Abstract
Multiple types and classes of medications are administered in the acute management of traumatic spinal cord injury. Prior clinical studies and evidence from animal models suggest that several of these medications could modify (i.e., enhance or impede) neurological recovery. We aimed to systematically determine the types of medications commonly administered, alone or in combination, in the transition from acute to subacute spinal cord injury. For that purpose, type, class, dosage, timing, and reason for administration were extracted from two large spinal cord injury datasets. Descriptive statistics were used to describe the medications administered within the first 60 days after spinal cord injury. Across 2040 individuals with spinal cord injury, 775 unique medications were administered within the two months after injury. On average, patients enrolled in a clinical trial were administered 9.9 ± 4.9 (range 0–34), 14.3 ± 6.3 (range 1–40), 18.6 ± 8.2 (range 0–58), and 21.5 ± 9.7 (range 0–59) medications within the first 7, 14, 30, and 60 days post-injury, respectively. Those enrolled in an observational study were administered on average 1.7 ± 1.7 (range 0–11), 3.7 ± 3.7 (range 0–24), 8.5 ± 6.3 (range 0–42), and 13.5 ± 8.3 (range 0–52) medications within the first 7, 14, 30, and 60 days post-injury, respectively. Polypharmacy was commonplace (up to 43 medications per day per patient). Approximately 10% of medications were administered acutely as prophylaxis (e.g., against the development of pain or infections). To our knowledge, this was the first time acute pharmacological practices have been comprehensively examined after spinal cord injury. Our study revealed a high degree of polypharmacy in the acute stages of spinal cord injury, raising the potential to impact neurological recovery. All results can be interactively explored on the RXSCI web site (https://jutzelec.shinyapps.io/RxSCI/) and GitHub repository (https://github.com/jutzca/Acute-Pharmacological-Treatment-in-SCI/).
Similar content being viewed by others
Introduction
Traumatic spinal cord injury is a neurological condition associated with varying degrees of motor, sensory and autonomic deficits. At present, there are no pharmacological interventions available to enhance the extent a person neurologically or functionally recovers from acute spinal cord injury1,2. In the absence of interventions that enhance neurological recovery, acute care of spinal cord injury chiefly focuses on managing neurological sequela (e.g., neuropathic pain) and secondary complications (e.g., infections). As spinal cord injury ultimately affects every organ system of the human body, a multidisciplinary treatment strategy is necessary. In accordance with existing treatment guidelines, these necessitate the administration of various drugs, including narcotics, analgesics, sympathomimetics, antibiotics, muscarinic antagonists, antithrombotics, anticonvulsants, and antidepressants to manage pain, infections, urinary tract dysfunction, deep venous thrombosis, and psychological disorders. To date, little is known to what degree common drugs used in the management of acute spinal cord injury have downstream and potentially unintended effects, which modify neurological recovery. This is surprising in light of the fact that numerous drugs are: (1) spinal cord blood barrier (SCBB) permeable and/or gain access to the central nervous system via a leaky SCBB after injury, (2) act on targets in the central nervous system, and (3) administered during the window of opportunity to promote neural repair and plasticity (i.e., in the initial hours to weeks post injury).
Recent observational studies have reported a potential beneficial effect of acutely administered gabapentionoid medications (but not other anticonvulsants) on long-term neurological outcomes after spinal cord injury3,4,5. Subsequent preclinical studies demonstrated a potential gabapentionoids-meditated mechanism for enhanced recovery, as well as confirmed behavioral benefits in animal models6,7. While efficacy awaits confirmation in prospective clinical trials, these collective observations point to the promise of a reverse translational approach (bedside-to-bench) to restore neurological function after spinal cord injury. Identifying other opportunities for drug repurposing depends, in part, on knowledge regarding specific medications commonly administered in the acute phase. Additionally, if promising pharmacologic agents are to be proposed for human evaluation in clinical trials of acute spinal cord injury, it is important to consider the spectrum of other concomitant medications that are routinely administered in the care of these patients, as they may have known interactions with the promising agent in question.
The aim of this study was to characterize what constitutes the “acute pharmacological management of spinal cord injury” leveraging available clinical trial and observational study data. Specifically, we determined the types of timing, and reason of administration for drugs commonly administered, alone or in combination, in the acute to subacute phase (i.e., first 2 months) of spinal cord injury.
Methods
Study design
The design and reporting of this analysis adhered to the relevant guidelines for observational studies8.
Data source and cohort definition
To quantify medications commonly administered in the acute management of spinal cord injury, we analyzed two sources of data. Both sources represent collections of data from the United States; the first (i.e., trial) between 1992 and 1998 and the second (i.e., observational) from 2007 to 2009.
The first source comprised details of concomitant medications administered in a clinical trial—the Sygen trial—delivering GM-1 ganglioside in acute spinal cord injury1,9. The Sygen trial was a randomized, prospective, phase III, placebo controlled, multi-center study testing the efficacy of GM-1 ganglioside therapy in acute, traumatic spinal cord injury1,9. Full design, recruitment, and enrollment details have been published previously10. Briefly, to be included in the Sygen trial patients were required to have at least one lower extremity with a substantial motor deficit. Patients with spinal cord transection or penetration, head trauma, major chest trauma, or intubation were excluded, as were patients with a cauda equina, brachial or lumbosacral plexus, or peripheral nerve injury. Multiple trauma cases were included as long as they were not so severe as to preclude neurologic evaluation. Patients were also excluded when they suffered from significant systemic disease such as lung, liver, gastrointestinal, or kidney disease; or active malignancy or any other condition as determined by history or laboratory investigation that could alter the distribution, accumulation, metabolism, or excretion of the study medication, cause a neurologic deficit, or result in the patient’s life expectancy being less than 2 years. The full list of inclusion and exclusion criteria can be found elsewhere10. All patients were to receive the NASCIS II dose regimen of methylprednisolone (MPSS) starting within eight hours after the SCI. To avoid any possible untoward interaction between MPSS and Sygen®11, the study medication was not started until after completion of MPSS administration. With 797 enrolled patients followed over the first year following injury, the Sygen trial was the largest clinical trial ever conducted in the field of spinal cord injury. The Sygen trial, which followed patients over the first year following injury, was clinically active from 1992 to 1998, and showed no differences between treatment and placebo groups in terms of neurological recovery12. The negative finding of the Sygen study is considered Class I Medical Evidence by the spinal cord injury Committee of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS)13,14. Subsequent analyses of the Sygen data have been performed to characterize the trajectory and extent of spontaneous recovery from acute spinal cord injury15,16.
The second source of data was from a large, observational study (i.e., SCIRehab), which abstracted information pertaining to medication use in the acute phase of spinal cord injury from patient medical records17. The SCIRehab study enrolled, upon consent, individuals aged ≥ 12 years with traumatic spinal cord injury who were rehabilitated at six participating rehabilitation centers from 2007 through 200918. Participating centers included Rocky Mountain Regional Spinal Injury System at Craig Hospital, Shepherd Center, Atlanta GA; Rehabilitation Institute of Chicago, Chicago, IL; Carolinas Rehabilitation, Charlotte, NC; the Mount Sinai Medical Center, New York, NY; and National Rehabilitation Hospital, Washington, DC. Patients were followed for the first-year post-injury and were excluded if they spent two or more weeks at a non-participating rehabilitation center. Details of more than 460,000 interventions provided to 1500 patients were documented by over 1000 clinicians at the six participating centers. Patient demographics and injury characteristics were extracted from the patient medical record (part of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Model Systems Form I). Design, recruitment, inclusion criteria, and enrollment details have been previously described in detail18.
To be included in our study, information on medications administered needed to be available for the patients.
Commonly administered medications
In the Sygen trial, alongside serious adverse events, concomitant medication information was routinely tracked following standardized case report forms by trained examiners in clinical trials as a measure of safety. For each concomitant medication administered during the trial, the reason for administration, dosage, dosing (i.e., start and end date, frequency), and reason for conclusion were recorded. It was also documented in case medications were administered for prophylactic reasons (e.g., to prevent deep vein thrombosis). Note that, although patients were randomized to GM-1 ganglioside therapy, individuals were not randomized to any concomitant medication administered and were managed according to the conventional care protocols of the enrolling center. The SCIRehab study documented the use of all commonly administered medications. For each medication administered, route, dosage, and dosing (i.e., start and end date, frequency) were abstracted directly from medical records. However, medication indication was not recorded.
Medication data cleaning and organizing
Medication data from the Sygen trial and SCIRehab study were separately cleaned and organized. From the medication files, which exist for each patient in the Sygen trial and SCIRehab, we extracted generic medication name and information on dosing (i.e., start and end date, frequency). As information on medication indication (i.e., reasons for administering a medication) was not entered in a standardized fashion during data collection, we classified the medication indication according to the Common Terminology Criteria for Adverse Events (CTCAE)19. Briefly, each indication was assigned to a System Organ Class (SOC)20, the highest level of the MedDRA hierarchy21. The SOC is identified by anatomical or physiological system, etiology, or purpose (e.g., SOC Investigations for laboratory test results) and comprises 26 different categories. We added a separate class for trauma-related pain (i.e., nociceptive and neuropathic). The rationale for this amendment stems from the fact that the CTCAE does not sufficiently cover this category. After carefully reviewing the medication list, we have also consulted study clinicians of both data sources to identify any discrepancies, including missing or duplicate medications, changes in dosages, and drug interactions (i.e., medication reconciliation).
Assessment of blood brain barrier (BBB) permeability
Leveraging the information from the DrugBank database (www.drugbank.ca), the permeability of medications to cross the blood brain barrier was determined. In case corresponding information was missing in the DrugBank, a PubMed search was performed to consider studies that have evaluated blood brain barrier permeability.
Statistical analysis and data visualization
R Statistical Software version 3.6.3 (Running under: macOS Mojave 10.14.4) was used for all analyses and to visualize the results. Descriptive statistics (mean, standard deviation, ranges, and proportions) were used to describe the patients’ demographics, injury characteristics, and medication information. For the latter, this included the number and type of medications administered, reason for administration, and how many medications each patient received per day (i.e., point prevalence). Type and frequency of medications that were administered prophylactically were also computed.
Interactive web platform R X SCI
In order to enable the spinal cord injury community, researchers, authorities, and policymakers to fully explore the data and results of this study (and beyond), we developed the freely available and open source RXSCI web platform. RXSCI was implemented with the Shiny framework22, which combines the computational power of the free statistical software R23 with friendly and interactive web interfaces. Both, the front- and back-end of RXSCI have been built using the shiny dashboard package24. RXSCI is available as an online application and is hosted at https://jutzelec.shinyapps.io/RxSCI/ and can be accessed via any web browser on any device (e.g., desktop computers, laptops, tablets, smartphones). RXSCI is published under the BSD 3-Clause License. The source code of RXSCI is available through Github at https://github.com/jutzca/Acute-Pharmacological-Treatment-in-SCI/tree/master/shinyapp.
Data sharing and code availability
Full anonymized data of both data sources will be shared at the request from any qualified investigator (please contact the Corresponding Author). The code for the data analysis and visualization is available in our GitHub repository (https://github.com/jutzca/Acute-Pharmacological-Treatment-in-SCI/).
Standard protocol approvals, registrations, and patient consents
Approval for this study (secondary analysis) was received by an institutional ethical standards committee on human experimentation at the University of British Columbia. The original Sygen clinical trial (results published elsewhere) also received ethical approval, but was conducted before clinical trials were required to be registered (i.e., no clinicaltrial.gov identifier available)12. Each participating center of the SCIRehab study received institutional review board approval for this study and obtained informed consent from each patient (or their parent/guardian).
Results
Patient characteristics and summary statistics
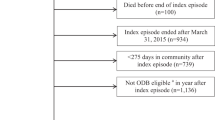
797 and 1243 patients from the Sygen clinical trial and SCIRehab observational study, respectively, were included in our analysis. While all patients from the Sygen study were included in our analysis, we had to exclude 257 patients from the SCIRehab study due to missing data on medications (n = 242) or spinal cord injuries with no sensory or motor impairments (i.e., AIS E, cauda equine or peripheral nervous system injuries, n = 15). In both cohorts, the ratio between male and female patients was approximately 4:1, the majority of the patients were injured at the cervical levels (Sygen: 75.2%; SCIRehab: 60.4%), and motor complete (Sygen: 65.7%; SCIRehab: 65.6%). The most frequent cause of injury was car accidents (Sygen: 47.9%; SCIRehab: 35.5%) followed by falls (Sygen: 16.2%; SCIRehab: 24.1%). Detailed description of both cohorts is provided in Table 1.
Acute pharmacological management after spinal cord injury
In total, 489 (trial) and 575 (observational study) unique medications were administered over the course of 60 days after spinal cord injury. More than a third (n = 289 [~ 37.3%]) of the medications administered were common to both data sources (for details see Supplementary Table 1). Medications were administered to manage secondary complications arising from 21 different system organ classes or to facilitate surgical and medical procedures (Fig. 1A and Supplementary Table 2). No medications were administered for the following five organ systems: (1) Congenital, familial and genetic disorders, (2) Injury; (3) hepatobiliary disorders; poisoning and procedural complications; (4) Pregnancy, puerperium and perinatal conditions; and (5) social circumstances. On average, patients enrolled in the Sygen trial received 9.9 ± 4.9 (range 0–34), 14.3 ± 6.3 (range 1–40), 18.6 ± 8.2 (range 0–58), and 21.5 ± 9.7 (range 0–59) medications within the first 7, 14, 30, and 60 days post-injury, respectively (Fig. 1B). Patients enrolled in the SCIRehab cohort study received on average 1.7 ± 1.7 (range 0–11), 3.7 ± 3.7 (range 0–24), 8.5 ± 6.3 (range 0–42), and 13.5 ± 8.3 (range 0–52) medications within the first 7, 14, 30, and 60 days post-injury, respectively (Fig. 1C). Supplementary Fig. 1 shows the absolute and cumulative number of unique drugs per day for the Sygen (Supplementary Fig. 1A) and the SCIRehab (Supplementary Fig. 1B). The disparity between Sygen and SCIRehab in the first month post injury can be attributed to different time-points of patient enrollment, with the Sygen trial enrolling patients within 72 h, compared to SCIRehab, which enrolled patients within days or weeks of injury (Table 1). As a result, medications for first-line trauma management (e.g., nitroglycerin, dopamine) as well as surgical and medical procedures (e.g., isoflurane, vecuronium bromide) are only captured by the Sygen trial. Acetaminophen (analgesic, n = 674 patients), morphine (analgesic, n = 664 patients), and heparin (anticoagulant, n = 505 patients) were the three most commonly administered medications in the Sygen trial (Fig. 1D). Similarly, in the SCIRehab study, the analgesic acetaminophen (n = 924 patients) was the most commonly administered medication, followed by the laxative docusate (n = 620 patients) and the analgesic combination medicine acetaminophen and oxycodone (n = 603 patients) (Fig. 1E).
Pharmacological management of acute spinal cord injury. (A) Secondary complications. Spinal cord injury is associated with a large number of secondary complications that arise from 20 organ systems as defined by Common Terminology Criteria for Adverse Events (CTCAE) published by U.S. Department of Health and Human Services19. Many medications were also administered to facilitate medical and surgical procedures, such as decompression surgeries, laminectomy, and computer tomography scans. (B) Number of medications administered to patients enrolled in the Sygen trial within the first 7, 14, 30, and 60 days post-injury. (C) Number of medications administered to patients enrolled in the SCIRehab study within the first 7, 14, 30, and 60 days post-injury. (D) Frequency of medications administered. The majority of patients enrolled in the Sygen trial received acetaminophen, morphine, and heparin to treat secondary complications, such as pain and deep venous thrombosis. (E) Frequency of medications administered. Pain killers (acetaminophen and acetaminophen oxycodone) as well as the laxative docusate were among the most frequently administered medications in the SCIRehab study.
The majority of patients enrolled in the Sygen trial required medications to treat secondary complications arising from the gastrointestinal system (n = 752, 95.1%), pain (n = 742, 93.8%), infections (n = 737, 93.2%), and psychiatric issues (n = 650, 82.2%) (Fig. 2A, Supplementary Table 3). A total of 150, 99, and 93 unique medications were administered to treat a variety of secondary complications arising from infections, respiratory system, and gastrointestinal system, respectively. Moreover, pain (e.g., musculoskeletal), gastrointestinal complications (e.g., heartburn, ulcers), and infections (i.e., bacteria, viral, and fungal) were the most frequently managed problems (Fig. 2B, Supplementary Table 4). This was also true when stratifying for injury severity (AIS grades, Supplementary Table 5). While infections were mainly treated with antibiotics, antifungal, and antiviral medications depending on their nature, complications arising from gastrointestinal tract were targeted with analgesics, antibiotics, antacids, antiulcer, anti-anemics, anticholinergics, and antispasmodics (see detailed overview in Supplementary Table 6).
Indication of medications administered. (A) Number of unique medications administered per organ system for patients enrolled in the Sygen clinical trial. Note the diversity of medications administered within each category of complications. For instance, over 100 different medications were administered to treat infections and infestations as well as for surgical and medical procedures. (B) Number of patients of the Sygen clinical trial that required treatment per organ system. The three most frequently treated secondary complications were pain, gastro-intestinal system disorders, as well as infections. The SCIRehab database did not track the indications for which medications were prescribed.
Polypharmacy
As illustrated in Fig. 3, polypharmacy was commonplace. Almost every patient enrolled the Sygen trial or the SCIRehab study received multiple medications per day (Fig. 3A). Patients with more severe injuries (AIS A and B) received more medications per day than those with less severe injuries (AIS D). The number of medications administered per day per patient ranged between 1 and 30 for patients enrolled in Sygen trial (Fig. 3B) and between 1 and 43 for patients enrolled in the SCIRehab study (Fig. 3B). Individual patient examples of the extend of polypharmacy is shown in Fig. 3C. The complexity of the combination of medications administered is illustrated in Fig. 3D. In the Sygen trial, the three most common combinations of medications were acetaminophen and morphine (n = 164 patients), morphine and ranitidine (n = 128 patients), as well as acetaminophen and heparin (n = 123 patients). In the SCIRehab study, acetaminophen and acetaminophen oxycodone was the most common combination of medications (n = 480 patients), followed by acetaminophen and acetaminophen hydrocodone (n = 407 patients), as well as acetaminophen and ibuprofen (n = 346 patients.) The complexity of the combination of medications administered to patients in the SCIRehab study is illustrated in Fig. 3E.
Polypharmacy. (A) Point prevalence of commonly administered medications. The number of medications administered per day per patient in the first 60 days post injury varied between 1 and 30 for the clinical trial and between 1 and 43 in the observational study. Each line represents one patient and the color white indicates that no medication was administered or no data was available for that time period. (B) Daily average number of medications administered. Patients with motor complete injuries (AIS A and B) received on average more medications per day compared to patients with motor incomplete injuries. The range medications administered varies quite drastically. The dashed line denotes the average number of medications and the solid lines the minimum and maximum number of medications, respectively. Patients with no information on AIS grades at baseline were grouped together in the category ‘unknown’. (C) Examples longitudinal medication profiles for four patients in the first 60 days post injury. Polypharmacy was commonplace across different injury severities and aetiologies. The pattern of medication administration varied between continuous, intermittent, and single-use indications. Medications were often co-administered bearing a high risk of pharmacological interactions between medications. While some are well-understood, the majority of these interactions (particularly combinations of three and more medications) have not yet been explored. (D) Network of medications administered in combination to patients enrolled in the Sygen trial. The nodes of the network represent the medications. The size of the nodes represents the number of patients that have received this particular medication on day 7 or 14, respectively. Medications that were administered together on a specific day, either 7 or 14, are connected via an edge. The width of the edge represents the number of patients that have received the two medications (acetaminophen and ketorolac) in combination on the day of interest. (E) Network of medications administered in combination to patients enrolled in the SCIRehab study. The nodes of the network represent the medications. The size of the nodes represents the number of patients that have received this particular medication on day 7 or 14, respectively.
Blood brain barrier (BBB) permeability
Out of the 775 unique medications, 59.4% (n = 460) have the ability to cross the BBB while 20.6% (n = 160) are not permeable for the BBB. No information regarding the BBB permeability was identified for the remaining 20.0% (n = 155). Detailed information on the permeability can be found in Supplementary Table 7.
Prophylactic administration of medications
Approximately 10% (n = 2838) of all recorded indications in the Sygen trial (Fig. 4A) were labelled ‘prophylactic’ or ‘preventative’. A total of 137 unique medications were administered for prophylactic treatment to prevent a wide range of secondary complications (Fig. 4B). The major medication groups included antihistamines (ranitidine, famotidine), anticoagulants (heparin, warfarin), and antibiotics (cefazolin, gentamicin) for the prevention of secondary complications arising from the gastrointestinal system (e.g., heart burn, gastric ulcers), blood and vasculature system (e.g., deep vein thrombosis), and infections, respectively (Fig. 4C). The majority of patient enrolled in the Sygen trial (n = 666 [83.6%]) received prophylactic treatments (meanmedications/patient = 3 [range 1–21]; meanindications/patient = 4.3 [range 1–33]) (Fig. 4D). Supplementary Table 8 provides a comprehensive overview of all medications (and their respective indications) that were administered prophylactically.
Prophylactic pharmacological treatment to prevent secondary complications from occurring. (A) Number of indications per organ system. The majority of prophylactic indications were related to the gastrointestinal and vascular system as well as infections of all sorts. (B) Number of unique medications administered to for disease prophylaxis. (C) Number of indications per medications. Anticoagulants, antihistamines, and antibiotics were amongst the most frequently administered medication classes. (D) Number of patients that received prophylactic treatment per organ system. The majority of the patients enrolled in the Sygen trial (n = 666 [83.6%]) received at least one medication for disease prophylaxis. The average number of medications per patient was 3 (range 1–21) and average number of indications per patient was 4.3 (1–33).
Interactive web platform R X SCI
The RxSCI web platform is hosted online (https://jutzelec.shinyapps.io/RxSCI/) and contains three main data visualization parts: (1) epidemiological features, including demographics and injury characteristic; (2) information on the pharmacological treatment of spinal cord injury patients on daily basis, including medication administration patterns; and (3) visualization of the polypharmacy. All data from the Sygen clinical trial and the SCIRehab study, which was used in this study, can be explored in a customized fashion (e.g., customized selection of patient groups). The platform is configured such that existing or newly generated data sets can be added if they comply with GDPR.
Discussion
The aim of the current study was to comprehensively evaluate pharmacological management practices in acute spinal cord injury. To this end, two large data sources were examined, one from a clinical trial and the other from an observational study. Our analysis revealed an incredibly high rate of polypharmacy spread over the course of the first 60 days’ post injury, which was administered to manage various health conditions arising directly or indirectly from acute spinal cord injury. Various medications were administered, including those that readily cross the BBB (e.g., pregabalin25, morphine26) to manage the sequela of spinal cord injury (e.g., neuropathic pain), as well as other complex medical complications. Drugs that cross the BBB may be more likely to have effects (positive or negative) on neural recovery pathways after injury.
To our knowledge, this was the first time acute pharmacological practices have been comprehensively examined after spinal cord injury. Even considering its extreme and traumatic nature, the sheer number of medications administered in a short window of time after spinal cord injury, over the course of the 2 months, was remarkably high. This led to a very high degree of polypharmacy. For comparison, polypharmacy in other complex health conditions is generally considered more than five medications27,28—the average for acute spinal cord injury patients was approximately double that threshold. While perhaps startling, the complexity of managing spinal cord injury requires aggressive pharmacological management. Nevertheless, the lack of attention paid to the question of “neurological safety” (i.e., whether use of a medication or its interaction with other medications in the acute phase of injury will have long-term and detrimental neurological consequences) is surprising, as is the fact that few attempts have been made to discern potential beneficial (or detrimental) effects of medications that readily cross the BBB. Furthermore, one must consider potential interactions between the high number of clinically used concomitant medications with novel medications and biologics being trialed for improving recovery from spinal cord injury.
The limited knowledge about the potential effects of acutely administered medications on recovery in humans becomes all the more curious considering that a number of these medications alter outcomes in animal studies. As an example, pregabalin, a potent calcium channel blocker and anticonvulsant administered for neuropathic pain, has been repeatedly shown to benefit recovery after spinal cord injury in animal and human spinal cord injury3,4,5,6. Detrimental effects were also observed for some medications, including opioids, which attenuated the recovery of locomotor function and exacerbated pathophysiological processes in rodent models of spinal cord injury29,30,31. A detrimental opioid effect is in line with beneficial effects of naloxone (i.e., opioid antagonist)29,32, and is highly concerning in light of the fact that opioids are ubiquitously administered for pain management in the early stages of injury (to > 80% of the patients). While completely removing or restricting opioids would be highly problematic and present with serious ethical concerns (i.e., weighing the management of acute pain with long-term neurological effects), opioids were among medications commonly administered to prevent the onset of pain. This suggests that opioids, at least in a proportion of patients, were prescribed with the intention to prevent the onset of pain, despite a lack of evidence33. Among these individuals, neurological recovery could perhaps be facilitated by minimizing the administration of opioids. Many other common medications (up to 10%) are prophylactically administered, including acetaminophen, cefazolin, and famotidine for pain/fever, infection, and ulcer prophylaxis, respectively.
Despite years of use in clinical routine, safety information with respect to neurological outcomes of many concomitant medications is currently not available. This is highly concerning because fundamental assumptions of pharmacokinetics and -dynamics may not apply as in other (healthy) individuals34. Alterations in physiology lead to prolonged absorption as a consequence of slowed gastric emptying and gastrointestinal motility34, altered distribution due to leaky blood spinal cord barrier35, hampered metabolism36,37, and slowed excretion are hallmarks of this altered physiology34,36,37. Examples of medications with changed pharmacokinetics are amikacin, baclofen, carbamazepine, cefotiam, ciprofloxacin, diazepam, diclofenac, doxycycline, ketamine, lorazepam, naproxen, and vancomycin. A major issue with these injury-induced modifications in pharmacokinetics is that some medications do not reach desired therapeutic effects, whereas others may reach potentially toxic levels. In addition to potential toxicity, also common side effects of medications (e.g., gastric emptying and gastrointestinal motility caused by opioids) may worsen the natural pathophysiology of injury. Post-marketing surveillance and risk assessment programs aim at detecting previously unrecognized positive or negative effects that may be associated with a medication—within real-world populations. To our knowledge, few of these studies have examined effects after spinal cord injury. An exception is a recent study that established neurological safety profile of baclofen, an antispasmodic to treat debilitating muscle spasms38. Cragg et al. performed a secondary analysis of clinical trial data to provide data reaffirming that baclofen is neurologically, hepatically, and renally safe to use in patients sustaining a spinal cord injury38. Complementing the existing safety profile, neurological safety medication profiles in the context of concomitant medications in real-world settings will enable health care providers to provide an informed, evidence-based response regarding the use of medications such as baclofen in the acute phase of spinal cord injury.
Limitations
There are multiple limitations that are noteworthy. Firstly, in this study, we compared two cohorts which were collected a decade apart. It cannot be excluded that changes in the management, in particular pharmacological management, of SCI occurred over this period. However, it has been shown that the recovery rate did not change43. Thus, we can hypothesize that the potential changes in the standard of care did not significantly improve or deteriorate the recovery of the SCI itself. Secondly, all medications administered after SCI were meticulously tracked in the Sygen trial. However, there is no information on medications prescribed prior to the injury. Thirdly, the two studies involve dissimilar populations of people with acute SCI and data from two drastically different periods (1992 versus 2007), both of which are dated. Another limitation was the differences between the two study cohorts in reporting of demographics (i.e., age, time since injury, etc.) at the time of enrollment. Thus, more contemporary studies are warranted to establish the extent to which polypharmacy during acute SCI management may have changed within the last 30 years. Lastly, there might be potential confounding factors that may undermine the legitimacy of the data used in this study, including comorbidities, patient characteristics (age, sex, race, or genetics), concomitant diseases or conditions, non-adherence of patients, variance in physician prescribing practices, timing and duration of concomitant medication use, and dosage and potency of concomitant medications. These confounding factors must be considered when analyzing the concomitant drug data of clinical trials and observational studies.
Conclusion and implications for other neurological disorders
Our study revealed a dramatic degree of polypharmacy after acute spinal cord injury that potentially impacts recovery and the potency of novel treatments of spinal cord injury. It should be noted that in the testing of novel drug agents in preclinical models of spinal cord injury, the experiments are typically designed to minimize (and of course standardize) the concomitant medications administered to the animals. How starkly different this is from clinical reality is revealed in our analysis. Spinal cord injury is a complex condition and as such, the pharmacologic needs are understandably high. While we are not arguing for an arbitrary “reduction” in the use of various medications in the management of these individuals, evaluating current standards of acute care and understanding what pharmacologic agents patients are typically exposed to does represent an intriguing alternative strategy to improve the lives of individuals with spinal cord injury. Knowledge gained from our study has major implications for other diseases hallmarked by polypharmacy, including Parkinson’s disease39, Alzheimer’s disease40, Multiple Sclerosis41, traumatic brain injury42,43, cancer44, and sepsis45. Similar to spinal cord injury, these diseases are complex conditions associated with a wide range of symptoms (e.g., functional impairment) and secondary complications (e.g., gastrointestinal and cardiovascular complications, pain) necessitating pharmacological treatment—at times simultaneously. Many of these diseases are not yet curable, but effective disease modifying treatments that relieve symptoms, slow down disease progression, and improve quality of life are available46,47,48,49. A cursory glance at the literature corroborates that the knowledge gap regarding the effect of commonly used medications on disease progression and their potential to alter the effectiveness of disease modifying treatments is not unique to spinal cord injury.
Data availability
Full anonymized data of both data sources will be shared at the request from any qualified investigator (please contact the Corresponding Author). The code for the data analysis and visualization is available in our GitHub repository (https://github.com/jutzca/Acute-Pharmacological-Treatment-in-SCI/).
References
Geisler, F. H., Coleman, W. P., Grieco, G., Poonian, D., Sygen Study Group. Recruitment and early treatment in a multicenter study of acute spinal cord injury. Spine (Phila Pa 1976) 26, S58–S67 (2001).
Segal, J. L. et al. Methylprednisolone disposition kinetics in patients with acute spinal cord injury. Pharmacotherapy 18, 16–22 (1998).
Warner, F. M. et al. Early administration of gabapentinoids improves motor recovery after human spinal cord injury. Cell Rep. 18, 1614–1618 (2017).
Cragg, J. J. et al. Effects of pain and pain management on motor recovery of spinal cord-injured patients: A longitudinal study. Neurorehabil. Neural Repair. 30, 753–761 (2016).
Warner, F. M. et al. The effect of non-gabapentinoid anticonvulsants on sensorimotor recovery after human spinal cord injury. CNS Drugs 33, 503–511 (2019).
Sun, W. et al. Gabapentinoid treatment promotes corticospinal plasticity and regeneration following murine spinal cord injury. J. Clin. Investig. 130, 345–358 (2020).
Tedeschi, A. et al. The calcium channel subunit Alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron 92, 419–434 (2016).
Vandenbroucke, J. P. et al. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. PLoS Med. 4, 1628–1654 (2007).
Geisler, F. H., Dorsey, F. C. & Coleman, W. P. Recovery of motor function after spinal-cord injury—A randomized, placebo-controlled trial with GM-1 ganglioside. N. Engl. J. Med. 324, 1829–1838 (1991).
Geisler, F. H., Coleman, W. P., Grieco, G. & Poonian, D. Recruitment and early treatment in a multicenter study of acute spinal cord injury. Spine (Phila Pa 1976) 26, S58–S67 (2001).
Bracken, M. B. et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury: Results of the Third National Acute Spinal Cord Injury randomized controlled trial. JAMA 277, 1597–1604 (1997).
Geisler, F. H., Coleman, W. P., Grieco, G. & Poonian, D. The Sygen multicenter acute spinal cord injury study. Spine (Phila Pa 1976) 26, S87-98 (2001).
Hadley, M. N. & Walters, B. C. Introduction to the guidelines for the management of acute cervical spine and spinal cord injuries. Neurosurgery 72, 5–16 (2013).
Hadley, M. N. et al. Guidelines for the management of acute cervical spine and spinal cord injuries. Clin. Neurosurg. 50, 407–498 (2002).
Fawcett, J. W. et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 45, 190–205 (2007).
Steeves, J. D. et al. Extent of spontaneous motor recovery after traumatic cervical sensorimotor complete spinal cord injury. Spinal Cord. 49, 257–265 (2011).
Whiteneck, G., Gassaway, J., Dijkers, M. & Jha, A. New approach to study the contents and outcomes of spinal cord injury rehabilitation: The SCIRehab project. J. Spinal Cord Med. 32, 251–259 (2009).
Whiteneck, G. et al. The SCIRehab project: Treatment time spent in SCI rehabilitation. Inpatient treatment time across disciplines in spinal cord injury rehabilitation. J. Spinal Cord Med. 34, 133–148 (2011).
Dueck, A. C. et al. Validity and reliability of the US national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. 1, 1051–1059 (2015).
Cancer Therapy Evaluation Program. Common terminology criteria for adverse events v5.0. UpToDate 2017.
MedDra. Medical Dictionary for Regulatory Activities.
Chang, W., Cheng, J., Allaire, J., Xie, Y., McPherson, J. Package ‘ shiny ’: Web Application Framework for R. R Packag. version 2020.
R Core team. R Core Team. R A Lang. Environ. Stat. Comput. R Found. Stat. Comput, Vienna, Austria. 275–286. ISBN 3-900051-07-0. http://www.R-project.org/. (2015).
Chang, W., Ribeiro, B. B. Package “ShinyDashboard”: Create Dashboards with “Shiny.” 27 (2018).
Ben-Menachem, E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia. (2004).
Wu, D., Kang, Y. S., Bickel, U. & Pardridge, W. M. Blood–brain barrier permeability to morphine-6-glucuronide is markedly reduced compared with morphine. Drug Metab. Dispos. 25, 768–771 (1997).
Viktil, K. K., Blix, H. S., Moger, T. A. & Reikvam, A. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br. J. Clin. Pharmacol. 63, 187–195 (2007).
Gnjidic, D. et al. Polypharmacy cutoff and outcomes: Five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J. Clin. Epidemiol. 65, 989–995 (2012).
Stampas, A. et al. The first 24 h: Opioid administration in people with spinal cord injury and neurologic recovery. Spinal Cord. 58, 1080–1089. https://doi.org/10.1038/s41393-020-0483-x (2020).
Hook, M. A. et al. Neurobiological effects of morphine after spinal cord injury. J. Neurotrauma. 34, 632–644 (2017).
Hook, M. A. et al. Intrathecal morphine attenuates recovery of function after a spinal cord injury. J. Neurotrauma. 26, 741–752 (2009).
Bracken, M. B. et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data: Results of the second National Acute Spinal Cord Injury Study. J. Neurosurg. 76, 23–31 (1992).
Goplen, C. M. et al. Preoperative opioid use is associated with worse patient outcomes after total joint arthroplasty: A systematic review and meta-analysis. BMC Musculoskelet. Disord. BMC Musculoskelet. Disord. 20, 1–12 (2019).
Mestre, H., Alkon, T., Salazar, S. & Ibarra, A. Spinal cord injury sequelae alter drug pharmacokinetics: An overview. Spinal Cord. 49, 955–960 (2011).
García-López, P., Martínez-Cruz, A., Guízar-Sahagún, G. & Castãeda-Hernández, G. Acute spinal cord injury changes the disposition of some, but not all drugs given intravenously. Spinal Cord. 45, 603–608 (2007).
Segal, L., Brunnemann, S. R., Eltorai, I. M. & Vulpe, M. Decreased systemic clearance of lorazepam in humans with spinal cord injury. J. Clin. Pharmacol. 31, 651–656 (1991).
Ibarra, A. et al. lteration of Cyclosporin-A pharmacokinetics after experimental spinal cord injury. J. Neurotrauma 13, 267–272 (1996).
Cragg, J. J. et al. A longitudinal study of the neurologic safety of acute baclofen use after spinal cord injury. Neurotherapeutics 16, 858–867 (2019).
McLean, G., Hindle, J. V., Guthrie, B. & Mercer, S. W. Co-morbidity and polypharmacy in Parkinson’s disease: Insights from a large Scottish primary care database. BMC Neurol. 17, 1–8 (2017).
Fereshtehnejad, S. M., Johnell, K. & Eriksdotter, M. Anti-dementia drugs and co-medication among patients with Alzheimer’s disease: Investigating real-world drug use in clinical practice using the Swedish Dementia Quality Registry (SveDem). Drugs Aging. 31, 215–224 (2014).
Thelen, J. M., Lynch, S. G., Bruce, A. S., Hancock, L. M. & Bruce, J. M. Polypharmacy in multiple sclerosis: Relationship with fatigue, perceived cognition, and objective cognitive performance. J. Psychosom. Res. 76, 400–404 (2014).
Cosano, G. et al. Polypharmacy and the use of medications in inpatients with acquired brain injury during post-acute rehabilitation: A cross-sectional study. Brain Inj. 30, 353–362 (2016).
Haddad, S. H. & Arabi, Y. M. Critical care management of severe traumatic brain injury in adults. Scand. J. Trauma. Resusc. Emerg. Med. 20, 1–15 (2012).
Kierner, K. A., Weixler, D., Masel, E. K., Gartner, V. & Watzke, H. H. Polypharmacy in the terminal stage of cancer. Support Care Cancer. 24, 2067–2074 (2016).
Mcallister, T. A. Polypharmacy in sepsis. Lancet (1973).
Ghezzi, L., Scarpini, E., Galimberti, D. Disease-modifying drugs in Alzheimer’s disease. Drug Des. Devel. Ther. (2013).
Cummings, J. & Fox, N. Defining disease modifying therapy for Alzheimer’s disease. J. Prev. Alzheimer’s Dis. 4, 109 (2017).
Lang, A. E. & Espay, A. J. Disease modification in Parkinson’s disease: Current approaches, challenges, and future considerations. Mov. Disord. 33, 660–677 (2018).
Schulenborg, T. et al. Proteomics in neurodegeneration—Disease driven approaches. J. Neural Transm. 113, 1055–1073 (2006).
Acknowledgements
The authors would like to acknowledge the participating centers in the Sygen trial and SCIRehab network that were involved in the patient care and collection of data necessary for this study.
Funding
This study was funded by research grants from the Swiss National Science Foundation (Ambizione Grant #PZ00P3_186101, Jutzeler), Wings for Life Research Foundation (#2017_044, Jutzeler and Kramer), and Craig H. Nielsen Foundation (Cragg and Jutzeler).
Author information
Authors and Affiliations
Contributions
C.R.J.: Study concept/design, data entry, data cleaning, data analyses, interpretation of data, and drafting the manuscript. L.B.: Data visualisation, interpretation of data and revising the manuscript for intellectual content. B.T.: Data cleaning, interpretation of data and revising the manuscript for intellectual content. E.R.: Interpretation of data and revising the manuscript for intellectual content. E.B.: Primary data collection, interpretation of data, and revising the manuscript for intellectual content. N.Y.H.: Data cleaning, interpretation of data, and revising the manuscript for intellectual content. F.G.: Data cleaning, interpretation of data, and revising the manuscript for intellectual content. K.B.: Interpretation of data, revising the manuscript for intellectual content. A.R.F.: Interpretation of data, revising the manuscript for intellectual content. B.K.K.: Interpretation of data, revising the manuscript for intellectual content. J.J.C.: Interpretation of data, revising the manuscript for intellectual content. L.G.: Study concept/design, interpretation of data, revising the manuscript for intellectual content. J.L.K.K.: study concept/design, interpretation of data, and revising the manuscript for intellectual content.
Corresponding author
Ethics declarations
Competing interests
CR. Jutzeler reports no disclosures relevant to the manuscript. L. Bourguignon reports no disclosures relevant to the manuscript. B. Tong reports no disclosures. E. Ronca reports no disclosures relevant to the manuscript. E. Bailey reports no disclosures relevant to the manuscript. NY. Harel reports no disclosures relevant to the manuscript. F. Geisler reports no disclosures relevant to the manuscript. AR. Ferguson reports no disclosures relevant to the manuscript. BK. Kwon reports no disclosures relevant to the manuscript. JJ. Cragg reports no disclosures relevant to the manuscript. L. Grassner reports no disclosures relevant to the manuscript. JLK. Kramer reports no disclosures relevant to the manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jutzeler, C.R., Bourguignon, L., Tong, B. et al. Pharmacological management of acute spinal cord injury: a longitudinal multi-cohort observational study. Sci Rep 13, 5434 (2023). https://doi.org/10.1038/s41598-023-31773-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31773-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.