Abstract
Existing literature on febrile neutropenia (FN) has categorized patients with acute leukemia or those undergoing allogeneic stem cell transplantation (SCT) as being high risk for severe infection, bacteremia, and poor outcomes. Comprehensive studies of infection risk in pediatric high-risk neuroblastoma (NB-HR) during induction chemotherapy are limited, and mostly merged within the solid tumor (ST) group. Therefore, it is unclear whether infectious complications and outcomes for NB-HR are the same as in other ST groups. We conducted a retrospective medical record review of pediatric FN patients in a single center from March 2009 to December 2016. FN episodes were categorized into five groups based on underlying diagnosis (acute myelogenous leukemia (AML), acute lymphocytic leukemia (ALL), NB-HR during induction chemotherapy, other solid tumors, and SCT). Comparative analyses of infectious complications between patients with NB-HR and those with other types of cancer diagnoses were performed. A total of 667 FN episodes (FNEs) were identified in 230 patients. FNEs occurred in 82 episodes with NB-HR. Bloodstream infection (BSI) occurred in 145 (21.7%) of total FN episodes. The most isolated organisms were the viridians group streptococci (VGS) (25%). NB-HR patients have higher rates of VGS bacteremia (OR 0.15, 95% [CI 0.04, 0.56]) and are more likely to be admitted to the Pediatric Intensive Care Unit (PICU) compared to patients with other solid tumors (OR 0.36, 95% [CI 0.15, 0.84]). Interestingly, there is no difference in VGS rates between patients with NB-HR and those with AML despite the fact that NB-HR patients do not receive a cytosine arabinoside (AraC)-based regimen. This large neuroblastoma cohort showed that patients with NB-HR during induction chemotherapy are at higher risk for VGS bacteremia and PICU admissions compared with patients with other solid tumors. Further prospective studies are needed to investigate infection-related complications in this high-risk group and to improve morbidity and mortality.
Similar content being viewed by others
Introduction
Neuroblastoma (NB) is the most prevalent childhood solid extracranial tumor in children and the third most common childhood cancer, accounting for around 15% of all pediatric cancer deaths1. Among infants younger than 12 months, NB is twice as common as leukemia2. In the United States alone, more than 600 cases are diagnosed every year and 50% are considered high-risk due to the adverse features of the tumor or an advanced disease course. The international neuroblastoma risk group (INRG) classifies high-risk NB (NB-HR) as tumors with MYCN amplification, metastatic tumors in children over 18 months, or in those less than 18 months with 11q aberrations3. The current chemotherapy regimen for NB-HR spans over 18 months and includes induction, consolidation [tandem autologous stem cell transplantation (SCT)], and maintenance therapy. The induction phase alone includes 5 to 8 cycles of intensive chemotherapy with platinum and alkylating agents4. Due to the high intensity of treatment, infectious complications are very common among this patient population.
It has been established that central line-associated bloodstream infections (CLABSIs) are associated with significant morbidity and mortality in pediatric cancers, particularly leukemia5,6,7,8. Over the past 10–15 years, VGS has accounted for 25 to 30% of bacteremia episodes among cancer patients and is the most common etiology for early BSI in recipients of allogeneic hematopoietic stem cell transplantation (HSCT)9,10,11. VGS is a commensal organism present in the gastrointestinal tract and female genital tract8. In the immunocompromised host, VGS could result in fever, hypotension, severe pulmonary compromise, as well as manifestations of viridans-related septic shock syndrome (VSSS)12. The risk of VSSS is more pronounced among pediatric patients with VGS bacteremia compared to their adult counterparts13 with fatality rates reaching as high as 12.5%12,14,15,16. Neutropenic oncology patients who have severe mucositis after receiving intensive chemotherapy are more at risk of developing VGS bacteremia17. Acute myelogenous leukemia (AML) patients have historically been known to be at higher risk for VGS bacteremia compared to patients with other malignancies, given that they receive high dose cytosine arabinoside (HD-AraC); however, studies looking at VGS bacteremia in NB-HR during induction chemotherapy are lacking limited.
Currently, most of the pediatric oncology literature focuses on infectious risk and bacteremia in acute leukemias and SCT recipients, with a paucity in the NB-HR population during induction chemotherapy. In most studies, NB-HR has been grouped with other solid tumors, for which the treatment regimens are often not as intensive. The question of whether the infectious risk in NB-HR patients during induction chemotherapy should be compared to patients with other solid tumors remains unclear18. Moreover, most of the available literature on NB-HR is from large clinical trials that lack information on the types of pathogens and infections encountered19,20,21,22.
In our study, we evaluated the spectrum of pathogens from isolated blood cultures in patients with NB-HR during induction chemotherapy. Our primary endpoint was to compare the VGS bacteremia rates in NB- HR patients during induction chemotherapy to that of bacteremia rates in patients with other solid tumors, acute lymphocytic leukemia (ALL), AML, and SCT recipients, and to define risk factors associated with VGS bacteremia in this group. Our secondary endpoint was to compare FN-associated morbidity and mortality in patients NB-HR during induction chemotherapy to that of patients with other diagnoses.
Methods
Study design and population
A retrospective medical record review was conducted on pediatric patients 21 years of age or younger from March 2009 to December 2016 who received oncologic care at the University of Chicago Medicine (UCM) Comer Children’s Hospital. The need for informed consent was formally waived by the Institutional Review Board of the University of Chicago. All methods were carried out in accordance with relevant guidelines and regulations. The experimental protocol was approved by the Institutional Review Boards at the University of Chicago. The need for informed consent was formally waived by the approving committee. The Clinical Research Data Warehouse was queried for hospitalized patients with FN from March 2009 to December 2016 with an International Classification for Disease-9th edition (ICD-9) or ICD-10 code. A retrospective electronic medical record (EMR) review was performed to verify that FN episodes were appropriate for inclusion based on the below characteristics. All episodes not meeting the above-mentioned criteria were excluded. Data for all variables listed below were extracted by EMR review. For patients with more than one admission for FN, each admission was counted as a separate episode. Patients included were pediatric oncology patients with different underlying diagnoses (ALL, AML, NB-HR during induction chemotherapy, other solid tumors (ST), or SCT recipients) who were hospitalized for FN (absolute neutrophil count (ANC) < 500/mm3) and had at least one blood culture obtained.
For analysis purposes, the following diseases were grouped together based on similar chemotherapy intensity: mixed leukemia and AML, ALL regardless of disease status, Hodgkin’s and non-Hodgkin’s lymphoma, and autologous and allogeneic SCT recipients. The term “NB-HR” in this paper referred only to high-risk neuroblastoma high risk during induction chemotherapy. FN episodes in NB-HR patients who underwent autologous transplants (during consolidation and maintenance) were grouped with other SCT recipients. Low and intermediate-risk neuroblastoma patients were excluded from this study. Also, febrile non-neutropenic episodes post-transplant were excluded. Patients with incomplete charts and those in remission or not actively receiving chemotherapy were excluded as well. There were no major changes during the study period in either empirical therapy and management in FN or NB-HR chemotherapy during induction. We separated NB-HR from all other solid tumors which included a heterogeneous group of tumors such as Wilms tumor, osteosarcoma, hepatoblastoma, Ewing sarcoma, rhabdomyosarcoma, intracranial malignancies, etc.
Definitions
Fever was defined as any recorded temperature ≥ 38.0 °C (≥ 100.4°F). Neutropenia was defined as an ANC of less than 500/mm3, thrombocytopenia was defined as a platelet of less than 50 per microliter23. Positive blood culture results (bacteria, fungal) were classified as a true pathogen (i.e., bloodstream infection) or a contaminant using the National Health Safety Network (NHSN) criteria for skin commensals23. IFD was stratified as possible, probable, and proven according to the latest European Organization for Research and Treatment of Cancer-Invasive Fungal Infections Cooperative Group/National Institute of Allergy and Infectious Diseases Mycosis Study Group (EORTC/MSG) criteria (2020). Mucositis was categorized into 5 grades based on National Cancer Institute/Common Toxicity Criteria (CTC) criteria; we included only only mucositis grade ≥ 2 was included in this study. FN-related PICU admissions were defined as the need to transfer to the PICU within 7 days of FN presentation24. Neuroblastoma high-risk patients were defined based International Neuroblastoma Risk Group (INRG) classification system. Patients who were already located in the PICU prior to FN onset were not counted as PICU admission. FN-associated mortality was defined as within 30 days from FN onset.
Clinical patient management
Pediatric FN patients were managed per standard institutional practice, which did not undergo any major changes during the study period. Ceftazidime is the initial empiric antimicrobial for FN patients with vancomycin ± gentamicin added based on clinical presentation (i.e., concern for central venous catheter infection or septic shock). Cefepime was administered instead of ceftazidime for selected patients with high-risk FN such as AML. Antibacterial prophylaxis was not routinely used except Trimethoprim-sulfamethoxazole for Pneumocystis jirovecii pneumonia (PJP) prophylaxis. Empiric antifungal therapy was added if the patient remained febrile on day 5 of antibiotics and if neutropenia was expected to last longer than 5 to 7 days.
Statistical analysis
SPSS version 27 was used for data analysis. Data were presented as frequency and percentages for categorical variables or as means and standard deviations for continuous variables. Chi-squared test or Fisher’s exact test was used when appropriate to compare different underlying cancer diagnoses. A p-value less than 0.05 was considered statistically significant.
Logistic regression was used to study the association between different outcomes and underlying cancer diagnoses and for adjusting to adjust for confounding variables including age, gender, ethnicity, ANC, absolute monocyte count (AMC), absolute lymphocyte count (ALC), platelet count, as well as chemotherapy within the last 2 weeks or during the FN episode. In this analysis, NB-HR was considered as the reference group in comparison to other types of underlying cancer.
Results
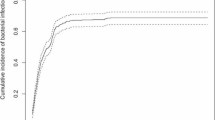
A total of 667 FN episodes were included in the study. About half were males (54.7%) and older than 10 years old (55.8%). They had different underlying cancer diagnoses including NB-HR (12.3%), ALL (30.3%), AML (12.3%), other ST (22%), and SCT (23.1%) (Fig. 1). Patients with NB-HR were primarily under 10 years old (80.5%) and males (54.7%) (Table 1).
Most of the included patients in this study received chemotherapy within the last 2 weeks or during the FN episode (72.1%). Gastrointestinal symptoms were the most common associated symptoms (34.5%), followed by upper respiratory tract infection (URI) symptoms (30.7%), mucositis (26.7%), and chills (5.7%). Additionally, 62.7% had low platelet levels and 21% had true bacteremia. Most of them had an ANC < 100/mm3 (69.9%) and an AMC < 100/mm3 (76.1%). Most patients with NB-HR had received chemotherapy within the last 2 weeks or during the FN episode (86.6%). Among patients with NB-HR, 51.2% had gastrointestinal symptoms, 32.9% had fever (> 39 °C) at the time of presentation, 32.9% had URI symptoms, 47.6% had mucositis, and 6.1% had chills. Table 2 presents the different outcomes of FN patients with NB-HR in comparison to those with other cancer diagnoses.
Patient outcomes
Bacteremia
One hundred forty-five positive blood cultures were identified as pathogens in the 667 FN episodes (21%). AML was the most common underlying cancer associated with bloodstream infections (BSI) compared with other types of cancer. Patients with NB-HR during induction chemotherapy had significantly higher rates of BSI compared with other solid tumors (OR 0.51, 95% CI 0.26–0.98). Additionally, BSI rates for both ALL and SCT groups were noted to be lower than in the NB group (OR 0.65, 95% CI [0.36, 1.19] and OR 0.72, 95% CI [0.38, 1.34], respectively).
VGS bacteremia
VGS was the most commonly isolated pathogen (37/145, 25% of all positive blood cultures) as shown in Table 3. Ten out of 37 cases of VGS bacteremia occurred in NB-HR patients during induction chemotherapy, and four had VSSS. VGS bacteremia rates in the NB-HR group were 3 times higher than in the ALL group (OR 0.34, 95% CI [0.13, 0.86]) and in SCT recipients (OR 0.34, 95% CI [0.13, 0.94]), and 7 times higher than in other ST patients (OR 0.15, 95% CI [0.04–0.56]). NB-HR during induction chemotherapy had slightly higher VGS bacteremia rates compared with AML, but this was not statistically significant (OR 0.78, 95% CI [0.29, 2.08]). Patients with NB-HR during induction chemotherapy also had higher rates of gram-positive (GP) BSI when compared with those with other STs (OR 0.17, 95% CI [0.06, 0.49]). After further adjusting for variables including fever, chemotherapy, mucositis, and gastrointestinal symptoms, the NB-HR during the induction chemotherapy cohort continued to have statistically significant greater VGS bacteremia rates than the patients with other STs.
PICU admission
PICU admission was required in 88/667 (13.2%) of FN episodes, and 14/88 (17%) were in NB-HR during induction chemotherapy. We found FNEs in NB-HR were more likely to be admitted to the PICU compared to other ST and ALL patients with FN (OR 0.36, 95% CI [0.15, 0.84] and OR 0.56, 95% CI [0.27, 1.17], respectively). There was no difference in PICU admissions compared with AML (OR 1.95% CI [0.44, 2.26]) and SCT recipients (OR 1.13, 95% CI [0.56, 2.28]). When the data was fit into a multivariate logistic regression model with adjusted variables, NB-HR during induction chemotherapy still had significantly higher rates of PICU admission compared with those with other solid tumors.
Mortality
Mortality associated with FN in this study was observed in only 15/667 (2.2%) FNEs, so there was no power in each category to conduct a meaningful analysis. There was no difference in mortality rates between NB-HR and other ST patients (OR 1.12, 95% CI [0.1, 12.5]), however, NB-HR during induction chemotherapy had a lower mortality rate than both AML patients (OR 3.08, 95% CI [0.31, 30.2]) and SCT recipients (OR 4.44, 95% CI [0.55, 36.12]), although these findings did not reach statistical significance.
Predictors of VGS infection
A multivariate logistic regression analysis was conducted to study predictors of VGS infection as shown in Table 4. The variables that fit the model included the type of malignancy and mucositis. It was found that patients with other solid tumors were less likely to have VGS bacteremia (aOR 0.167; 95% CI [0.044, 0.630]; p = 0.008) in comparison to patients with NB-HR. On the other hand, patients with mucositis are twice as likely to have VGS bacteremia [aOR 2.1; 95% CI [1.041, 4.297]; p = 0.038) as those without mucositis.
Discussion
In this large cohort study of pediatric FN, we examined the burden, risk factors, and outcomes of bacteremia in pediatric patients with NB-HR. We found that NB-HR patients have higher rates of VGS bacteremia and are more likely to be admitted to the PICU compared to patients with other solid tumors. This finding indicates the importance of stratifying infectious risks in NB-HR during induction chemotherapy compared to patients with other solid tumors in both future studies and clinical practice, which will help guide the approach to managing infections in this patient population.
In this study, 26.8% of patients with NB-HR during induction chemotherapy were found to have a BSI (all of them bacterial infection) during induction chemotherapy. Although this finding is lower than the 36% incidence of bacteremia that has been previously reported among pediatric NB-HR patients by Castagnola et al., this difference in rates may be explained by the fact that our study investigated bacteremia in FN episodes during induction therapy in this patient population, while Castagnola et al. included all NB-HR patients throughout their entire treatment course (including during consolidation with high-dose chemotherapy followed by autologous stem cell rescue)25. The few other studies that have looked at infectious complications in induction therapy for NB-HR have used varying definitions for infections26,27. Whittle et al. report nearly 40% (including 9% fungemia) of NB-HR during induction had at least one BSI, and nearly 80% had at least one episode of febrile neutropenia for which they were hospitalized26. These findings may mirror our study as we found at least 36% of NB-HR had one BSI despite the total BSI rate being 26.8%. Another study by Garaventa et al. found statistical differences in infectious risks, (5% versus 25% (P 0.011) between 2 two induction regimens for NB-HR Memorial Sloan Kettering Cancer Center N5 (MSKCC-N5) versus the rapid treatment (cisplatin [C], vincristine [O], carboplatin [J], etoposide [E], and cyclophosphamide [C], known as COJEC) COJEC respectively26. Regarding the rapid COJEC study, it is unclear what infectious etiologies were included in this study, which makes it challenging to compare it with our results.
VGS was determined to be the most isolated pathogen in this cohort of pediatric oncology patients with FN. This finding is similar to other studies in which VGS has been shown to account for the majority of first episodes of bacteremia in pediatric NB-HR patients throughout treatment25. The present study further demonstrated that patients with NB-HR were more likely to have VGS bacteremia during induction chemotherapy than patients with other solid tumors, thus highlighting that pediatric NB-HR patients are considered at higher risk for VGS bacteremia.
In a multivariate analysis, patients with mucositis were twice as likely to have VGS bacteremia, and both NB-HR during induction chemotherapy and mucositis were found to be predictors of VGS bacteremia. Treatment with high-dose cytosine arabinoside (HD-ARAC) is known to be associated with cytarabine-induced mucositis, prolonged neutropenia, and VGS bacteremia in AML pediatric patients10,28,29,30,31,32,33. However, a more recent study on AML showed that the high rate of VGS bacteremia was irrespective of the dose of cytarabine and rather dependent on the intensive chemotherapy treatment causing immunosuppression, cumulative mucosal damage, and sulfamethoxazole/trimethoprim prophylaxis34, the latter of which is believed to increase the frequency of VGS infections by allowing the overgrowth of resistant gram-positive organisms16,30,35,36. The induction chemotherapy regimen of NB-HR consists of five to six 5–6 cycles of intensive myelosuppressive chemotherapy including high-dose cyclophosphamide, etoposide, cisplatin, doxorubicin, and etoposide meant to alleviate the primary metastatic tumor burden4. Although this is not an AraC-based regimen, this regimen is associated with the high rate of VGS BSI in pediatric NB-HR during induction chemotherapy. These findings emphasize that NB-HR induction regimens involve intensive chemotherapy leading to both prolonged neutropenia between each cycle of induction and severe GI mucositis between each cycle of induction, which in turn leads to a higher VGS translocation rate to the bloodstream during periods of severe neutropenia. Mucositis rates in NB-HR patients are higher than in those with other cancer types, including AML and SCT patients. Although the present study investigated BSI in NB-HR during induction chemotherapy patients with FN, other studies have found that neuroblastoma patients who present with non-neutropenic fever may also be at risk of bloodstream infections, although with lower rates of VGS bacteremia37. One study in non-neutropenic febrile patients with NB found only one case of VGS bacteremia out of 39 positive blood cultures36, which may underscore the role of profound neutropenia as an additional risk factor in VGS bacteremia.
Patients with NB-HR in this study had significantly more PICU admissions compared to patients with other solid tumors. This is consistent with findings from a similar study comparing NB-HR to other cancers, where 70% of the patients had on average two additional admissions for infectious complications during induction when compared to other malignancies26. NB-HR during induction chemotherapy patients had higher FN admission rates between cycles compared with other solid tumors. This is consistent with previous studies where NB-HR has also been shown to be a risk factor for unplanned readmissions within 30 days of hospital discharge38. This increase in complications is likely linked to the increase in the to the increased intensity and number of chemotherapy cycles during the induction phase of NB-HR treatment. The prolonged central venous catheter uses in this population, particularly externalized catheters, could also expose patients to increased infection risk26,37. This high intensity of upfront therapy and central venous catheter use in NB-HR during induction chemotherapy highlights the importance of separating it from other solid tumors when considering the risk of infection.
The present study is limited by the retrospective nature of its design. As such, other potential risk factors for bacteremia in this patient population, including G-CSF administration20,39, antibiotic prophylaxis36,40, type of central venous catheter26,37, and the treatment of bacteremia episodes, which was at the discretion of the primary team, cannot be fully addressed. Additionally, this is a single-center study in an urban city, so these results may not be generalizable to other environments. Despite its limitations, this study includes a relatively large cohort (82 patients) of NB-HR patients during induction chemotherapy with FN and is the first to compare outcomes and infections in pediatric NB-HR patients during induction chemotherapy with those in patients with other cancer types. These This study’s findings led to an institutional change to empirical antibiotics of FN in NB-HR patients during induction to cefepime given a high rate of VGS given ceftazidime has poor VGS coverage. Also, given NB-HR during induction chemotherapy is more intensive with frequent neutropenia intervals during induction chemotherapy, so patients and their families should be educated about potential infectious burdens and complications during the induction phase to avoid any delay from SCT and improve overall outcomes. Future prospective studies are still needed to confirm these results and to further delineate risk factors for and understand the etiology of VGS bacteremia in this patient population.
Conclusion
NB-HR during induction chemotherapy patients during induction chemotherapy typically receives high-intensity of treatment, which may render them to be more severely and chronically immunocompromised and thereby predisposed to infections, particularly VGS bacteremia. It is advisable to start distinguishing NB-HR from other solid tumors due to the worse outcomes Further research is needed to define the infectious risk of NB-HR during induction chemotherapy and so forth to improve their outcomes.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- FN:
-
Febrile neutropenia
- SCT:
-
Stem cell transplantation
- ST:
-
Solid tumors
- NB-HR:
-
Neuroblastoma high risk
- AML:
-
Acute myelogenous leukemia
- ALL:
-
Acute lymphocytic leukemia
- FNE:
-
Febrile neutropenia episode
- VGS:
-
Viridans group streptococci
- PICU:
-
Pediatric intensive care unit
- NB:
-
Neuroblastoma
- INRG:
-
International neuroblastoma risk group
- CLABSI:
-
Central line associated bloodstream infections
- BSI:
-
Blood stream infection
- HSCT:
-
Hematopoietic stem cell transplantation
- VSSS:
-
Viridans-related septic shock syndrome
- UCM:
-
University of Chicago medicine
- ICD-9:
-
International classification for disease-9th edition
- EMR:
-
Electronic medical record
- NHSN:
-
National health safety network
- ANC:
-
Absolute neutrophil count
- ALC:
-
Absolute lymphocyte count
- AMC:
-
Absolute monocyte count
- URI:
-
Upper respiratory tract infection
- GP:
-
Gram positive
- BMT:
-
Bone marrow transplant
- HD-ARAC:
-
High-dose cytosine arabinoside
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
References
Goodman, M. T., Smith, M. A. & Olshan, A. F. Sympathetic Nervous System Tumors. Cancer Incidence and Survival Among Children and Adolescents 35 (United States SEER Program, 1999).
Gurney, J. G. et al. Infant cancer in the US: Histology-specific incidence and trends, 1973 to 1992. J. Pediatr. Hematol. Oncol. 19(5), 428–432 (1997).
Cohn, S. L. et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG task force report. J. Clin. Oncol. 27(2), 289 (2009).
Smith, V. & Foster, J. High-risk neuroblastoma treatment review. Children 5(9), 114. https://doi.org/10.3390/children5090114 (2018).
Rogers, A. E. et al. Risk factors for bacteremia and central line-associated blood stream infections in children with acute myelogenous leukemia: A single-institution report. Pediatr. Blood Cancer 64(3), e26254 (2017).
Shafey, A., Ethier, M. C., Traubici, J., Naqvi, A. & Sung, L. Incidence, risk factors, and outcomes of enteritis, typhlitis, and colitis in children with acute leukemia. J. Pediatr. Hematol. Oncol. 35(7), 514–517 (2013).
Sung, L. et al. Infections and association with different intensity of chemotherapy in children with acute myeloid leukemia. Cancer 115(5), 1100–1108 (2009).
Lewis, V. et al. Predictors and outcomes of viridans group streptococcal infections in pediatric acute myeloid leukemia: From the Canadian infections in AML research group. Pediatr. Infect. Dis. J. 33(2), 126–129 (2014).
Ahmed, R., Hassall, T., Morland, B. & Gray, J. Viridans streptococcus bacteremia in children on chemotherapy for cancer: An underestimated problem. Pediatr. Hematol. Oncol. 20(6), 439–444 (2003).
Gamis, A. S. et al. Alpha hemolytic streptococcal infection during intensive treatment for acute myeloid leukemia: A report from the Children’s cancer group study CCG-2891. J. Clin. Oncol. 18(9), 1845–1855 (2000).
Almyroudis, N. G. et al. Pre-and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 7(1), 11–17 (2005).
Gassas, A. et al. Predictors of viridans streptococcal shock syndrome in bacteremic children with cancer and stem-cell transplant recipients. J. Clin. Oncol. 22(7), 1222–1227 (2004).
Martino, R. et al. Viridans streptococcal bacteremia and viridans streptococcal shock syndrome in neutropenic patients: Comparison between children and adults receiving chemotherapy or undergoing bone marrow transplantation. Clin. Infect. Dis. 20(2), 476–477 (1995).
Okamoto, Y. et al. Viridans streptococcal sepsis: Clinical features and complications in childhood acute myeloid leukemia. J. Pediatr. Hematol. Oncol. 25(9), 696–703 (2003).
Wisplinghoff, H., Reinert, R. R., Cornely, O. & Seifert, H. Molecular relationships and antimicrobial susceptibilities of viridans group streptococci isolated from blood of neutropenic cancer patients. J. Clin. Microbiol. 37(6), 1876–1880 (1999).
Bochud, P. Y., Calandra, T. & Francioli, P. Bacteremia due to viridans streptococci in neutropenic patients: A review. Am. J. Med. 97(3), 256–264 (1994).
Curra, M., Soares Junior, L. A., Martins, M. D. & Santos, P. S. Chemotherapy protocols and incidence of oral mucositis: An integrative review. Einstein 16(1), e4007 (2018).
Delebarre, M. et al. Differential risk of severe infection in febrile neutropenia among children with blood cancer or solid tumor. J. Infect. 79(2), 95–100 (2019).
Pearson, A. D. et al. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: A randomised trial. Lancet Oncol. 9(3), 247–256 (2008).
Ladenstein, R. et al. Randomized trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: The European HR-NBL1/SIOPEN study. J. Clin. Oncol. 28(21), 3516–3524 (2010).
Matthay, K. K. et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N. Engl. J. Med. 341(16), 1165–1173 (1999).
Castel, V. et al. Outcome of high-risk neuroblastoma using a dose intensity approach: Improvement in initial but not in long-term results. Med. Pediatr. Oncol. 37(6), 537–542 (2001).
Freifeld, A. G. et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin. Infect. Dis. 52(4), e56-93 (2011).
US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Accessed 27 Nov 2017.
Castagnola, E. et al. Incidence of bacteremias and invasive mycoses in children with high risk neuroblastoma. Pediatr. Blood Cancer 49(5), 672–677 (2007).
Whittle, S. B., Williamson, K. C. & Russell, H. V. Incidence and risk factors of bacterial and fungal infection during induction chemotherapy for high-risk neuroblastoma. Pediatr. Hematol. Oncol. 34(5), 331–342 (2017).
Garaventa, A. et al. Randomized trial of two induction therapy regimens for high-risk neuroblastoma: HR-NBL1. 5 International Society of Pediatric Oncology European Neuroblastoma Group study. J. Clin. Oncol. 39(23), 2552–2563 (2021).
Sotiropoulos, S. V. et al. Alpha-streptococcal septicemia in leukemic children treated with continuous or large dosage intermittent cytosine arabinoside. Pediatr. Infect. Dis. J. 8(11), 755–758 (1989).
Rossetti, F., Cesaro, S., Putti, M. C. & Zanesco, L. High-dose cytosine arabinoside and viridans streptococcus sepsis in children with leukemia. Pediatr. Hematol. Oncol. 12(4), 387–392 (1995).
Weisman, S. J., Scoopo, F. J., Johnson, G. M., Altman, A. J. & Quinn, J. J. Septicemia in pediatric oncology patients: The significance of viridans streptococcal infections. J. Clin. Oncol. 8(3), 453–459 (1990).
Engelhard, D. et al. Cytosine arabinoside as a major risk factor for Streptococcus viridans septicemia following bone marrow transplantation: A 5-year prospective study. Bone Marrow Transplant. 16(4), 565–570 (1995).
Kern, W., Kurrle, E. & Schmeiser, T. Streptococcal bacteremia in adult patients with leukemia undergoing aggressive chemotherapy: A review of 55 cases. Infection 18(3), 138–145 (1990).
Shenep, J. L. Viridans-group streptococcal infections in immunocompromised hosts. Int. J. Antimicrob. Agents 14(2), 129–135 (2000).
Johannsen, K. H., Handrup, M. M., Lausen, B., Schrøder, H. & Hasle, H. High frequency of streptococcal bacteraemia during childhood AML therapy irrespective of dose of cytarabine. Pediatr. Blood Cancer 60(7), 1154–1160 (2013).
Elting, L. S., Bodey, G. P. & Keefe, B. H. Septicemia and shock syndrome due to viridans streptococci: A case-control study of predisposing factors. Clin. Infect. Dis. 14(6), 1201–1207 (1992).
Hammond, S. P. & Baden, L. R. Antibiotic prophylaxis during chemotherapy-induced neutropenia for patients with acute leukemia. Curr. Hematol. Malig. Rep. 2(2), 97–103 (2007).
Moskalewicz, R. L., Isenalumhe, L. L., Luu, C., Wee, C. P. & Nager, A. L. Bacteremia in nonneutropenic pediatric oncology patients with central venous catheters in the ED. Am. J. Emerg. Med. 35(1), 20–24 (2017).
Hoenk, K.A.-O. et al. Multicenter study of risk factors of unplanned 30-day readmissions in pediatric oncology. Cancer Rep. 4(3), e1343 (2021).
Whittle, S. B. et al. Is high-risk neuroblastoma induction chemotherapy possible without G-CSF? A pilot study of safety and treatment delays in the absence of primary prophylactic hematopoietic growth factors. Pediatr. Blood Cancer 67(10), e28417 (2020).
Ramphal, R. Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated pathogens. Clin. Infect. Dis. 39(Supplement 1), S25–S31 (2004).
Author information
Authors and Affiliations
Contributions
Conceptualization: MA. Data Curation: MA. Formal Analysis: OE, MK. Methodology: OE, MK. Supervision: MA, CP, LE. Writing - original draft: MA, OE. Writing - Review and Editing : MA, CP, LE. Figure and Table Preparation: OE, MK.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Kebbi, O., Prather, C.S., Elmuti, L. et al. High frequency of viridians group streptococci bacteremia in pediatric neuroblastoma high-risk patients during induction chemotherapy. Sci Rep 13, 5627 (2023). https://doi.org/10.1038/s41598-023-31805-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31805-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.