Abstract
Apex predators typically affect the distribution of key soil and vegetation nutrients through the heterogeneous deposition of prey carcasses and excreta, leading to a nutrient concentration in a hotspot. The exact role of central-place foragers, such as tropical raptors, in nutrient deposition and cycling, is not yet known. We investigated whether harpy eagles (Harpia harpyja) in Amazonian Forests—a typically low soil fertility ecosystem—affect soil nutrient profiles and the phytochemistry around their nest-trees through cumulative deposition of prey carcasses and excreta. Nest-trees occurred at densities of 1.5–5.0/100 km2, and each nest received ~ 102.3 kg of undressed carcasses each year. Effects of nests were surprisingly negative over local soil nutrient profiles, with soils underneath nest-trees showing reductions in nutrients compared with controls. Conversely, canopy tree leaves around nests showed significant 99%, 154% and 50% increases in nitrogen, phosphorus and potassium, respectively. Harpy eagles have experienced a 41% decline in their range, and many raptor species are becoming locally extirpated. These are general examples of disruption in biogeochemical cycles and nutrient heterogeneity caused by population declines in a central-place apex predator. This form of carrion deposition is by no means an exception since several large raptors have similar habits.
Similar content being viewed by others
Introduction
In his seminal work (Animal Ecology, 19271), Charles Elton states: “It is usual to speak of an animal as living in a certain physical and chemical environment, but it should always be remembered that strictly speaking we cannot say exactly where the animal ends and the environment begins”. Elton meant that animals can only be interpreted if their ecological interactions are considered. This was followed by a profusion of twentieth-century ecological research built around this idea. Elton then continued: “unless it is dead, in which case it has ceased to be a proper animal at all”. In the twenty first century, ecologists have explored in detail the notion that even after death, animals continue to influence their physical and chemical environments, sometimes at unexpected spatial scales2. Nothing exerts a stronger connection between life and death, the two conditions discussed by Elton, than an apex predator3. In particular, the rapacious nature of apex predators renders them inextricably intertwined with their chemical and physical environments.
Animals can influence biogeochemical cycles via two main processes: concentration of nutrients into hotspots4 and diffusion of nutrients against natural gradients5. Since big and fierce animals are rare6, concentration in hotspots is the main pathway through which they influence biogeochemical cycles. Nutrient subsidies can be defined as a donor-controlled resource from one habitat to a recipient (such as a plant) in another habitat. This increases the productivity (as population growth or larger body size) of the recipient, potentially altering the consumer–resource relationship in the recipient ecosystem7. For apex predators, those resources are usually prey carcasses or prey-derived detritus (scats or excreta) that are locally concentrated because of landscape traits4,8.
Amazonian forest soils are susceptible to changes in the distribution of key nutrients because they are usually nutrient-poor9, as most nutrients are generally concentrated in the aboveground forest biomass. Modest changes in soil nutrient profiles can profoundly affect biodiversity, as in mineral licks visited by geophagous vertebrates in search of sodium10. However, good apex predator candidates for the role of nutrient concentrators in the megadiverse Amazonian ecosystem are hard to predict. Apex predators include black caiman (Melanosuchus niger), green anaconda (Eunectes murinus), puma (Puma concolor), jaguar (Panthera onca) and harpy eagle (Harpia harpyja)11. Therefore, single-species effects over any phenomena should be rare or non-existent as predicted by ecological theory applied to tropical ecosystems12.
Harpy eagles (Fig. 1) are particularly interesting in their nutrient concentration role. They typically nest in the same giant emergent tree for decades13. The harpy eagle's breeding cycle is the longest of all birds, during which they bring prey to their eaglets for 30–36 months13. They feed extensively on medium-sized canopy vertebrates14. Bird excreta is often rich in limiting nutrients15, and harpy eagle excreta often taints the nest tree's surrounding canopy foliage and branches. Harpy eagles are, therefore, central place foragers, as they maximise foraging rates while travelling through a discrete resource concentration while maintaining the key distinction of a forager travelling from a home base (the nest tree) to a distant foraging location16,17. These traits render them an ideal model to test the nutrient concentration hypothesis in Amazonian forests. Although harpy eagles are Earth’s largest eagles, they are surpassed by other apex predators that suit the role of affecting nutrient distribution in the landscape—such as wolves and bears—by one or two orders of magnitude in body mass18. Contrary to those predators, harpy eagles do not rely on landscape traits to increase prey kill rate and are obligatory central-place foragers once they reach breeding age. This raises the question: can harpy eagles influence soil chemistry and, therefore, nutrient cycling in their ecosystem?
Our objectives were to test the hypothesis that harpy eagles serve as accumulation agents of nutrients by concentrating decaying remains of prey items at nest sites, thereby biomagnifying soil and foliage nutrients in the undergrowth, canopy vegetation and nest-tree (Fig. 2). We predicted that harpy eagles would increase the nutrient profiles of both soils and foliage. In providing these ecosystem-level insights, we attempt to show how local extinctions of this apex predator may result in disruptions in biogeochemical cycles that modulate soil and vegetation nutrient heterogeneity across the Amazon Basin.
Schematic representation of the nutrient concentration role of nesting harpy eagles through central-place prey carcass and excreta deposition on different vegetation strata, including soil (a), the understorey foliage (b), canopy (c) and the nest tree itself. Lower panel represents the landscape-scale dynamic of nutrient concentration from commuting distances of up to 3 km from the nest. Magnitude of nutrient concentration is indicated by increasingly green colours. Illustration: Paula Viana.
Results
We collected soil and vegetation samples from 20 harpy eagle nests; 10 active, 10 inactive, plus one nest that we sampled while both active and inactive. Those 21 samples were paired with 47 conspecific control trees at which we collected comparable soil and vegetation samples. Nest tree species included 16 Brazil nut trees (Bertholletia excelsa Lecythidaceae), one self-standing Ficus spp. (Moraceae), one Astronium lecointei (Anacardiaceae), one Cariniana spp. (Lecythidaceae) and one Apuleia leiocarpa (Fabaceae). Although we selected the largest available emergent individuals for non-nest trees, tree diameter at breast height (dbh) was significantly larger in nest trees (mean ± SD, 148.3 ± 25.5 cm) than in control trees (134 ± 25 cm; β = 0.62, t = 5.94, p < 0.01).
Carcass input
Using camera-traps to monitor 10 harpy eagle nests (or 20 adult eagles), we recorded 212 prey items amounting to an estimated 411 kg of prey delivered per nest per nesting cycle (Table 1). Although adults continue to deliver prey at low rates to their nests after 24 months, eaglets usually consume these elsewhere, in addition to hunting independently. We, therefore, labelled nesting cycles older than 24 months as “nutrient-inactive” and excluded these data from our carcass biomass input estimates. This resulted in a total of 307 kg of prey delivered per nesting cycle (36 months), or approximately 102.3 kg per year.
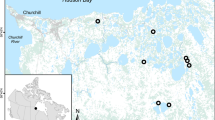
Using the maximum packed nest density, we estimated 1.97–4.84 nests/100 km2 throughout our study area (Supplementary Information, Table S1, Fig. S1). The polygon method produced densities of 1.55–3.30 nests/100km2. Consequently, as a central-place hunter, harpy eagles concentrated 102.3 kg/year of prey captured over 20–64 km2 into a single carcass hotspot, over an approximate density of 1.5–5.0 carcass hotspots per 100 km2.
Soil nutrients
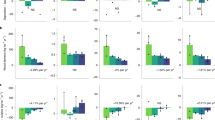
Contrary to our expectations (Supplementary Information Table S2: soil stratum; Fig. 3: soil panel), four of the five measured soil nutrients underneath nest trees had a reduced concentration compared to controls: (1) phosphorus (50% reduction; β = − 0.11, t = − 2.29, p < 0.03); (2) calcium (32% reduction; β = − 0.42, t = − 3.07, p < 0.01); (3) magnesium (21% reduction; β = − 0.11, t = − 2.71, p < 0.01); (4) aluminium (50% reduction; β = − 0.08, t = − 2.29, p < 0.03); (5) while not for potassium (β = − 2.65, t = − 0.67, p = 0.50). We did not detect any main effect of nest activity for any nutrient type (p > 0.05; i.e. nest effects were detected irrespective of activity), nor any interaction between nest presence and nest activity (p > 0.05). Positive effects of tree size (i.e. diameter at breast height) were important as a covariate for phosphorus (β = 0.13, t = 1.83, p < 0.01), potassium (β = 3.68, t = 2.27, p < 0.03) and aluminium (β = 0.06, t = 2.62, p < 0.01), but not for the other nutrients (p > 0.05).
Understorey vegetation
We detected an effect of eagle nest presence only for potassium concentration (16% increase; β = 2.15, t = 2.22, p < 0.03) in the foliage samples in undergrowth vegetation (Supplementary Information Table S2: undergrowth stratum, Fig. 3: undergrowth panel). Nitrogen (β = 2.03, t = 1.23, p = 0.22) and phosphorous (β = 0.03, t = 1.35, p = 0.17) concentrations were unaffected by nest presence. We did not find an effect of nest activity on any nutrient concentration (p > 0.05), nor the interaction between nest presence and nest activity (p > 0.05; Table S2), except for potassium. In the case of potassium concentration, a positive effect of nest presence occurred around active nests but not around inactive nests (interaction term; β = − 3.17, t = − 2.29, p < 0.03). Tree girth was also unimportant in determining the magnitude of nutrient concentrations (p > 0.05).
Forest canopy around nest-trees
Nest presence had a positive effect on foliage nutrient concentration of canopy trees adjacent to nest-trees for nitrogen (87% increase; β = 16.95, 13.03, p < 0.01), phosphorus (142% increase; β = 0.31, t = 12.24, p < 0.01), and potassium (79% increase; β = 10.11, t = 8.32, p < 0.01; Supplementary Information Table S2: surrounding canopy trees, Fig. 3: canopy panel). However, although we failed to detect a main effect of nest activity per se (p > 0.05), nest activity magnified the positive effect of nests on the concentration of nitrogen (interaction term; β = 4.20, t = 1.95, p < 0.04) and potassium (interaction term; β = 7.50, t = 4.09, p < 0.01), but not phosphorus (interaction term; β = − 0.01, t = − 0.31, p = 0.75). Nest activity also magnified the positive effect of nests on the canopy foliage for nitrogen and phosphorus by 24% and 74%, respectively, as observed for potassium. Tree size was unrelated to any of the foliar nutrients (p < 0.05).
Nest-trees
Long-term presence of harpy eagles at the nest exerted a strong positive effect on nutrient concentrations of nest-tree foliage for nitrogen (80% increase; β = 5.60, t = 9.73, p < 0.01), phosphorus (25% increase; β = 0.14, t = 23.54, p < 0.01), and potassium (47% increase; β = 3.04, t = 12.44, p < 0.01) (Table S2: nest tree; Fig. 3: nest-tree panel). However, we did not detect a main effect of nest activity (p > 0.05) on foliage nutrient concentrations, nor the interaction between nest presence and nest activity (p > 0.05), with the exception of potassium. For potassium, nest activity amplified the positive effect of nest presence by 50% (interaction term; β = 1.51, t = 4.13, p < 0.01). There were no effects of tree girth on any of the nutrient levels (p < 0.05).
Discussion
Apex predators exert decisive effects on prey distribution, demography and behaviour11,19, but whether these extend to the base of food-webs remains uncertain. Here, we have shown that a rare central-place apex carnivore enhances nutrient availability for local plant communities in Amazonian forests. These findings are of prime interest to food web and carrion ecology. Our study highlights a new mechanism through which an apex predator exhibiting long-term site-fidelity can affect soil heterogeneity. This process is driven by increasing key nutrients in vegetation through the cumulative deposition of both carrion and excreta that is neither ephemeral nor constrained by landscape traits. This site-specific trophic connectivity, although now seemingly obvious, has remained hitherto undocumented because the ecology of raptor and plant communities represent opposite extremes of food webs and may appear to be hardly intertwined.
Our estimates of harpy eagle nest densities of 1.5–5.0 nests/100 km2 allow us to hypothesize that these effects are important at the landscape scale, generating a heterogeneous nutrient pump that favours the uptake of rare nutrients into the vegetation on otherwise oligotrophic Amazon soils. Subalusky and Post20 highlighted that carcass input rates are one of the most frequently missing links in carcass subsidy studies. The direct and sustained nutrient carcass inputs per nest tree shown here—roughly 102.3 kg per year—challenges one of the central notions in carcass ecology: that the effects of animal carcasses are ephemeral21. We have shown a mechanism by which carcasses are consistently deposited at the same spot for periods that may extend for several decades. This deposition is conditional on the harpy eagle's reproductive phenology, which fluctuates over time, resulting in different vegetation nutrient profiles between active and inactive nest sites. Nest activity resulted in 24% and 74% increases in nitrogen and phosphorus in canopy foliage around the nest-tree, and an 50% increase in potassium for the nest-tree itself. The “rare and unpredictable” availability of large vertebrate carrion is therefore challenged by this study, as seen in the consistent and frequent nutrient deposition through space and time of an otherwise limited resource.
Carrion is known to result in intense interspecific competition, and this form of interaction has been shown to occur at harpy eagle nests22. This mechanism of carrion deposition is widespread in raptors, even if the magnitude of carcass deposition by smaller raptors that consume smaller prey is more modest. On the other hand, several large-bodied raptors are known to exhibit similar philopatric habits, including bald (Haliaeetus leucocephalus), crowned (Stephanoaetus coronatus), martial (Polemaetus bellicosus), golden (Aquila chrysaetos), and Philippine (Pithecophaga jefferyii) eagles23,24,25,26. This poses the question of how these species affect nutrient mosaics of soils in their range.
The amount, quality and duration of carcass inputs are influenced by how the input takes place. Prey remains underneath harpy eagle nests are mostly skeletal material, with few or no soft body parts. Bones are the main sources of phosphorus but exhibit the slowest decomposition rate of all carcass parts, taking 170 times longer than muscle to decompose in large mammalian carcasses27. Harpy eagles primarily prey on medium-sized vertebrates (which have a lower proportion of phosphorus28), and arboreal termites often cover skeletal remains within hours after they fall to the ground. This likely accelerates assimilation rates (EPBM, pers. obs.), and consequently provides relatively little opportunity for the diffusion of phosphorus and other nutrients into the topsoil. On the other hand, harpy eagles regularly provide soluble nutrient-rich excreta, which are easily assimilated by vegetation29 through the leaves, making excreta a high-quality resource.
Nevertheless, we found that active harpy eagle nests had a puzzling impact on soil quality, resulting in lower soil nutrient availability. Except for potassium, soil nutrients decreased in soil samples underneath nest-tree crowns (by − 50% for phosphorus, − 32% for calcium, − 21% for magnesium, and − 50% for aluminium). In contrast, other cases of clumped deposition of faecal/excreta material typically boosts soil nutrients. For instance, manganese and potassium concentrations are elevated in soils beneath the latrines of frugivorous spider monkeys30 (Ateles spp.). Howler monkeys (Alouatta spp.)—which are folivore-frugivores—also increase the fertility of soil nutrient profiles, with phosphorus content increasing by 3.8–6.0 times in latrines beneath sleeping sites compared with control sites31. Since foliage and fruit pulp are relatively poorer than carcasses in nitrogen and phosphorus32, how can these species positively affect soil nutrients while harpy eagles do not?
The strong and consistent response of canopy foliage chemistry to deposition of harpy eagle excreta (i.e. 99%, 154% and 51% increases in nitrogen, phosphorus and potassium, respectively) suggests that the apparent soil nutrient sink we observed is a consequence of canopy trees shortcutting access to excreta nutrients through direct leaf uptake, rather than from the soil per se. Excreta ejected by adults and eaglets usually smears much of the canopy foliage underneath the nest-tree, which is not the case for most detrital resources in tropical forests, such as primate dung, that fall on the ground. By intercepting an important limiting nutrient—phosphorus—before it reaches the ground, canopy trees putatively absorb other nutrients in more significant amounts, including phosphorus. In a classic case of Liebig's Law of the minimum33, nest trees likely fill the “barrel gap” through leaf uptake, consequently reducing soil nutrient profiles.
Nest-trees also showed the effects of harpy eagle nesting. While those effects may be moderate compared with the canopy, nest-trees may also remove nutrients from soils underneath their crowns by shortcutting the phosphorus uptake pathway. Some nutrient absorption likely occurs via the bark (the position of harpy eagle nests allows excreta to reach terminal foliage in only 2.1% of nests34), but bark nutrient uptake is, at best, poorly understood35. This is likely the reason for the weaker and inconsistent increase in the nest-tree foliage nutrient profile compared with the surrounding canopy. Harpy eagle nests are large renewable structures averaging 152 × 99 cm34. This large platform of piled dead branches accumulates much of the carcass and skeletal remains while adults continue to add new green branches. This accumulated nutrient source—a metaphorical carrion-enriched compost pile—possibly becomes directly available to the nest-tree through bark absorption, given the runoff of nutrients dissolved in rainwater through > 30 m boles (or an average bark surface of 143.2 m2 per nest-tree bole) before reaching the ground. While water absorption through bark has been shown in several trees36,37, the degree to which this occurs in tropical emergent trees remains unknown. Nevertheless, this form of nutrient enrichment is the single most likely mechanism for increasing foliar nutrient profiles of nest-trees observed here, thereby enabling a sustained physicochemical mutualism between harpy eagles and their long-term nest-trees. To what degree this may affect the reproductive fitness and longevity of individual emergent “mega-trees”38, and thereby ensuring the long-term availability of suitable nest platforms, remains unclear.
Therefore, these findings suggest non-obligatory reciprocal benefits between harpy eagles and their nest-trees. Harpy eagles require particular morphological traits for emergent trees to be selected as suitable nest-trees, particularly exceptionally high crown stature and T-shaped primary branching34. Trees with this crown architecture may, in turn, benefit from nesting harpy eagles through the significant, long-term deposition of limiting nutrients. These effects may also feedback to influence the donor ecosystem: direct nutrient augmentation in the canopy may affect the primary tree productivity via increased foliage and fruit production. To what degree active nests increase nest tree demographics, including growth rates, fecundity (seed production) and longevity remain largely open questions. Given the extremely low density of large raptors throughout Neotropical forests39, this also creates a patchy mosaic of rare but sustained nutrient hotspots in a forest landscape otherwise dominated by nutrient-poor soils. Since heterogeneity is one of the main spatial correlates of landscape-scale plant and animal diversity, this could represent a mechanism by which the understorey in the vicinities of harpy eagle nests show higher floristic diversity40. This illustrates how this trophic cascade generates a wealth of testable hypotheses41 for future studies.
The undergrowth vegetation underneath harpy eagle nest trees had higher potassium content even if rooting in nutrient-poor soils. Significant increases of 16% for potassium have also likely resulted from the direct deposition of harpy eagle excreta on leaves, ensuring direct nutrient absorption while circumventing below-ground root uptake. Although we selected understorey foliage samples that lacked clear signs of excreta staining, this may be masked by the detritus runoff during the frequent downpours during the wet season, leaving no evidence of recent animal excreta. Foliar absorption is a well-known phenomenon that occurs in > 85% of all plant species tested42,43, and the time-lag required for 50% absorption of phosphorus is estimated at 7–15 days, which is a plausible interval between consecutive rains. Other nutrients have a much shorter absorption time, estimated at 1–24 h for nitrogen and 1–4 days for potassium42. Elevated root nutrient uptake is frequently induced by foliar fertilisation in several plant species44.
Large hypercarnivores are rare and becoming rarer worldwide45,46, so the degree to which local extinctions disrupt ecosystem processes should be explored in further detail. Harpy eagles have succumbed to a 41% decline in their distribution range47,48, and have been locally extirpated over vast landscapes of the Brazilian Atlantic Forest and Mesoamerica, which now lack this apex predator and its long-term forest nutrient transport and aggregation, and downstream bottom-up effects on vegetation. The restoration plans dedicated to the species in the Atlantic Forest47,49 will provide an opportunity to test how the return of those species affects this phenomenon.
The markedly philopatric nature of large birds of prey suggests that other declining raptor species can perform similar ecological roles. We can only speculate on the magnitude of nutrient hotspots generated by the now-extinct mega-raptors that once hunted many of the World’s large islands. Large raptors that were (pre)historically driven to extinction by humans, such as the Cuban terrestrial owl (Ornimegalonyx oteroi) and New Zealand’s Haast eagle (Hieraaetus moorei), were much larger than harpy eagles and likely performed similar central-place nutrient inputs into soils and vegetation. This is another example of how historical and ongoing large vertebrate extinctions can sever nutrient transport systems, severely affecting biogeochemical cycles and nutrient redistribution5.
In conclusion, we suggest that harpy eagles impact soil and vegetation nutrient heterogeneity by regularly depositing excreta and carrion from their nests over decades. This not only enhances our understanding of the role of natural predation and carrion deposition in sustaining nutrient cycles, but also elucidates the role of large raptors in ecosystem processes. Finally, our findings pose further questions regarding how far the effects triggered by multi-annual site-specific prey delivery by large raptors reverberate over animal and vegetation communities in tropical forests, and the degree to which trophic downgrading results from the increasingly widespread spate of apex-predator extinctions.
Methods
Study area
Our study was conducted in the southern Amazon Forest—known in conservation circles as the Arc of Deforestation—in the northern part of the state of Mato Grosso, Brazil (Supplementary information Fig. S2). Koeppen50 classifies the regional climate as “tropical wet climate” or Amazonian (tropical monsoon climate). Rainfall averages 2350 mm/yr, and the ambient temperature averages 24.5 °C, combined with a high relative air humidity (80–85%51). As in most of the Amazon Basin, soils in our study region are highly acidic9. Levels of phosphorus, potassium, calcium, and magnesium are generally low, while aluminium and H + Al—which are toxic to plant life—are generally high. Phosphorus limitation is particularly severe in the region5.
Carcass input
We installed 2–3 camera-traps at each harpy eagle nest site to sample their food delivery rates, thereby enabling estimates of nutrient input into the soil underneath the nests from remains of prey carcasses. To do so, we used nests without signs of food stress resulting from habitat fragmentation. Since prey delivery rates decreased with eaglet development, we defined four categories of prey delivery rates: 0–6 months (unfledged), 7–12 months (recently fledged eaglets), 13–24 months (late-fledged eaglets) and 25–36 months (individuals at the onset of dispersal). We inferred eaglet age from known hatchling dates, or feather colours and nest degradation status when the hatchling date was unknown52. We defined nests as unoccupied as those in disuse because the pair was active at an alternative nest (a minority of 16% of harpy eagles have alternative nests;39) and those with eaglets above 24 months.
We estimated prey biomass delivered per nest based on the average body size described for each prey species. Subadult prey were attributed two-thirds of the adult body mass. For infant or juvenile prey (such as ungulates that were killed almost exclusively as newborns53), we attributed one-fifth of the adult body mass. Sloths received a further reduction of one-third, because of the large amount of foliage representing ~ 30% of the body mass in living sloths54 that harpy eagles discard soon after kills. As prey items are usually dismembered when delivered to nests, we applied approximate reductions in body mass as follows: 10% (head or viscera), 20% (per member missing or single-member delivered), 50% (lower or upper body missing), and 90% (tail of Atelinae primates, porcupines) of the total prey body mass23.
We also calculated harpy eagle nest density to infer nutrient input rates at the landscape scale. We found harpy eagle nests by actively distributing brochures advertising a financial reward of c. USD100 (BRL500) to anyone who could locate a harpy eagle nest. We found a total of 38 nests over four years. After excluding alternative nests, we calculated active nest density using two methods: maximum packed nest density (MPND), and the polygon method. We selected these methods to maximise comparability with previous studies39. The MPND55 was calculated as:
where A is defined as half the distance to the nearest neighbouring nest in a cluster of nests. This distance is then considered as the radius for a circular breeding territory centred at the nest. The 1.158 is a constant designed to fill the interstitial space between breeding territory circumferences. The polygon method uses half the average distance to the nearest neighbouring nest to establish a polygon around all nests within a cluster, from which we estimated the total area. We calculated the two density estimates for our study area based on two known clusters of nests (with five and six nests each). We concentrated the most intensive sampling effort on those clusters over the last four years, thereby deriving a high nest detection rate. We then divided the values that resulted from both estimates by 100 km2 of available forest habitat to derive a nest density estimate.
Soil sampling
We collected five soil samples underneath each harpy eagle nest-tree at 5–15 cm depth using a mechanical auger, at 1–10 m distance from the nest tree, where excreta and prey bones typically fall. We paired each nest-tree with one to three same-species, emergent-sized control trees. Local peculiarities made only one or two control trees of the same species available in some locations. This phylogenetic control was done to ensure that any nutrient effects were related to harpy eagle nesting activity, rather than tree species identity. We added tree circumference at breast height to the models to warrant that the effects were not from the mega-trees since they can alter soil composition by deposition of leaves, bark, and branches. We excluded any emergent trees frequently used as perches by adults and fledged eaglets as control sites.
We quantified soil aluminium, potassium, calcium and magnesium using the 1 M KCl extraction method56. Concentrations of these nutrients were determined by atomic absorption spectrophotometry. Phosphorus was extracted using the Mehlich 1 method57, and phosphorus concentrations were determined by spectrophotometry at 725 nm56.
Vegetation sampling
At each nest-tree and control tree site, we collected undergrowth vegetation samples randomly at 1–10 m from the focal tree bole. We selected foliage from mature branches of three healthy stems from different individuals of up to 1 m in height that were free from direct signs of harpy eagle excreta. We did not select foliage from any particular plant species. Samples were stored in paper envelopes and dehydrated naturally. We also collected foliage samples from three branches of 25–30 m canopy-height trees at 5–15 m from the nest, as well as foliage from three branches from nest-trees, using a rope chainsaw.
We rinsed vegetation samples in distilled water to remove detrital material and dehydrated them in an oven, and then ground them until homogenised. We analysed the nitrogen, phosphorus and potassium content of each sample using methods described by Embrapa56. Nitrogen was extracted using sulphuric acid (total Kjeldahl N). Nitricperchloric extract was used for the other elements: phosphorus (colorimetry) and potassium, which were subsequently determined by spectrophotometry at 725 nm56.
Statistical analyses
We used Generalised Linear Mixed Models to test the effect of nest presence and nest occupancy (0 or 1), as well as their interaction, on nutrient profiles in the soil and leaf samples across the vertical stratification of foliage. We ran a model for each nutrient in each stratum (five nutrients for soil and three nutrients for each forest stratum: foliage in the undergrowth, canopy trees around the nest-tree, and the nest-tree). Because we strictly adopted a case–control design—i.e. nest-tree samples were paired with nearby non-nest tree samples—we included the identity of each case–control pair as a random intercept. In our case, including this random intercept aimed to mimic a repeated measure analysis. We also included the circumference at breast height (CBH) of each nest and non-nest tree as a covariate to account for uncontrolled trait differences between the nest-tree and non-nest tree, even though all paired trees were, by definition, conspecific emergents (i.e. belonging to the same tree species). Models were run using the nlme package58 available in R 4.0.3, assuming a Gaussian residual distribution. Each model’s residuals were visually checked for normality and homoscedasticity.
Data availability
The data is available through the repository Mendeley Data (https://data.mendeley.com/9hgvn9s4nx).
References
Elton, C. Animal Ecology (Sidgwick & Jackson, 1927).
Turner, W. C. et al. Fatal attraction: Vegetation responses to nutrient inputs attract herbivores to infectious anthrax carcass sites. Proc. R. Soc. B Biol. Sci. 281, 20141785 (2014).
Terborgh, J. & Estes, J. Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature (Island Press, 2013).
Holtgrieve, G. W., Schindler, D. E. & Jewett, P. K. Large predators and biogeochemical hotspots: Brown bear (Ursus arctos) predation on salmon alters nitrogen cycling in riparian soils. Ecol. Res. 24, 1125–1135 (2009).
Doughty, C. E., Wolf, A. & Malhi, Y. The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia. Nat. Geosci. 6, 761–764 (2013).
Carbone, C. & Gittleman, J. L. A common rule for the scaling of carnivore density. Science (80-.) 295, 2273–2276 (2002).
Polis, G. A., Anderson, W. B. & Holt, R. D. Toward an integration of landscape and food web ecology: The dynamics of spatially subsidized food webs. Annu. Rev. Ecol. Syst. 28, 289–316 (1997).
Bump, J. K., Peterson, R. O. & Vucetich, J. A. Wolves modulate soil nutrient heterogeneity and foliar nitrogen by configuring the distribution of ungulate carcasses. Ecology 90, 3159–3167 (2009).
Moreira, A. & Fageria, N. K. Soil chemical attributes of Amazonas state, Brazil. Commun. Soil Sci. Plant Anal. 40, 2912–2925 (2009).
Molina, E., León, T. E. & Armenteras, D. Characteristics of natural salt licks located in the Colombian Amazon foothills. Environ. Geochem. Health 36, 117–129 (2014).
Terborgh, J. et al. Ecological meltdown in predator-free forest fragments. Science (80-.) 294, 1923–1926 (2001).
Odum, E. P. & Barrett, G. W. Fundamentals of Ecology (Saunders, 1971).
Muñiz López, R. Biología y Conservación del Águila Harpía (Harpia harpyja) en Ecuador (Universidad de Alicante, 2016).
Aguiar-Silva, F., Sanaiotti, T. & Luz, B. Food habits of the Harpy Eagle, a top predator from the Amazonian rainforest canopy. J. Raptor Res. 48, 24–45 (2014).
Sekercioglu, C. H. Increasing awareness of avian ecological function. Trends Ecol. Evol. 21, 464–471 (2006).
Orians, G. H. & Pearson, N. E. On the theory of central place foraging. In Analysis of Ecological Systems (eds Horn, D. J. et al.) 154–177 (The Ohio State University Press, 1979).
Vargas González, Jd. J. et al. Predictive habitat model reveals specificity in a broadly distributed forest raptor, the Harpy Eagle. J. Raptor Res. 54, 349–363 (2020).
Walker, E. P. Walker’s Mammals of the World (Johns Hopkins University Press, 1991).
Gil-da-Costa, R., Palleroni, A., Hauser, M. D., Touchton, J. & Kelley, J. P. Rapid acquisition of an alarm response by a neotropical primate to a newly introduced avian predator. Proc. Biol. Sci. 270, 605–610 (2003).
Subalusky, A. L. & Post, D. M. Context dependency of animal resource subsidies. Biol. Rev. 94, 517–538 (2019).
Barton, P. S. & Bump, J. K. Carrion decomposition. In Carrion Ecology and Management (eds Olea, P. et al.) 101–124 (Springer, 2019).
Aguiar-Silva, F. H. et al. Camera trapping at active Harpy Eagle nests: Interspecies interactions under predation risk. J. Raptor Res. 51, 72–29 (2017).
Van der Meer, T., McPherson, S. & Downs, C. Temporal changes in prey composition and biomass delivery to African Crowned Eagle nestlings in urban areas of KwaZulu-Natal, South Africa. Ostrich 83, 241–250 (2018).
Abaño, T. R. C., Salvador, D. J. & Ibañez, J. C. First nesting record of Philippine Eagle Pithecophaga jefferyi from Luzon, Philippines, with notes on diet and breeding biology. Forktail 32, 86–88 (2016).
Killengreen, S. T., Strømseng, E., Yoccoz, N. G. & Ims, R. A. How ecological neighbourhoods influence the structure of the scavenger guild in low arctic tundra. Divers. Distrib. 18, 563–574 (2012).
van Eeden, R., Whitfield, D. P., Botha, A. & Amar, A. Ranging behaviour and habitat preferences of the Martial Eagle: Implications for the conservation of a declining apex predator. PLoS ONE 12, e0173956 (2017).
Subalusky, A. L., Dutton, C. L., Rosi, E. J. & Post, D. M. Annual mass drownings of the Serengeti wildebeest migration influence nutrient cycling and storage in the Mara River. Proc. Natl. Acad. Sci. U. S. A. 114, 7647–7652 (2017).
Elser, J. J., Dobberfuhl, D. R., MacKay, N. A. & Schampel, J. H. Organism size, life history, and N: P stoichiometry: Toward a unified view of cellular and ecosystem processes. Bioscience 46, 674–684 (1996).
Vanni, M. J. Nutrient cycling by animals in freshwater ecosystems. Annu. Rev. Ecol. Syst. 33, 341–370 (2002).
Whitworth, A. et al. Spider Monkeys Rule the roost: Ateline sleeping sites influence rainforest heterogeneity. Animals 9, 0–16 (2019).
Feeley, K. The role of clumped defecation in the spatial distribution of soil nutrients and the availability of nutrients for plant uptake. J. Trop. Ecol. 21, 99–102 (2005).
D’Alessandro, C., Piccoli, G. B. & Cupisti, A. The, “phosphorus pyramid”: A visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 16, 1–6 (2015).
von Liebig, J. Principles of Agricultural Chemistry with Special Reference to the Late Researches Made in England (Walton & Maberly, 1855).
Miranda, E. B. P., Peres, C. A., Marini, M. Â. & Downs, C. T. Harpy Eagle (Harpia harpyja) nest tree selection: Logging in Amazonian forests threatens Earth’s largest eagle. Biol. Conserv. 1, 108754 (2020).
Aubrey, D. P. Relevance of precipitation partitioning to the tree water and nutrient balance. in Precipitation Partitioning by Vegetation 147–162 (Springer, 2020).
Mayr, S. et al. Uptake of water via branches helps timberline conifers refill embolized xylem in late winter. Plant Physiol. 164, 1731–1740 (2014).
Mason Earles, J. et al. Bark water uptake promotes localized hydraulic recovery in coastal redwood crown. Plant. Cell Environ. 39, 320–328 (2016).
Pinho, B. X., Peres, C. A., Leal, I. R. & Tabarelli, M. Critical role and collapse of tropical mega-trees: A key global resource. Tropical Ecosystems in the 21st Century. in Tropical Ecosystems in the 21st Century 253 (2020).
Vargas-González, Jd. J. & Vargas, F. H. Nesting density of Harpy Eagles in Darien with population size estimates for Panama. J. Raptor Res. 43, 199–210 (2011).
Vargas González, Jd. J., Vargas, F. H. & McClure, C. Características de la vegetación en sitios de anidación del águila arpía (Harpia harpyja) en Darién, Panamá. Ornitol. Neotrop. 25, 207–218 (2014).
McClure, C. J. et al. Toward scoping reviews of individual bird species. Ibis (Lond. 1859) 164, 835–845 (2022).
Wittwer, S. H. & Teubner, F. G. Foliar absorption of mineral nutrients. Annu. Rev. Plant Physiol. 10, 13–30 (1959).
Goldsmith, G. R., Matzke, N. J. & Dawson, T. E. The incidence and implications of clouds for cloud forest plant water relations. Ecol. Lett. 16, 307–314 (2013).
Geetah, A. Physiology of foliar spray uptake and its importance in some of the commercial crops. In Advances in Agriculture Sciences (ed. Naresh, R. K.) 01–28 (AkiNik Publications, 2019).
Sutton, L. J. et al. Reduced range size and Important Bird and Biodiversity Area coverage for the Harpy Eagle (Harpia harpyja) predicted from multiple climate change scenarios. Ibis (Lond. 1859) 164, 649–666 (2022).
Zuluaga, S., Vargas, F. H., Kohn, S. & Grande, J. M. Top-down local management, perceived contribution to people, and actual detriments influence a rampant human-top predator conflict in the Neotropics. Perspect. Ecol. Conserv. 20, 91–102 (2022).
Miranda, E. B. P., Menezes, J. F. S., Farias, C. C., Munn, C. & Peres, C. A. Species distribution modeling reveals strongholds and potential reintroduction areas for the world’s largest eagle. PLoS ONE 14, e0216323 (2019).
Sutton, L. J. et al. Range-wide habitat use of the Harpy Eagle indicates four major tropical forest gaps in the Key Biodiversity Area network. Ornithol. Appl. 124, 1–16 (2022).
Oliveira, M. J. et al. Ex situ population of the Harpy Eagle and its potential for integrated conservation. Zookeys 1083, 109–128 (2022).
Koeppen, W. Climatologia: Con Un Estudio de Los Climas de la Tierra. (1948).
Radam-Brasil. Projeto Radam-Brasil: Levantamento de Recursos Naturais. (1983).
Rettig, N. Breeding behavior of the harpy eagle (Harpia harpyja). Auk 95, 629–643 (1978).
Miranda, E. B. P. Prey composition of harpy eagles (Harpia harpyja) in Raleighvallen, Suriname. Trop. Conserv. Sci. 11, 1–8 (2018).
Goffart, M. Function and Form in the Sloth (Pergamon Press, 1971).
Brown, D. A test of randomness of nest spacing. Wildfowl 26, 102–103 (1975).
Embrapa. Manual de Análises Químicas de Solos, Plantas E Fertilizantes. (Embrapa Informação Tecnológica, 2009).
Mehlich, A. Determination of P, Ca, Mg, K, Na and NH4. (1953).
Pinheiro, J. et al. Package ‘nlme’. Linear and nonlinear mixed effects models, version, 3(1). 336 (2017).
Acknowledgements
EBPM would like to thank Prof Vinicius Farjalla for the inspiring ecosystem ecology classes during undergrad school, where he had the insight that led to this research. CTD is grateful to the University of KwaZulu-Natal and the National Research Foundation (ZA, Grant 98404) for funding. We greatly appreciate the generous financial support of the following donors: Rufford Small Grants Foundation (18743-1, 23022-2 and 31091-B), Rainforest Biodiversity Group, Idea Wild, The Mamont Scholars Program of the Explorer’s Club Exploration Fund, Cleveland Metroparks Zoo, and the SouthWild.com Conservation Travel System. Logistical support was given by the Peugeot-ONF Carbon Sink Reforestation Project, based at the São Nicolau Farm in Cotriguaçu, Mato Grosso, Brazil. This project is a Peugeot initiative to fulfil some of the Kyoto Protocol directions and is run by the ONF-Brasil enterprise. English review was kindly performed by Luke Sutton. We thank the Laboratory of Soil Analysis at Unemat—Alta Floresta, in the figure of Gustavo Caione, for timely performing soil analysis. Finally, whereas no live animals were used in the study, we thank ICMBio for authorising the nest access for project activities under the permit SISBIO 58533.
Author information
Authors and Affiliations
Contributions
E.B.P.M. formulated research ideas, goals, aims, produced the figures, managed the project, collected, curated and stored the field data, raised funds and wrote the first draft. L.G.R.O.S. performed the analyses, produced the figures, and reviewed the final draft. C.A.P. co-designed research ideas and the sampling methodology, and implemented multiple revisions of the manuscript. C.T.D. provided useful comments on the original draft, its critical review and approval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Miranda, E.B.P., Peres, C.A., Oliveira-Santos, L.G.R. et al. Long-term concentration of tropical forest nutrient hotspots is generated by a central-place apex predator. Sci Rep 13, 4464 (2023). https://doi.org/10.1038/s41598-023-31258-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31258-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.