Abstract
Pulmonary fibrosis (PF) is a special type of pulmonary parenchymal disease, with chronic, progressive, fibrosis, and high mortality. There is a lack of safe, effective, and affordable treatment methods. Qilongtian (QLT) is a traditional Chinese prescription that is composed of Panax notoginseng, Earthworm, and Rhodiola, and shows the remarkable clinical curative effect of PF. However, the mechanism of QLT remains to be clarified. Therefore, we studied the effectivity of QLT in treating Bleomycin (BLM) induced PF mice. 36 C57BL/6 J mice were randomized into the control group, the model group, the low-, medium- and high-dose QLT group, and Pirfenidone group. After establishing a model of pulmonary fibrosis in mice, the control and model groups were infused with a normal saline solution, and the delivery group was infused with QLT. Pulmonary function in the mice from each group was detected. Pulmonary tissue morphologies and collagen deposition were stained by HE and Masson. The content of hydroxyproline (HYP) was detected by alkaline hydrolysis and the mRNA and protein expression of related genes in pulmonary tissues were detected by using q-PCR, ELISA, and Western blot. Our studies have shown that QLT significantly reduced the inflammatory injury, hydroxy-proline content, and collagen deposition of pulmonary tissue in BLM-induced PF mice and down-regulated the cytokine related to inflammation and fibrosis and PF expression on the mRNA and protein level in PF mice. To identify the mechanism of QLT, the Transcriptome was measured and the IL-17 signal pathway was screened out for further research. Further studies indicated that QLT reduced the mRNAs and protein levels of interleukin 17 (IL-17), c–c motif chemokine ligand 12 (CCL12), c-x-c motif chemokine ligand 5 (CXCL5), fos-like antigen 1 (FOSL1), matrix metalloproteinase-9 (MMP9), and amphiregulin (AREG), which are inflammation and fibrosis-related genes in the IL-17 signal pathway. The results indicated that the potential mechanism for QLT in the prevention of PF progression was by inhibiting inflammation resulting in the IL-17 signal pathway. Our study provides the novel scientific basis of QLT and represents new therapeutics for PF in clinical.
Similar content being viewed by others
Introduction
The COVID-19 chest CT scan in severe patients shows more changes in PF, and that change may persist1. PF pathogenesis involves theories of inflammatory response imbalance, extracellular matrix deposition, epithelial-mesenchymal transformation, and oxidative stress. The proliferation of interstitial fibroblasts and fibrotic alterations of interstitial tissues are characteristics of PF2,3,4,5. Repetition of inflammation leads to abnormal expression of the downstream gene and differentiation of fibroblasts, resulting in the repair of excessively fibrous tissue6,7,8. Treatment methods such as glucocorticoids, immunosuppressants, antioxidants, anti-fibrosis medications, etc., delay disease progression and lead to quality of life for patients9,10,11,12,13,14. Therefore, there is an urgent need to find new therapeutic agents to treat PF. Currently, new therapeutic strategies are needed to treat PF.
For thousands of years, Traditional Chinese Medicine (TCM) has long been used for disease prevention and treatment based on systematic approaches15. Research in Chinese medicine and modern medicine concluded that it was an option for treating respiratory diseases. Qilongtian (QLT) is a traditional Chinese medicine prescription with Panax notoginseng, earthworm, and Rhodiola. The main chemical composition of QLT and the role of PF have been shown in Table 116,17,18. In our clinical efficiency observation study showed that QLT reduced inflammatory factor and fiber factor transforming growth factor β (TGF-β) and matrix metalloproteinase-9 (MMP9) to improve anti-infection ability of respiratory tract in chronic obstructive pulmonary disease (COPD) patients19. The animal experimental research indicated that QLT reduced the inflammatory factor interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α) to inhibit the inflammatory reaction of the airway in a pulmonary hypertension rat model20,21. In recent years, Pro. Yi Fu finds that QLT delays the process of PF, improves lung function and life quality of PF patients. However, systematic investigations of the mechanisms by which QLT exerts beneficial effects in PF, including analyses of potential targets, biological processes, and metabolic pathways, are lacking.
Multiple signaling pathways contribute to the development of pulmonary fibrosis as the IL-17 signaling pathway plays a direct or indirect role22,23,24. Interleukin 17 (IL-17) is a cytokine mainly derived from Th17 cells. Its main biological effect is the promotion of inflammatory reaction. After IL-17 is attached to the receptor, it exerts biological activity by activating the mitogenic-activated protein kinase18,19. The stimulation of IL-17 signaling pathway promotes the inflammation and fibrosis in lung20,21,22,23,24. One study reported25 that molecular, immunohistochemical, and flow cytometric analyses of human and mouse specimens determine the immune response before collagen deposition. The results showed that PD-1+CD4+ T cells were detected in CD4+ T cells with decreased proliferative capacity and increased expression of transforming growth factor-β (TGF-β)/interleukin-17 (IL-17). PD-1+ T helper 17 cells are the major CD4+ T cell subpopulation expressing TGF-β. PD-1+ CD4+ T cells co-cultured with human lung fibroblasts co-cultured to induce collagen production. Blocking the expression of TGF-β and IL-17 was followed by a reduction in collagen production by fibroblasts. This points to an unrecognized critical role for TGF-β and IL-17 in pulmonary fibrosis. Additionally, genome-scale association studies have demonstrated that the role of the IL-17 signalling pathway in sarcoidosis pulmonary progression is associated with mediation of multiple cytokines26,27,28,29. Analysis of the transcriptome revealed that adaptive immune dysfunction is associated with PF30,31. In the current study, we used QLT to intervene in the mouse PF model, observed the treatment effect, and explore the possible anti-fibrosis mechanism, to provide the experimental basis for QLT in the clinical treatment of PF.
Materials
Experimental animals and feeding
36 male C57BL/6 J mice, SPF grade, 4 weeks old, body weight (20 ± 2) g, (Chengdu Dashuo experimental animal Co., Ltd., Chengdu, China, regular animal No.: SCXK (Sichuan) 2020-030). Laboratory conditions: temperature (22 ± 3 °C), 12 h light–dark cycle. In this study, mice were fed ad libitum and ingested water. All methods were carried out in accordance with the care and use of experimental animals published by the National Institutes of Health and the guidelines of the Animal Care & Welfare Committee of Yunnan University of Chinese Medicine. According to the guidelines for, the mouse experiments have been approved and implemented (Approval Institution: Animal Experiment Ethics Review Committee of Yunnan University of Traditional Chinese, Medicine animal experiment license No.: SYXK (Dian) K2022-0004, animal ethics license No.: R-06202023). All methods are reported in accordance with ARRIVE guidelines.
Experimental drugs
QLT capsule: Panax notoginseng, Earthworm, Rhodiola; 0.4 g per capsule, equivalent to 1.53 g of decoction pieces. The positive drug was selected as pirfenidone (PFD, H20133376, Beijing Contini Pharmaceutical Co., Ltd., Beijing, China). Pirfenidone is a drug for IPF with good anti-inflammatory properties. Hydrochloride Bleomycin (Hanhui Pharmaceutical Co., Ltd. Hangzhou, China) for injection: 15,000 bleomycin units (equivalent to 15 mg)/bottle, purchased from, national medicine permission number (NMPN) H20055883. Before use, add 7.5 ml of normal saline to each bottle to obtain a solution with a concentration of 2 mg/ml.
Modeling and administration
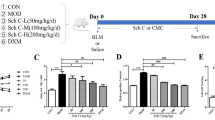
36 mice were randomly divided into control group, model group, QLT low, medium, high administration group and pirfenidone group. Modeling method: the model was made by endotracheal intubation and injection of bleomycin (5 mg/kg)25,26, the control group and model group are gavaged with normal saline, and the QLT low, medium, and high dose groups were gavaged with 0.39 g/kg, 0.78 g/kg and 1.56 g/kg respectively, and Pirfenidone group was gavaged with 0.78 g/kg. The drug was administered at 14 days after modeling, and the materials were taken 21 days after administration.
Invasive pulmonary function test
After administration, mice were anesthetized intraperitoneally with 1% pentobarbital sodium (0.4 g/kg). After fixation, skin preparation, and disinfection, the exposed trachea was gently and passively separated. The trachea was severed horizontally between the tracheal cartilaginous rings. The trachea was lifted after threading, the intubation needle was inserted, and the pulmonary function instrument (model: crfm100, EMMS, UK) was connected, the related indexes of pulmonary function were recorded.
Specimen collection
Three weeks after administration, the middle lobe of the right lung was taken and fixed in tissue fixation solution (Wuhan Servicebio Technology Co., Ltd., Wuhan, PR China) for HE and Masson staining. The left lung was placed in 1.5 ml EP tube and frozen at – 80 °C.
HE staining
Paraffin sections were used for dewaxing, hematoxylin staining, eosin staining, and dehydration sealing. After the above operations, the sections were dried, sealed with neutral gum, microscopically observed, photographed, captured, and analysed.
Masson staining
Paraffin sections were dehydrated, stained with potassium dichromate, ferrochrome, Ponceau red, acid fuchsin, phosphoric acid, and aniline blue, identified, transparent, sealed with neutral glue, observed under a microscope, photographed, and images collected and analyzed.
Hydroxyproline (HYP) detection
The HYP assay was measured with hydroxyproline determination kit (Cat#: A030-2-1, Nanjing Jiancheng Bioengineering Institute), following the product instruction book. The absorbance of each tube was measured with a microplate reader (BioTek SynergyH1, Agilent Technologies, Inc., USA) at a wavelength of 550 nm.
Transcriptome sequencing
The transcriptome sequencing was detected by Metabo-Profile Biotechnology (Shanghai) Co., Ltd., the mRNA with Polya structure in total RNA was enriched by oligo (DT) magnetic beads, and the RNA was interrupted to a fragment with a length of about 300 bp by ion interruption. Using RNA as template, the first strand of cDNA was synthesized with 6-base random primers and reverse transcriptase, and the second strand of cDNA was synthesized with the first strand of cDNA as template. After construction library, the library fragments were enriched by PCR amplification, and then the library was selected according to the fragment size. After RNA extraction, purification, and library construction, these libraries were sequenced by next-generation sequencing (NGS) based on the Illumina sequencing platform.
Elisa
Samples were tested according to the Elisa Kit instructions. The kits were Mouse IL-17 ELISA Kit (PI545, Beyotime), Mouse CCL12 Elisa Kit (SEKM-0164, Solarbio), Mouse FOSL1 Elisa Kit (E03C0735, BlueGene Biotech Co., Ltd.), Mouse MMP9 Elisa Kit (ARG81241, Arigo Biolaboratories Corp.), Mouse CXCL5 Elisa Kit (EMCXCL5, Thermo Fisher), Mouse AREG Elisa Kit (EMAREG, Thermo Fisher). The absorbance was measured, and the concentration was calculated using an enzyme marker (Synergy, BioTek, USA) at 550 nm.
qRT-PCR
The total tissue mRNA was extracted with Invitrogen™ TRIzol™ Reagent (Thermo Fisher Scientific Inc.). The primer sequences were listed in Table 2 and were synthesized by Sangon Biotech (Shanghai) Co., Ltd. The qPCR assay was measured by using Applied Biosystems QuantStudio 5 Real-Time PCR System (A28569, Thermo Fisher Scientific Inc.). The relative mRNA levels of each gene were calculated by using the 2−ΔΔCt method.
Immunofluorescence assay
The experimental operation steps of immunofluorescence were as follows. Dewaxing paraffin sections to water, circle, serum blocking, added COL-I antibody: add Goat anti rabbit IgG HRP, After the sections were slightly dried and incubated for 50 min, added Cy3 fluorescence enhancer, added COL-III antibody: add Goat anti rabbit IgG HRP, added FITC fluorescence enhancer, added dropwise into the slice circle and incubated. DAPI staining, autofluorescence quenching, sealing slide, microscopic photography. DAPI staining was blue, COL-I was red, and COL-III was green.
Western blot
Samples were homogenized with Ripa lysate (Cat#: P0013B, Beyotime Biotech-nology), and the total protein concentration was measured with BCA method (Cat#: P0010S, Beyotime Biotechnology). The total protein (20 μg) was separated with 10% SDS–polyacrylamide gel electrophoresis. Then, the protein was transferred to PVDF membrane, blocked with 5% skimmed milk, washed in tris buffer solution containing 0.1% Tween-20 (TBST), and the primary antibody was incubated at 4 ℃ overnight. The primary antibody was used as follows: TGF-β antibody (EPR18163, Cat#: ab215715, Abcam), α-SMA antibody (Cat#: af1032, Affinity), COL-I antibody (EPR22894-89, Cat#: ab260043, Abcam), COL-III antibody (RM1028, Cat#: ab283694, Abcam), TNF-α antibody (EPR20972, Cat#: ab215188, Abcam), β-actin antibody (Cat#:GB12001, Affinity). Goat anti-rabbit IgG (Cat#: bl033a, Biosharp) and Goat anti-mouse IgG (Cat#: bl001a, Biosharp) were used as secondary antibodies. The images were obtained by chemiluminescence systems chemiluminescence systems (GeneGnome XRQ, Syngene, A Division of Synoptics Ltd.), with exposure time of 30–90 s. Use Image J 18.0 software to analyze the gray value of each strip.
Statistical analysis
The experimental data were statistically processed by using SPSS (IBM, Armonk, USA) and GraphPad Prism 10.0 (GraphPad Software, San Diego, CA). The measurement data were expressed by mean ± Standard error of mean (SEM) unless otherwise specified. All endpoints are representative of at least 3 independent experiments and analyzed using one-way ANOVA with the post hoc Bonferroni test. The difference was statistically significant when P < 0.05.
Institutional review board statement
The animal study protocol was approved by the Animal Care & Welfare Committee of Yunnan University of Chinese Medicine (protocol code: R-06202023, June 15th, 2020).
Results
QLT improved the morphology of pulmonary tissue and reduce collagen deposition in PF mice
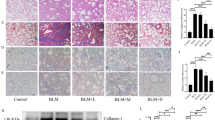
Bleomycin induced PF in mice is a widely recognized PF model, which lead to pulmonary fibrosis in mice. Based on previous studies showing that mice begin to form collagen on days 3–7 and that inflammation coexists with collagen deposition on day 1438,39, low, medium and high doses of QLT were given to PF mice with intragastric administration within 14–35 days. The morphology of pulmonary tissue was observed by HE staining and Masson staining. Compared with model group, QLT significantly inhibited the infiltration of inflammatory cells and the expansion of alveolar space. In addition, QLT prevented the accumulation of collagen in the lungs in a dose-dependent way, with the high dose being more effective (Fig. 1a). Additionally, QLT treatment decreased the pulmonary collagen deposition area (Fig. 1b,c). The model group’s lung tissue contains significantly more hydroxyproline than the normal group did. QLT treatment resulted in a dose-dependent decrease in hydroxyproline content (Fig. 1d). The results discussed above show that QLT could effectively reduce PF (Fig. 1).
Therapeutic effect of QLT in PF mice. (a) HE staining was used to indicate the morphology of pulmonary tissue (400 × magnification). (b) Masson staining was used to indicate collagen deposition in pulmonary tissue (400 × magnification). (c) The area percentage of blue collagen fibers in Masson pathological sections. (d) The level of hydroxyproline in pulmonary tissue. (n = 3; data are expressed as mean ± SD. ∗P < 0.05, compared with Model group).
QLT improved the pulmonary function of PF mice
As a necessary examination method for respiratory diseases, pulmonary function could be used as one of the diagnostic methods of PF. Quasi-static lung compliance (Cchord), mean mid-expiratory flow (MMEF), peak expiratory flow (PEF), 50 ms first expiratory volume/forced vital capacity (FEV 50/FVC), functional residual capacity (FRC) and total lung capacity (TLC) reflect the pulmonary function severity of PF. The results show that compared with model group, QLT significantly improved Cchord, MMEF, PEF, FEV 50/FVC, FRC and TLC value with dose-dependent, indicated that QLT improved pulmonary function in PF mice. (Fig. 2).
Effects of pulmonary function in each group. (a) Cchord: an index reflecting alveolar compliance and expansibility, representing the effect of changes in thoracic pressure on lung volume. It also reflects pulmonary tissue elasticity and airway resistance. (b) MMEF: It reflects the detection index of alveolar diffusion function. (c) PEF: it mainly reflects whether the large airway is blocked. (d) FEV 50/FVC: it is the volume of exhaled volume after maximum deep inhalation and maximum exhalation for 50 ms in mice, Obstructive or mixed type is slightly reduced to significantly reduced. (e) FRC: The decrease indicates a reduction in alveolar function. (f) TLC: The decrease is mostly related to restrictive ventilation disorder, suggesting that it is related to PF. (n = 3; data are expressed as mean ± SD. ∗P < 0.05, ∗∗P < 0.01, compared with Model group).
QLT reduced the inflammation and collagen deposition in the pulmonary tissue of PF mice
Collagen I (COL-I) and collagen III (COL-III) are indicators of the deposition of extracellular matrix. The mRNA relative expression levels of COL-I and COL-III in the pulmonary tissue were increased in the model group compared to the control group. The mRNA levels of COL-I and COL-III were decreased in the pulmonary tissue of QLT treatment PF mice, indicating that QLT decreased the extracellular matrix deposition in PF animals. We also examined the mRNA levels of TGF-β, TNF-α, and α-smooth muscle actin (α-SMA) in lung tissue. TGF- is regarded as a major factor in the promotion of fibrosis, while TNF- is a critical element in the promotion of inflammation and fibrosis. α-SMA is a marker protein for the transformation of fibroblasts into myofibroblasts. The results confirmed that after the treatment with QLT, the mRNA levels of α-SMA, TGF-β and TNF-α were significantly down-regulated in lung, comparing with that of the model group (Fig. 3a).
Effects of inflammation and collagen-related indexes in each group. (a) The relative expression level of COL-I, COL-III, α-SMA, TGF-β, and TNF-α mRNA levels in pulmonary tissues. (b) The location and expression levels of COL-I and COL-III in pulmonary tissues were detected by using an immunofluorescence assay. (c) The protein levels of α-SMA, TGF-β, and TNF-α were detected by western blot. (d) The quantification and statistical analysis of (b–d). (n = 3; data are expressed as mean ± SD. ∗P < 0.05, ∗∗P < 0.01, compared with Model group).
To further confirm the influence of inflammation and collagen deposition level of QLT, the protein level of COL-I, COL-III, α-SMA, TGF-β, and TNF-α was evaluated. The results showed that the protein level of COL-I and COL-III in the pulmonary tissue of the model group was increased when compared with the control group and was decreased in the QLT treatment group when compared with the model group (Fig. 3b,d). Meanwhile, the protein level of α-SMA, TGF-β, and TNF-α was determined by ELISA and Western Blot. The results demonstrated that QLT decreased the protein level of α-SMA, TGF-β, and TNF-α in the pulmonary tissue of PF mice, and the high dose was more effective (Fig. 3c,d). These results suggested that QLT efficiently down-regulation inflammation and collagen deposition of PF.
QLT interfere in inflammation and collagen deposition in PF mice through IL-17 signal pathway
By sequencing the transcriptome of pulmonary tissue in the model and control group, 94 meaningful differential Expressed Genes (DEGs) were obtained after the comparison of the two groups. 72 DEGs were up-regulated and 22 were down-regulated in the model group (Fig. 4a,b). Through analyzing in database Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG), we found that the IL-17 signal pathway was important to PF (Fig. 4c). And in these two pathways, c–c motif chemokine ligand 12 (CCL12), c-x-c motif chemokine ligand 5 (CXCL5), fos-like antigen 1 (FOSL1), matrix metalloproteinase-9 (MMP9), and amphiregulin (AREG) were enriched with fold change (Fig. 4d).
IL-17 signal pathway is prominent in the development of pulmonary fibrosis and the influence of QLT on IL-17 signal pathway40. (a) Heat map of differential genes clustering in pulmonary tissues. (b) Volcano plot distribution of PM 2.5 differential genes cluster heat map in pulmonary tissues. (c) KEGG analysis bubble diagram of differential genes in pulmonary tissue (X-axis represents the P-value of the enrichment factor, and the Y-axis represents the names of the 12 pathways with significant differences. The size of the dots represents the number of target genes, and the color of the dots represents the range of the false discovery rate (FDR), which represents the ratio of target genes of a specific pathway to the number of all annotated genes in that pathway). (d) The 5 differential genes in pulmonary tissue were enriched into the genes table of IL-17 signal pathway.
By sequencing the transcriptome of pulmonary tissue in the model and to confirm the role of the IL-17 pathway in PF mice and the influence of the related genes of QLT, the mRNA level of IL-17, CCL12, FOSL1, MMP9, CXCL5, and AREG in pulmonary tissue was determined. The results showed that compared with the control group, the relative mRNA expression levels of IL-17, CCL12, FOSL1, MMP9 CXCL5, and AREG were significantly upregulated in the model group. Compared with the model group, the relative mRNA expression levels of IL-17, CCL12, FOSL1, MMP9, CXCL5, and AREG in the QLT group was significantly down-regulated, indicating that QLT reduced the expression of genes that related to IL-17 signal pathway (Fig. 5a). To further study, the protein levels of IL-17, CCL12, FOSL1, MMP9, CXCL5, and AREG were measured. Compared with the model group, the expression levels of IL-17, CCL12, FOSL1, MMP9, CXCL5 and AREG were much higher than those of the control group, while the protein levels of IL-17, CCL12, FOSL1, MMP9, CXCL5 and AREG were significantly decreased in the QLT group (Fig. 5b). These results suggested that QLT efficiently inhibited the IL-17 signal pathway (Figs. 4, 5).
The influence of QLT on IL-17 signal transduction pathway. qRT-PCR was used to verify the mRNA levels of (a) IL-17, (b) CCL12, (c) FOSL1, (d) MMP9, (e) CXCL5 and (f) AREG in pulmonary tissues. Elisa was used to verify the protein levels of (g) IL-17, (h) CCL12, (i) FOSL1, (j) MMP9, (k) CXCL5 and (l) AREG in pulmonary tissues. The data shown are Mean ± SEM of 3 independent experiments. (n = 3; data are expressed as mean ± SD. ∗P < 0.05∗∗P < 0.01, compared with Model group).
Discussion
The main pathological feature of PF is the acute inflammatory reaction of the lower respiratory tract at the early stage, accompanied by pathological proliferation and transformation of a large number of fibroblasts, resulting in the deposition of extracellular matrix (ECM) components in alveoli and stroma at the later stage. The excessive repair of fibrous tissue leads to the disorder of pulmonary tissue structure, alveolar injury, and collagen deposition, which eventually leads to PF3,41. The incidence rate of PF is high in middle-aged and elderly people. PF is mostly the pathological result of respiratory diseases, and also one of the common complications of COVID-19 patients. The accumulation of inflammatory cells and the release of cytokines increase collagen fibers, resulting in pulmonary tissue remodeling, reduction of alveolar number, deformation, atresia, and loss of pulmonary function, which are the main pathological changes of PF42,43. Previous research found that Yifei Sanjie Recipe alleviated pulmonary fibrosis by reducing the phosphorylation level of phosphatidylinositol3-kinase (PI3K) and protein kinase B (AKT), upregulating the expression of autophagy marker protein, enhancing autophagy, reducing the content of HYP and the expression of COL-I and COL-III in pulmonary tissue38. Further study indicated that curcumin and curcumol reduced fibroblasts α-SMA expression and decreased the contents of HYP and COL-I/III, which could reduce collagen synthesis. Curcumin and curcumol increased the expression levels of Beclin1, recombinant autophagy-related protein 7 (ATG7), and microtubule-associated protein 1 light chain 3B-II (Lc3b-II), promoted the formation of autophagy bodies, and activate fibroblast autophagy44. Previous report45 also found that Panax notoginseng saponins down regulated the expression of fibrosis related factors, reduced collagen fiber deposition and alleviate bleomycin induced pulmonary fibrosis in mice. Through HPLC experiments, we found that the main chemical components of QLT include Salidroside and ginsenoside Rg1 (Rg1) play an important role in anti-inflammatory and anti-fibrosis46 (Fig. S1). In this study, the pathological section results showed that there was a large infiltration of inflammatory cells in the lung tissue and an enlarged alveolar space in the PF model group. Meanwhile, the expression level of hydroxyproline, one of the main components of collagen deposition, was significantly increased. After the administration of QLT, the lung tissue was repaired with a significant decrease in the expression level of hydroxyproline, indicating that QLT could reduce the deposition of collagen and inhibit the expression of hydroxyproline. These results suggest that QLT has the effect of repairing the pathological changes in PF.
Pulmonary function examination is one of the necessary examinations for respiratory diseases and is mainly used to detect the patency of respiratory tract and the size of lung volume. It is of great value for detecting lung diseases, evaluating the severity and prognosis of diseases, and evaluating the efficacy of drugs or other treatment methods. This study showed that compared with PF model group, taking QLT could improve Cchord, MMEF, PEF, FEV50/FVC, FRC and TLC (P < 0.05), and improved pulmonary function, which is consistent with relevant research reports on pulmonary function47.
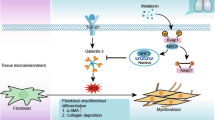
Inflammatory factors could repeatedly mediate the injury and repair of alveolar cells48. In this process, fibroblasts migrate go through the damaged basement membrane, proliferate and transform into myofibroblasts49. Large amounts of inflammatory and fibrotic factors produced by myofibroblasts are released into the extracellular matrix (ECM)50. These factors lead to the pathological processes such as cell structure remodeling, airway wall structure destruction, basement membrane thickening and interstitial hyperplasia, and resulting in pulmonary fibrosis, finally51. In process of ECM formation, TGF-β has the activity to induce fibroblasts divided to myofibroblasts and specifically promotes the secretion of α-SMA and plays an important role in formation of pulmonary fibrosis52. In addition, collagen I/ III secreted by myofibroblasts also promote ECM deposition and accelerate the progress of pulmonary fibrosis53,54. In this study, the expression of fibrosis related factors was detected. The results showed that QLT administration group significantly reduced the mRNA and protein expression level of TGF-β, α-SMA, COL-I, COL-III and TNF-α in pulmonary tissue of model mice. It indicated that QLT inhibited the activation of TGF-β, α-SMA and TNF-α, reduced pulmonary inflammatory response and collagen deposition, to delay pulmonary fibrosis.
Transcriptome sequencing is a high-throughput sequencing of the sum of all RNA that could be transcribed by a specific cell in a certain functional state55,56. In this study, the high-throughput sequencing of the transcriptome of the pulmonary tissue of mice in control group and model group was used to explore the relevant mechanism of PF. Through transcriptome sequencing, the differentially expressed genes were found, and the KEGG signal pathway were enriched and analyzed. We found that the differentially expressed genes were involved in a variety of biological processes of the body, especially the regulation of the immune system, signal transduction pathway. Through the calculation of FDR and P value, we ranked the pathways with significant differences. In these pathways, we found that IL-17 signal pathway was important to PF in this study.
IL-17 is an early promoter of T cell and induces inflammatory response. After IL-17 binds to the IL-17 receptor, IL-17 plays its biological activity through the pathway and effectively mediates the inflammatory response in tissues and cells. Previous studies57 have reported that the IL-17-driven signaling pathway plays a critical role in cell survival and tissue growth. Aberrant signaling of IL-17 implicates the pathogenesis of several autoimmune diseases, including idiopathic pulmonary fibrosis, acute lung injury, chronic airway disease, and cancer58. Literature research on the enrichment gene of the IL-17 signaling pathway in the first place showed that CCL12 chemokine produced by macrophages and epithelial cells is a fibrogenic medium, and CCL12 could promote the development of fibrosis59,60,61. Pena-Philippides et al.62 reported that CCL12 and CXCL5 cytokine expression affect the secretion of IL-17. FOSL1 could regulate biological behaviors such as proliferation, apoptosis, and migration of various cells, which is closely related to the inflammatory signal pathway63. A previous study of hepatocyte inflammation and immune response also found that the FOSL1 gene, identified based on bioinformatics techniques, is one of the central targets of action of the IL-17 signaling pathway64. MMP9, a member of the MMPs family, plays an important role in collagen hydrolysis, and they can promote collagen hydrolysis in the myocardial interstitium. TIMP1 and TIMP2, specific inhibitory molecules of MMPs, can bind to MMP molecules and reduce their hydrolytic activity, favoring collagen deposition, and suggesting that the IL-17 gene can disrupt the MMPs/TIMPs balance65,66. The previous reports indicated that IL-17 stimulates the migration of carotid artery vascular smooth muscle cells in an MMP-9-dependent manner67,68. CXCL5 chemokine is a member of the CXC chemokine family, produced by various cells, and has strong granulocyte chemotaxis69. As component of the inflammatory environment, CXCL5 has an important role in tumorigenesis, invasion, metastasis, and progression70. Previous studies have reported that IL-17 can promote inflammation and cancer development by inducing the production of factors such as CXCL571. AREG can be secret by various cells, which can cause the proliferation and differentiation of fibroblasts and protect pulmonary tissue by activating the EGFR signaling pathway to inhibit TNF-α and mediating the apoptotic signaling pathway to reduce damage to alveolar epithelial cells72,73. Previous studies have reported that AREG, a target gene of IL-17, promotes the development of keratin-forming cell proliferation74. Our studies indicated that QLT reduced the levels of IL-17, CCL12, CXCL5, FOSL1, MMP9, and AREG, which are inflammation and fibrosis-related genes in the IL-17 signal pathway.
Conclusion
In this study, we have confirmed the role of QLT in the prevention and treatment of pulmonary fibrosis and revealed the underlying mechanisms of QLT. The results suggest that QLT can improve pulmonary fibrosis in multiple ways, and the IL-17 signaling pathway is likely to be the most potential target of QLT. Our study provides a new strategy for the clinical treatment of PF.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Change history
12 July 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-023-38495-x
Abbreviations
- AKT:
-
Protein kinase B
- AREG:
-
Amphiregulin
- ATG7:
-
Recombinant autophagy related protein 7
- cAMP:
-
Cyclic adenosine monophosphate
- Cchord:
-
Quasi-static lung compliance
- CCL12:
-
C-C motif chemokine ligand 12
- COPD:
-
Chronic obstructive pulmonary disease
- CXCL5:
-
C-X-C motif chemokine ligand 5
- DEGs:
-
Differential expressed genes
- DL:
-
Drug likeness
- ECM:
-
Extra cellular matrix
- Elisa:
-
Enzyme linked immunosorbent assay
- FDR:
-
False discovery rate
- FEV50/FVC:
-
50Ms first expiratory volume/forced vital capacity
- FOSL1:
-
Fos-like antigen 1
- FRC:
-
Functional residual capacity
- GO:
-
Gene ontology
- HPLC:
-
High performance liquid chromatography
- HYP:
-
Hydroxyproline
- IL-17:
-
Interleukin 17
- IL-1β:
-
Interleukin 1β
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- Lc3b-II:
-
Microtubule-associated protein 1 light chain 3B-II
- MAPK:
-
Mitogen-activated protein kinase
- MMEF:
-
Mean mid-expiratory Flow
- MMP9:
-
Matrix metalloproteinase-9
- OB:
-
Oral bioavailability
- PEF:
-
Peak expiratory flow
- PF:
-
Pulmonary fibrosis
- PFD:
-
Pirfenidone
- PI3K:
-
Phosphatidylinositol 3-kinase
- QLT:
-
Qilongtian
- q-PCR:
-
Real-time fluorescence quantitative PCR
- Rg1:
-
Ginsenoside Rg1
- TCM:
-
Traditional Chinese medicine
- TGF-β:
-
Transforming growth factor β
- TLC:
-
Total lung capacity
- TNF-α:
-
Tumor necrosis factor α
- α-SMA:
-
α-Smooth muscle actin
References
Zhan, X., Liu, B. & Tong, Z. Current situation and thinking of pulmonary fibrosis after New Coronavirus pneumonia inflammation. Chin. J. Tuberculosis Respir. 43, 728–732. https://doi.org/10.3760/cma.j.cn112147-20200317-00359 (2020).
Guzy, R. D., Li, L., & Smith, C., et al. Pulmonary fibrosis requires cellautonomous mesenchymal fibroblast growth factor (FGF) signaling. J. Biol. Chem. 292, 10364–10378. https://doi.org/10.1074/jbc.M117.791764 (2017).
Yang, C. M., Zhang, H. C. & Liu, D. Systematic evaluation of Supplementing Qi, nourishing yin and dredging collaterals in the treatment of idiopathic pulmonary fibrosis. J. China Jpn. Friendship Hosp. 35, 172–174. https://doi.org/10.13463/j.cnki.cczyy.2018.01.027 (2021).
Rahmati, S. et al. Insights into the pathogenesis of psoriatic arthritis from genetic studies. Semin. Immunopathol. 43, 21–234. https://doi.org/10.1007/s00281-021-00843-2 (2021).
Meyer, K. C. Pulmonary fibrosis, part I: epidemiology, pathogenesis, and diagnosis. Expert. Rev. Respir. Med. 11, 343–359. https://doi.org/10.1080/17476348.2017.1312346 (2017).
Thannickal, V. J. et al. Mechanisms of pulmonary fibrosis. Annu. Rev. Med. 55, 395–417. https://doi.org/10.1146/annurev.med.55.091902.103810 (2004).
Guo, S. S. et al. Research progress of idiopathic pulmonary fibrosis. Chin. Folk Ther. 28, 97–100. https://doi.org/10.19621/j.cnki.11-3555/r.2020.0348 (2020).
Kinoshita, T. & Goto, T. Molecular mechanisms of pulmonary fibrogenesis and its progression to lung cancer: A review. Int. J. Mol. Sci. 20, 1461. https://doi.org/10.3390/ijms20061461 (2019).
Hu, X. et al. Liver X receptor agonist To901317 attenuates paraquat-induced acute lung injury through inhibition of NF-κB and JNK/p38MAPK signal pathways. Biomed. Res. Int. 49, 693–699. https://doi.org/10.1155/2017/4652695 (2017).
Li, F. Z. et al. Crosstalk between cal-pain activation and TGF-β1 augments collage-I synthesis in pulmonary fibrosis. Biochim. Biophys. Acta 1852, 1796–1804. https://doi.org/10.1016/j.bbadis.2015.06.008 (2015).
Wu, J., Yang, X. J., & Wang, J. et al. TGF-β/Immunomodulatory effect of Smads signaling pathway on pulmonary tissue injury induced by PM2.5 in rats. Northw. J. Pharm. 32, 316–321 (2017).
Chen, X., Li, C., & Liu, J.L. et al. Inhibition of ER stress by targeting the IRE1α-TXNDC5 pathway alleviates crystalline silica-induced pulmonary fibrosis. Int. Immunopharmacol. 95, 107792. https://doi.org/10.1016/j.intimp.2021.107519 (2021).
Noble, P. W., Barkauskas, C.E., & Jiang, D. Pulmonary fibrosis: Patterns and perpetrators. J. Clin. Invest. 122, 2756–2762. https://doi.org/10.1172/JCI60323 (2012).
Smith, M. L. Update on pulmonary fibrosis: Not all fibrosis is created equally. Arch. Pathol. Lab. Med. 140, 221–229. https://doi.org/10.5858/arpa.2015-0288-SA (2016).
Chao, J. et al. Major achievements of evidence-based traditional Chinese medicine in treating major diseases. Biochem. Pharmacol. 139, 94–104. https://doi.org/10.1016/j.bcp.2017.06.123 (2017).
Wu, Y. et al. SymMap: an integrative database of traditional Chinese medicine enhanced by symptom mapping. Nucleic Acids Res. 47(D1), D1110–D1117. https://doi.org/10.1093/nar/gky1021 (2019).
Ru, J. et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Chem. Inform. 6, 13–20. https://doi.org/10.1186/1758-2946-6-13 (2014).
Chen, C. Y. TCM Database@Taiwan: the world's largest traditional Chinese medicine database for drug screening in silico. PLoS One 6(1), e15939–e15945. https://doi.org/10.1371/journal.pone.0015939 (2011).
An, P. Effects of Yunyao "Qilongtian" on pulmonary function and TGF-β1, MMP9 serum level in stable COPD patients. Kunming: Yunnan University of traditional Chinese medicine. link: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1020086510.nh&DbName=CMFD2021 (2020).
Leng, P. et al. Effect of Qilongtian on IL-1β and TNF-α in rats with influence of hypoxic pulmonary hypertension. J. Beijing Univ. Trad. Chin. Med. 11, 915–919 (2020).
Fu, X. et al. Qi-Long-Tian formula extract alleviates symptoms of acute high-altitude diseases via suppressing the inflammation responses in rat. Respir. Res. 22, 52. https://doi.org/10.1186/s12931-021-01645-8 (2021).
Zhang, J. et al. Profibrotic effect of IL-17A and elevated IL-17RA in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated lung disease support a direct role for IL-17A/IL-17RA in human fibrotic interstitial lung disease. Am. J. Physiol. Lung Cell Mol. Physiol. 316(3), L487–L497. https://doi.org/10.1152/ajplung.00301.2018 (2019).
Nie, Y. J. et al. Role of IL-17 family cytokines in the progression of IPF from inflammation to fibrosis. Mil Med. Res. 9(1), 21–57. https://doi.org/10.1186/s40779-022-00382-3 (2022).
Ritzmann, F., Lunding, L. P., Bals, R., Wegmann, M. & Beisswenger, C. IL-17 cytokines and chronic lung diseases. Cells 11(14), 2132–2133. https://doi.org/10.3390/cells11142132 (2022).
Celada, L. J. et al. PD-1 up-regulation on CD4+ T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β production. Sci. Transl. Med. 10(460), 8356–8399. https://doi.org/10.1126/scitranslmed.aar8356 (2018).
Kolodsick, J. E., Toews, G. B., & Jakubzick, C., et al. Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J. Immunol. 172(7), 4068–4076. https://doi.org/10.4049/jimmunol.172.7.4068.
Lee, C. G. et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta (1). J. Exp. Med. 194(6), 809–821. https://doi.org/10.1084/jem.194.6.809 (2001).
Chung, S. I. et al. IL-13 is a therapeutic target in radiation lung injury. Sci. Rep. 6, 39714–39748. https://doi.org/10.1038/srep39714 (2016).
Fischer, A. et al. Identification of immune-relevant factors conferring sarcoidosis genetic risk. Am. J. Respir. Crit. Care Med. 192(6), 727–736. https://doi.org/10.1164/rccm.201503-0418OC (2015).
Herazo-Maya, J. D. et al. Validation of a 52-gene risk profile for outcome prediction in patients with idiopathic pulmonary fibrosis: An international, multicentre, cohort study. Lancet Respir. Med. 5(11), 857–868. https://doi.org/10.1016/S2213-2600(17)30349-1 (2017).
Herazo-Maya, J. D., Noth, I., & Duncan, S. R., et al. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci. Transl. Med. 5(205), 205ra136–205ra170. https://doi.org/10.1126/scitranslmed.3005964 (2013).
Wu, X. et al. The molecular mechanism of Ligusticum wallichii for improving idiopathic pulmonary fibrosis: A network pharmacology and molecular docking study. Medicine (Baltimore) 101(6), e28787–e28797. https://doi.org/10.1097/MD.0000000000028787 (2022).
Wang, C., Chen, L., & Sun, Y., et al. Design, synthesis and SAR study of Fluorine-containing 3rd-generation taxoids. Bioorg Chem. 119, 105578. https://doi.org/10.1016/j.bioorg.2021.105578 (2022).
Park, Y. J. et al. Effects of β-sitosterol from corn silk on TGF-β-induced epithelial-mesenchymal transition in lung alveolar epithelial cells. J. Agric. Food Chem. 67(35), 9789–9795. https://doi.org/10.1021/acs.jafc.9b02730 (2019).
Mo, Z., Xu, P. & Li, H. Stigmasterol alleviates interleukin-1beta-induced chondrocyte injury by down-regulatingsterol regulatory element binding transcription factor 2 to regulateferroptosis. Bioengineered 12(2), 9332–9340. https://doi.org/10.1080/21655979.2021.2000742 (2021).
Li, H. et al. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer 18(1), 579. https://doi.org/10.1186/s12885-018-4299-4 (2018).
Gui, D. et al. Salidroside attenuates hypoxia-induced pulmonary arterial smooth muscle cell proliferation and apoptosis resistance by upregulating autophagy through the AMPK-mTOR-ULK1 pathway. BMC Pulm Med. 17(1), 191. https://doi.org/10.1186/s12890-017-0477-4 (2017).
Chen, D. Preliminary study on the mechanism of lung fibrosis in mice by Yilong Dispersing Knot formula. (Master dissertation, Yunnan College of Traditional Chinese Medicine, 2018: 1–9. link: http://cdmd.cnki.com.cn/Article/CDMD-10680-1018090629.htm.
Zhenyou, S. et al. Expression of inflammatory microRNAs in lung of mice with pulmonary fibrosis induced by bleomycin. Snakes 33(04), 389–393 (2021).
Kanehisa, M. et al. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51(D1), D587–D592. https://doi.org/10.1093/nar/gkac963 (2023).
Rizza, A. et al. Laxtobacillus plantarum reduces strepocaecus pyogenes viulence by modulting the IL-17, IL-23 and Tall like receptar 2/4 expressions in human epithelial cells. Int. Immunophamacal. 17, 453–461. https://doi.org/10.1016/j.intimp.2013.07.005 (2013).
Gramley, F. J. et al. Atrial fibrosis and atrial fibrillation: The role of the TGF-β1 signaling pathway. Int. J. Cardiol 143, 405–413. https://doi.org/10.1016/j.ijcard.2009.03.110 (2010).
Nakerakanti, S. M., & Trojanowska, A. The role of TGF-β1 receptors in fibrosis. Open Rheumatol. J. 6, 156–162. https://doi.org/10.2174/1874312901206010156 (2012).
Li, N., Liu, T.H., & Liu, Y., et al. Curcumin and curcumol inhibit NF-κB and TGF-β1 /Smads signaling pathways in CSE-Treated RAW246.7 cells. Evid. Based Complem. Altern. Med. eCAM 3035125. https://doi.org/10.1155/2019/3035125 (2019).
Sun, C. B. et al. Study of panax notoginseng saponins activate autophagy to alleviate pulmonary fibrosis in mice via PI3K/AKT/mTOR signaling pathway. Lishizhen Med. Mater. Med. 31, 2872–2876 (2020).
Fu, P. et al. Study on HPLC fingerprint of Qilongtian capsule and determination of four components. Chin. J. Trad. Chin. Med. 36, 1594–1597 (2021).
Milton, P. L. et al. Assessment of respiratory physiology of C57BL/6 mice following bleomycin administration using barometric plethysmography. Respiration 83, 253–266. https://doi.org/10.1159/000330586 (2012).
Hayek, H., Kosmider, B. & Bahmed, K. The role of miRNAs in alveolar epithelial cells in emphysema. Biomed. Pharmacother. 143, 112216. https://doi.org/10.1016/j.biopha.2021.112216 (2021).
He, M. et al. PM2.5-induced lung inflammation in mice: Differences of inflammatory response in macrophages and type II alveolar cells. J. Appl. Toxicol. 37(10), 1203–1218. https://doi.org/10.1002/jat.3482 (2017).
Al-Qahtani, A. A. et al. SARS-CoV-2 modulates inflammatory responses of alveolar epithelial type II cells via PI3K/AKT pathway. Front Immunol. 13, 1020624. https://doi.org/10.3389/fimmu.2022.1020624 (2022).
Yang, W. H. & Dong, H. Y. Clinical efficacy analysis of N-acetylcysteine combined with glucocorticoid in the treatment of COPD complicated with pulmonary interstitial fibrosis. Huaxia Med. 32, 23–25. https://doi.org/10.19296/j.cnki.1008-2409.2019-03-005 (2019).
Ji, Y. et al. Paeoniflorin suppresses TGF-β mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. Acta Pharmacol. Sin. 37(6), 794–804. https://doi.org/10.1038/aps.2016.36 (2016).
Liu, X. et al. Type I collagen induces mesenchymal cell differentiation into myofibroblasts through YAP-induced TGF-β1 activation. Biochimie 150, 110–130. https://doi.org/10.1016/j.biochi.2018.05.005 (2018).
Huang, C. Y. et al. Attenuation of magnesium sulfate on CoCl2-induced cell death by activating ERK1/2/MAPK and inhibiting HIF-1αvia mitochondrial apoptotic signaling suppression in a neuronal cell line. Chin. J. Physiol. 58, 244–253. https://doi.org/10.4077/CJP.2015.BAD296 (2015).
Xie, Y., Luo, J., & Wang, Y., et al. Transcriptomics analysis of key genes and signaling pathways in sepsis-related exogenous acute respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 34(11), 1154–1160 (2022). https://doi.org/10.3760/cma.j.cn121430-20211223-01914
Khorramdelazad, M. et al. Transcriptome profiling of lentil (Lens culinaris) through the first 24 hours of Ascochyta lentis infection reveals key defence response genes. BMC Genomics 19, 108. https://doi.org/10.1186/s12864-018-4488-1 (2018).
Nakerakanti, S. M. & Trojanowska, A. The role of TGF-β1 receptors in fibrosis. Open Rheumatol. J. 6, 156–162. https://doi.org/10.2174/1874312901206010156 (2012).
Mi, S. et al. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J. Immunol. 187(6), 3003–3014. https://doi.org/10.4049/jimmunol.1004081 (2011).
Rex, D. A. B. et al. A comprehensive network map of IL-17A signaling pathway. J. Cell Commun. Signal. 15, 1–7. https://doi.org/10.1007/s12079-022-00686-y (2022).
Li, X. et al. Immunomodulatory activity of a novel, synthetic beta-glucan (β-glu6) in murine macrophages and human peripheral blood mononuclear cells. PLoS ONE 8(11), e80399. https://doi.org/10.1371/journal.pone.0080399 (2013).
Popiolek, B. K. et al. The CCL2 /CCL7 /CCL12 /CCR2 pathway is substantially and persistently upregulated in mice after traumatic brain injury, and CCL2 modulates the complement system in microglia. Mol. Cell. Probes 54, 101671. https://doi.org/10.1016/j.mcp.2020.101671 (2020).
Pena-Philippides, J. C. et al. In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response. J. Neuroinflam. 13(1), 287. https://doi.org/10.1186/s12974-016-0753-x (2016).
Shetty, A. et al. Interactome networks of FOSL1 and FOSL2 in human Th17 cells. ACS Omega 6, 24834–24847. https://doi.org/10.1021/acsomega.1c03681 (2021).
Sobolev, V. et al. Analysis of PPARγ signaling activity in psoriasis. Int. J. Mol. Sci. 22(16), 8603. https://doi.org/10.3390/ijms22168603 (2021).
Caballero, E. P., Santamaría, M. H. & Corral, R. S. Endogenous osteopontin induces myocardial CCL5 and MMP-2 activation that contributes to inflammation and cardiac remodeling in a mouse model of chronic Chagas heart disease. Biochim. Biophys. Acta 1864(1), 11–23. https://doi.org/10.1016/j.bbadis.2017.10.006 (2018).
Wang, L. L., Zhang, A. N. & He, Y. M. Correlation of peripheral blood IL-17A gene polymorphism with myocardial injury and MMPs/TIMPs balance in patients with viral myocarditis. J. Hainan Med. College 24(13), 17–20 (2018).
Chen, X. et al. Tc17/IL-17A up-regulated the expression of MMP-9 via NF-κB pathway in nasal epithelial cells of patients with chronic rhinosinusitis. Front. Immunol. 9, 2121. https://doi.org/10.3389/fimmu.2018.02121 (2018).
Cheng, G. et al. IL-17 stimulates migration of carotid artery vascular smooth muscle cells in an MMP-9 dependent manner via p38 MAPK and ERK1/2-dependent NF-kappaB and AP-1 activation. Cell Mol. Neurobiol. 29(8), 1161–1168. https://doi.org/10.1007/s10571-009-9409-z (2009).
Chang, T. T., Liao, L. Y. & Chen, J. W. Inhibition on CXCL5 reduces aortic matrix metalloproteinase 9 expression and protects against acute aortic dissection. Vasc. Pharmacol. 1, 106926–106926. https://doi.org/10.1016/j.vph.2021.106926 (2021).
Jia, X. B. et al. Effect of Malus asiatica Nakai leaf flavonoids on the prevention of esophageal cancer in C57BL/6J mice by regulating the IL-17 signaling pathway. Onco Targets Ther. 13, 6987–6996. https://doi.org/10.2147/OTT.S261033 (2020).
Guo, M. X. et al. Expressions of IL-17 signaling pathway-related factors mRNA in esophageal tissue of mouse with esophageal cancer. J. Zhengzhou Univ. (Med Sci). 54(4), 492–495. https://doi.org/10.13705/j.issn.1671-6825.2019.02.046 (2019).
Zhang, M. Y. et al. A critical role of AREG for bleomycin-induced skin fibrosis. Cell Biosci. 11, 40. https://doi.org/10.1186/S13578-021-00553-0 (2021).
Zhang, X. F. et al. Electroacupuncture inhibits IL-17/IL-17R and post-receptor MAPK signaling pathways in a rat model of chronic obstructive pulmonary disease. Acupunct. Med. 39, 662–672. https://doi.org/10.1177/0964528421996720 (2021).
Yu, Z. et al. IL-17A promotes psoriasis-associated keratinocyte proliferation through ACT1-dependent activation of YAP-AREG axis. J. Invest. Dermatol. 142(9), 2343–2352. https://doi.org/10.1016/j.jid.2022.02.016 (2022).
Acknowledgements
This research was funded by Research on the comprehensive efficacy evaluation of "Tian Long Dao" stage-based treatment program on IPF (The key project of Yunnan Provincial Science and Technology of Chinese medicine association) [2017FF117 (-007)]. This research was supported by Yunnan Provincial Key Laboratory of Molecular Biology for Sinomedicine, China (No. 2019DG016). Thanks to Associate Professor Xiaoling Yu (Third Affiliated Hospital of Yunnan University of Traditional Chinese Medicine: Kunming Municipal Hospital of Traditional Chinese Medicine, Kunming, 650500, China) for providing the HPLC data of QLT.
Author information
Authors and Affiliations
Contributions
Q. Z. and T. L. did the conception and designed of the study. D. -Z. Y. and J. L. drafted the article and revised it critically for important intellectual content. Y. F. and J. -L. Y. approved of the version to be submitted. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-023-38495-x
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Q., Luo, T., Yuan, D. et al. RETRACTED ARTICLE: Qilongtian ameliorate bleomycin-induced pulmonary fibrosis in mice via inhibiting IL-17 signal pathway. Sci Rep 13, 6002 (2023). https://doi.org/10.1038/s41598-023-31439-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31439-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.