that included baseline value, sex, age (< 60 years, ≥ 60 years), smoking status, body mass index (BMI; < 25 kg/m2, ≥ 25 kg/m2), and blood eosinophil (EOS) count (< 200 cells/mm3, ≥ 200 cells/mm3) as fixed effects, and time as a random variable.
Detection of factors associated with rapid decliners
Logistic regression and gradient boosting decision tree (XGBoost) were applied to predict the factors associated with rapid FEV1 decline, as described in our previous report15. Models were constructed in the training sets and validated for model evaluation in the test datasets. The training dataset that consisted of eligible subjects was first generated and then randomly split into 80% for model construction and the remaining 20% for evaluation of model performance. A test dataset was also created. The ratio of training and test sets was 4:1. The process from data split to model evaluation was repeated 5 times for cross-validation (fivefold cross-validation). The most fitted model was investigated by area under the curve (AUC). The contributions of each predictor to the constructed model were examined by calculating the feature importance. Statistical analyses were performed using R version 3.6 (R foundation for Statistical Software) and Python 3.6 (Python Software Foundation). p values are shown in all comparisons, and p-value that shows less than 5% was considered a statistically significant difference.
Ethics approval and consent to participate
The study protocol was reviewed and approved by the ethics committee of MINS (a non-profit organization in Tokyo, Japan) and the Research and Development Group and Corporate Hospital Group of Hitachi, Ltd. (Tokyo, Japan) prior to the start of data analysis. Individual informed consent was obtained using an opt-out model in agreement with the Institutional Review Board at Hitachi, Ltd. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Results
Patient disposition
Of 26,101 subjects, 24,807 were excluded (Fig. 2) as they met the exclusion criteria. In total, 1294 were included in the study; 241 (18.6%) were classified as rapid decliners, 332 (25.7%) as slow decliners, and 721 (55.7%) as sustainers.
Characteristics of subjects diagnosed with COPD in each group
Patient characteristics are shown in Table 1. Fewer current smokers were observed in the sustainers than in the rapid or slow decliners. There were more ex-smokers and nonsmokers in the sustainers compared with the others. Lower means of BMI (22.6 kg/m2 vs 23.05 kg/m2), waist circumference (80.9 cm vs 82.5 cm), and body fat percentage (20.2% vs 21.5%) were seen in the rapid decliners compared with the sustainers group (Table 1).
Rapid decliners had higher lung function (mean ± standard error) at the year of COPD diagnosis in comparison with sustainers (FEV1 = 2.82 ± 0.04 L vs 2.67 ± 0.02 L [p = 0.0004], FVC = 4.18 ± 0.04 L vs 3.93 ± 0.05 L [p = 0.0003], and percentage vital capacity (%VC) = 110% vs 103.8% [p < 0.000001]).
The answers obtained through a questionnaire at the year of COPD diagnosis are summarized in Table 2. A statistically significant difference in sleep duration between rapid decliners and sustainers was observed (p < 0.000001). A total of 21 (8.71%) rapid decliners reported sleeping for over 7 h during the past months vs 113 (15.67%) sustainers. Most rapid decliners slept in a range of 5–6 h during the past month vs 6–7 h reported by sustainers. Regarding the degree of physical activity at work, a significant difference was observed between rapid decliners and sustainers, wherein rapid decliners engaged in increased workplace activity as compared to sustainers. Similarly, decliners’ daily consumption of breakfast (rapid decliners: p < 0.000001 and slow decliners: p = 0.000001) and regular exercise (rapid decliners: p = 0.000018 and slow decliners: p = 0.000002) varied significantly from the sustainers.
Lung function trajectories across the diagnosis of COPD
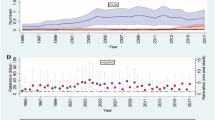
The time profile of lung function across the diagnosis of COPD is shown in Fig. 3. The time profile in rapid decliners was statistically different from that in slow decliners or sustainers (p = 0.0001).
Rapid decliners showed the highest mean ± standard error FEV1 of 2.82 ± 0.04 L in year 0 among the 3 groups; this mean value sharply declined to 2.41 ± 0.05 L in year 5. Slow decliners presented a mean FEV1 of 2.71 ± 0.03 L in year 0, which steadily declined to 2.52 ± 0.03 L in year 5. The mean FEV1 of sustainers was 2.67 ± 0.02 L in year 0, which was slightly lower compared with that of slow decliners, increased to 2.70 ± 0.02 L in year 2, and then continued to increase to 2.72 ± 0.02 L in year 5. The time profile of FEV1 was created for 3 years prior to the onset of COPD (Fig. 1). Annual rates for the decline were similar from year − 3 to year 0, except for sustainers with the highest mean FEV1 decline at index (− 0.07 mL). There was a large decline in sustainers from year − 1 to year 0.
Factors associated with a rapid decline in FEV1
The gradient boosting decision tree model (AUC = 0.516) identified factors associated with a rapid FEV1 decline that showed higher importance (see Supplementary Fig. S1 including the top 30 factors) and those based on answers to questionnaires (see Supplementary Fig. S2). Mean FVC decline has been presented in Supplementary Fig. S3. The results of the logistic regression model indicated that vital capacity, mean corpuscular hemoglobin, smoking status, cough, platelet count, total protein, HbA1c, FEV1% (see Supplementary Fig. S4), and uric acid were predictive of rapid decliners. In contrast, arrhythmia, other diseases, FEV1, mean corpuscular volume, BMI, mean corpuscular hemoglobin concentration, albumin, C reactive protein, smoking duration, hematocrit, aspartate aminotransferase, percentage VC, and duration of smoking cessation were unlikely to predict rapid decliners (see Supplementary Table 2). Generally consistent with these results were those obtained using a logistic regression model, including questionnaire-obtained data comprising lifestyle, symptoms, and treatment-related information (see Supplementary Table 3).
Discussion
Studies have reported the rate of FEV1 decline among the total or male Japanese population20,21. In contrast, our study evaluated the time profile of FEV1 capacity grouped by 3 categories (rapid decliners, slow decliners, and sustainers) using datasets, which included working-age Japanese adults, with information collected for over 20 years, providing further characterization for the time profile of FEV1 among subjects with varying rates of decline over time, consistently measured over the course of 8 years.
In the current study, the proportion of rapid decliners among COPD subjects, particularly those newly diagnosed with COPD, remains largely unclear, and 241 (18.6%) were classified according to the Hokkaido cohort as rapid decliners19. Subjects with a higher basal FEV1 showed a steeper decline in lung function, possibly because early-stage COPD subjects with preserved FEV1 might “have more lung function to lose” than those with a more advanced stage of COPD22. Although some similarities were noted, the declines in FEV1 were not exactly parallel among the three groups; for rapid decliners, the greatest decline in lung function was observed during the second year of follow-up, reflecting the accelerated loss of lung function usually observed in the early stage of COPD14,22. The onset of COPD was unpredictable based on the level of annual FEV1 decline between year − 3 and year 0, which were similar between all 3 groups except for the sharp decline in the sustainers between year − 1 and 0. Since no relevant difference was observed in the annual FEV1 decline among the 3 groups, the onset of COPD cannot be determined based on this factor; thus, regular lung function tests are necessary to detect the onset of COPD in a timely manner.
In our study, current smokers were more prevalently rapid decliners and slow decliners compared with sustainers. Although the ML model had a low AUC, it was able to identify smoking status, pack-year, smoking duration, and duration of smoking cessation as potential risk factors for a rapid FEV1 decline. Multiple studies have validated cigarette smoking as a risk factor for accelerated lung function decline7,20,23,24. Smoking cessation may reverse or alleviate the damage incurred over time and restore lung function to near-normal FEV1 values7,20,23.
BMI and body fat percentage were lower in rapid decliners compared with sustainers at the diagnosis of COPD. Similar to previous reports, our ML results support an inverse relationship between BMI and a rapid FEV1 decline25,26,27. This finding has been described as the “obesity paradox” in COPD; a high BMI shows a protective effect, whereas a low BMI has been identified as a factor associated with accelerated lung function decline28.
A rapid FEV1 decline was associated with several lifestyle-related factors. The characteristics describing rapid decliners were short sleeping hours, omission of breakfast, increased alcohol consumption, lack of regular exercise, and high level of physical activity at work. Of note, ML results supported these findings, except for omission of breakfast which showed an opposite trend in ML. Although the discrepancy between patient characteristics and ML findings remains unknown, our findings possibly indicate a role of frequent activity, adequate sleep, and moderate alcohol consumption in preventing a rapid FEV1 decline.
Our analysis has several limitations. First, complete medical and medication history of the participants was not available, which may have impacted the degree of COPD progression; nevertheless, pre-BD lung test was conducted as part of annual medical health checkup. The impact of longitudinal changes in lifestyle on disease trajectory remains a limitation of database research. The reasons for missing data vary broadly, including unrecorded collection during an office visit29,30. Hence, our collected data might not necessarily fully encompass the natural clinical course. For example, the potential contributions of specific comorbidities in the decline in lung function could not be established. Second, using a single database may give biased results, such as overlooking the influence of occupational exposure and the development of COPD31,32. Consequently, to generalize these results and obtain a broader vision of the general population, it is necessary to include multiple databases, especially those from other countries; thus, further studies will be required to evaluate the factors associated with a rapid FEV1 decline in subjects at risk of developing COPD across a diverse population. Although sensitivity analysis based on FEV1 percentile showed similar results (data not shown), we were not able to categorize patients into the 3 groups based on age-matched lower-limit of normality values using this data. Finally, we obtained our results based on the available pre-BD FEV1 values in the absence of post-BD FEV1 values. However, COPD prevalence significantly differs when COPD was diagnosed based on pre-BD compared with post-BD spirometry measurements33. Biological variability and/or measurement errors can give rise to known and expected variations in spirometry values upon repeated testing34,35. Decline in FEV1 values can also be seen in cardiovascular and respiratory disorders36. Although airflow limitation (pre-BD FEV1) is not paramount for COPD diagnosis as systemic inflammation and other clinical signs may not be captured by FEV1, data suggest a link between airflow limitation and prognosis34,37. Nonetheless, pre-BD FEV1 values are known to possibly overestimate COPD prevalence through high false-positive rates; thus, post-BD FEV1 values are usually a more accurate predictor of COPD outcomes, and the data here need to be interpreted with caution38,39,40. Unfortunately, we could not clarify the importance of “rapid decline of FEV1 over time” as a definitive patient characteristic. Ideally, COPD diagnosis requires serial longitudinal spirometry assessments, which should be accompanied by a comprehensive clinical assessment34.
Conclusions
FEV1 declined yearly before diagnosis in rapid decliners, slow decliners, and sustainers. The time profiles of FEV1 were different in the 3 groups after COPD diagnosis. Therefore, appropriate treatment of the 3 groups with regular lung function tests is necessary to follow the FEV1 decline after COPD onset in a timely manner.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- EOS:
-
Eosinophil
- FEV1 :
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- ML:
-
Machine learning
- pre-BD:
-
Pre-bronchodilator
- VC:
-
Vital capacity
References
Choi, J. Y. & Rhee, C. K. Diagnosis and treatment of early chronic obstructive lung disease (COPD). J. Clin. Med. 9, 3426 (2020).
Iheanacho, I., Zhang, S., King, D., Rizzo, M. & Ismaila, A. S. Economic burden of chronic obstructive pulmonary disease (COPD): A systematic literature review. Int. J. Chron. Obstruct. Pulmon. Dis. 15, 439–460 (2020).
Pérez-Padilla, R. et al. Lung function decline in subjects with and without COPD in a population-based cohort in Latin-America. PLoS One 12, e0177032 (2017).
Kesten, S., Celli, B., Decramer, M., Liu, D. & Tashkin, D. Adverse health consequences in COPD patients with rapid decline in FEV1—Evidence from the UPLIFT Trial. Respir. Res. 12, 129 (2011).
Cerveri, I. et al. The rapid FEV(1) decline in chronic obstructive pulmonary disease is associated with predominant emphysema: A longitudinal study. COPD 10, 55–61 (2013).
Bhatt, S. P. et al. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 194, 178–184 (2016).
Wise, R. A. The value of forced expiratory volume in 1 second decline in the assessment of chronic obstructive pulmonary disease progression. Am. J. Med. 119, 4–11 (2006).
Donaldson, G. C., Seemungal, T. A. R., Bhowmik, A. & Wedzicha, J. A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 57, 847–852 (2002).
Mannino, D. M., Reichert, M. M. & Davis, K. J. Lung function decline and outcomes in an adult population. Am. J. Respir. Crit. Care Med. 173, 985–990 (2006).
Celli, B. R. et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 178, 332–338 (2008).
Silvestre, O. M. et al. Declining lung function and cardiovascular risk. J. Am. Coll. Cardiol. 72, 1109–1122 (2018).
Vestbo, J. et al. Changes in forced expiratory volume in 1 second over time in COPD. N. Engl. J. Med. 365, 1184–1192 (2011).
Suzuki, M. et al. Annual change in FEV1 in elderly 10-year survivors with established chronic obstructive pulmonary disease. Sci. Rep. 9, 2073 (2019).
Chen, S. et al. Risk factors for FEV1 decline in mild COPD and high-risk populations. Int. J. Chron. Obstruct. Pulmon. Dis. 12, 435–442 (2017).
Muro, S. et al. Machine learning methods for the diagnosis of chronic obstructive pulmonary disease in healthy subjects: Retrospective observational cohort study. JMIR Med. Inform. 9, e24796 (2021).
Tojo, N., Suga, H. & Kambe, M. Lung function testing—The Official Guideline of the Japanese Respiratory Society. Rinsho Byori 53, 77–81 (2005) (Japanese).
Terzikhan, N. et al. Prevalence and incidence of COPD in smokers and non-smokers: The Rotterdam Study. Eur. J. Epidemiol. 31, 785–792 (2016).
Wurst, K. E. et al. A comparison of COPD patients with and without ACOS in the ECLIPSE study. Eur. Respir. J. 47, 1559–1562 (2016).
Nishimura, M. et al. Annual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 185, 44–52 (2012).
Omori, H., Nonami, Y. & Morimoto, Y. Effect of smoking on FEV1 decline in a cross-sectional and longitudinal study of a large cohort of Japanese males. Respirology 10, 464–469 (2005).
Luoto, J., Pihlsgård, M., Wollmer, P. & Elmståhl, S. Relative and absolute lung function change in a general population aged 60–102 years. Eur. Respir. J. 53, 1701812 (2019).
Tantucci, C. & Modina, D. Lung function decline in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 7, 95–99 (2012).
Leem, A. Y. et al. Longitudinal decline in lung function: A community-based cohort study in Korea. Sci. Rep. 9, 13614 (2019).
Lindberg, A. et al. Decline in FEV1 in relation to incident chronic obstructive pulmonary disease in a cohort with respiratory symptoms. COPD 4, 5–13 (2007).
Sun, Y. et al. BMI is associated with FEV1 decline in chronic obstructive pulmonary disease: A meta-analysis of clinical trials. Respir. Res. 20, 236 (2019).
Tirado-Conde, G. et al. Factors associated with the evolution of lung function in patients with alpha-1 antitrypsin deficiency in the Spanish registry. Arch. Bronconeumol. 47, 495–503 (2011).
Watson, L. et al. Predictors of lung function and its decline in mild to moderate COPD in association with gender: Results from the Euroscop study. Respir. Med. 100, 746–753 (2006).
Sun, Y. & Zhou, J. New insights into early intervention of chronic obstructive pulmonary disease with mild airflow limitation. Int. J. Chron. Obstruct. Pulmon. Dis. 14, 1119–1125 (2019).
Haneuse, S., Arterburn, D. & Daniels, M. J. Assessing missing data assumptions in EHR-based studies: A complex and underappreciated task. JAMA Netw. Open 4, e210184 (2021).
Wells, B. J., Nowacki, A. S., Chagin, K. & Kattan, M. W. Strategies for handling missing data in electronic health record derived data. EGEMS 1, 1035 (2013).
Mehta, A. J. et al. Occupational exposure to dusts, gases, and fumes and incidence of chronic obstructive pulmonary disease in the Swiss Cohort Study on Air Pollution and Lung and Heart Diseases in Adults. Am. J. Respir. Crit. Care Med. 185, 1292–1300 (2012).
Balmes, J. et al. American Thoracic Society Statement: Occupational contribution to the burden of airway disease. Am. J. Respir. Crit. Care Med. 167, 787–797 (2003).
Tilert, T., Dillon, C., Paulose-Ram, R., Hnizdo, E. & Doney, B. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: The National Health and Nutrition Examination Survey (NHANES) 2007–2010. Respir. Res. 14, 103 (2013).
Andreeva, E. et al. Spirometry is not enough to diagnose COPD in epidemiological studies: A follow-up study. NPJ Prim. Care Resp. Med. 27, 62 (2017).
Miller, M. R. et al. ATS/ERS Task Force. General considerations for lung function testing. Eur. Respir. J. 26(1), 153–161 (2005).
Duong, M. et al. Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): An international, community-based cohort study. Lancet Glob. Health 7, e613–e623 (2019).
Akkermans, R. P. et al. COPD prognosis in relation to diagnostic criteria for airflow obstruction in smokers. Eur. Respir. J. 43, 54–63 (2014).
Vollmer, W. M. et al. Comparison of spirometry criteria for the diagnosis of COPD: Results from the BOLD study. Eur. Respir. J. 34, 588–597 (2009).
Enright, P. & Brusasco, V. Counterpoint: Should we abandon FEV1/FVC < 0.70 to detect airway obstruction? Yes. Chest 138, 1040–1042 (2010).
Fortis, S., Eberlein, M., Georgopoulos, D. & Comellas, A. P. Predictive value of prebronchodilator and postbronchodilator spirometry for COPD features and outcomes. BMJ Open Respir. Res. 4, e000213 (2017).
Acknowledgements
Medical writing and editorial assistance were provided by Elbalejandra Baquero, M.Sc., of Cactus Life Sciences (part of Cactus Communications) and funded by AstraZeneca. All authors retained control of the manuscript and approved the final draft.
Funding
This study was funded by AstraZeneca K.K.
Author information
Authors and Affiliations
Contributions
M.S., S.M., and T.K. contributed to data interpretation and reviewed the manuscript. I.M. drafted the manuscript. Y.H. planned the analyses, contributed to data interpretation, and drafted the manuscript. M.I. designed the study and drafted the manuscript. H.B., W.T., and S.N. conducted the analyses. T.N. corrected and provided the data and reviewed the analysis, including data cleansing and preprocessing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
MS has received honoraria from AstraZeneca K.K., Boehringer Ingelheim Japan, GlaxoSmithKline K.K., and Novartis Pharma K.K. IM, MI and YH are employees of AstraZeneca K.K. HB, WT, SN, and TN are employees of Hitachi, Ltd. TK has received honoraria from AstraZeneca KK. SM has received honoraria from AstraZeneca K.K., Boehringer Ingelheim Japan, GlaxoSmithKline K.K., Novartis Pharma K.K., Meiji Seika Pharma Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Teijin Pharma Ltd., CHEST M.I., Inc, Daiichi Sankyo Co., Ltd., Chugai Pharmaceutical Co., Ltd., Sanofi K.K., Actelion Pharmaceuticals Japan Ltd., and Olympus Corporation.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suzuki, M., Matsumoto, I., Ishida, M. et al. Investigation of time profile of FEV1 across the onset of potential COPD: a retrospective cohort study using medical checkup data in Japan. Sci Rep 13, 5454 (2023). https://doi.org/10.1038/s41598-023-32205-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32205-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.