Abstract
NTRK gene fusions are rare somatic mutations found across cancer types with promising targeted therapies emerging. Healthcare systems face significant challenges in integrating these treatments, with uncertainty in prevalence and optimal testing methods to identify eligible patients. We performed a systematic review of NTRK fusion prevalence to inform efficient diagnostic screening and scale of therapeutic uptake. We searched Medline, Embase and Cochrane databases on 31/03/2021. Inclusion criteria were studies reporting fusion rates in solid tumours, English language, post-2010 publication and minimum sample size. Critical appraisal was performed using a custom 11-item checklist. Rates were collated by cancer type and pooled if additional synthesis criteria were met. 160 studies were included, with estimates for 15 pan-cancer and 429 specific cancer types (63 paediatric). Adult pan-cancer estimates ranged 0.03–0.70%, with higher rates found in RNA-based assays. In common cancers, rates were consistently below 0.5%. Rare morphological subtypes, colorectal microsatellite instability, and driver mutation exclusion cancers had higher rates. Only 35.6% of extracted estimates used appropriate methods and sample size to identify NTRK fusions. NTRK fusion-positive cancers are rare and widely distributed across solid tumours. Small-scale, heterogeneous data confound prevalence prediction. Further large-scale, standardised genomic data are needed to characterise NTRK fusion epidemiology.
Similar content being viewed by others
Introduction
Rationale
Genomics enabled precision oncology continues to drive improved and additional treatment options for patients through the development of targeted therapies designed for cancers harbouring specific biomarkers. A significant advancement of this approach is the emergence of ‘Pan Cancer’ or ‘histology independent’ therapies where biomarker positive patients receive a biomarker targeted therapy, irrespective of the physical site of origin of a tumour. Cancer patients whose tumours harbour a gene fusion in the Neurotrophic Tyrosine Receptor Kinase genes 1, 2 or 3 (NTRK1, NTRK or NTRK3) are clinically recommended for treatment in the advanced and refractory setting with targeted therapy in the form of Tyrosine Receptor Kinase (TRK) inhibitors1. These drugs demonstrated impressive response rates across cancer types in phase 3 clinical trials and both Larotrectinib and Entrectinib received regulatory approvals in recent years with the Food and Drug Administration in the United States, and conditional approval with the European Medicines Agency2,3.
Despite growing clinical support for use of TRK inhibitors1, integration of these drugs into public health systems presents significant challenges for health technology assessment (HTA) agencies who must consider the health economic impact of providing public access to TRK inhibitors in a setting of limited resources and growing cancer disease burden4. Novel targeted drugs are relatively costly and accurate prevalence estimates are required to inform the relevant cost-effectiveness analyses and health system budget impact of identifying and treating these rare patient groups. Though commonly cited at a prevalence of ‘up to 1% of all cancers’2, epidemiological data is extremely limited due to only recent interest in NTRK fusions and limited large scale genomic studies using next generation sequencing (NGS) technologies. Specific NTRK fusions have been found at a high prevalence in a handful of rare cancer types, but otherwise are widely dispersed and uncommon across other cancer types. It is therefore important to establish the histological types of cancer which are associated with fusion prevalence5,6. The rarity of NTRK fusions complicates health economic evaluations as uncertainty in the estimated prevalence (precision interval) is greater and minor variations in estimates can substantially impact on projections of drug uptake and cost. Importantly, biomarker testing, often approved as part of access to targeted therapies, requires screening of an extremely large population for a small yield, with impacts to the cost-effectiveness of providing NTRK fusion targeted treatment.
Estimation of population prevalence is further confounded by the use of multiple molecular diagnostic tests for identification, with varying diagnostic accuracy, accessibility and cost5,7,8. Briefly, NTRK fusions occur when the NTRK 1, 2 or 3 genes form a chromosomal rearrangement with one of many different genes (fusion partner) and result in the oncogenic expression of a TRK fusion protein that drives cancer growth9,10. An international expert review recommended DNA or RNA NGS testing for identifying NTRK fusions outside of high prevalence cancer types as these broad assays can identify a variety of known and novel fusion partners with high confidence1. Alternative molecular tests such as fluorescence in situ hybridization (FISH) and reverse transcription polymerase chain reaction (RT-PCR) are more limited in performance and the range of fusions identifiable, and a Pan-TRK immunohistochemistry antibody is increasingly used as an efficient (cheaper) screen for aberrant TRK protein expression, which would suggest a fusion is present, but has shown poor sensitivity and specificity that varies by cancer type relative to NGS and is not recommended as a sufficient diagnostic method1,10. Currently consensus is lacking clinically, and by extension at the regulatory level, for optimal testing and screening algorithms as different approaches to who, how and when to test for these fusions present trade-offs between cost, accessibility and accuracy of various methods1,11,12,13,14. Given diagnostics with lower sensitivity may underestimate prevalence in a population, this issue is an important consideration for mapping prevalence data.
To date, the most comprehensive estimates for NTRK fusion prevalence include those sourced from the largest single genomic testing cohort (Foundation Medicine) with a recent publication interrogating over 200,000 tested patients for NTRK fusions15. However, there are several potential sources of bias in this cohort such as the selected and likely enriched advanced stage of patients referred for testing. Additionally, the significant majority (~ 95%) of this cohort was tested with a DNA panel, which may underestimate NTRK fusion prevalence due to low sensitivity for NTRK 2 and 3 fusions7,10. Most reviews detailing NTRK fusion prevalence cite a convenience selection of one or two data sources, with smaller cancer cohorts of several hundred patients which are not large enough to detect a prevalence < 1% with confidence16,17. Only one systematic review of NTRK fusion prevalence was identified18 which reported prevalence across solid tumours but this search only covered literature up to 2019 and synthesised cancer type cohorts tested for NTRK fusions using any method, with a minimum of 20 patients. Little detail was reported to explore the variation and bias in NTRK fusion epidemiology, which was noted in discussing the limitations of the current evidence. An exploration of bias and sources of variation in prevalence estimates along with an updated inclusion of more recent data can provide much needed nuanced data to inform health economic evaluations and translation of NTRK fusion treatments into routine care.
Objective
The objective of this review was to extract, synthesise and critically evaluate the prevalence of NTRK 1/2/3 fusions in adult and paediatric solid tumour cohorts through a broad review of studies reporting rates Pan-Cancer and across cancer types. Due to the rarity and complexity in identification of these biomarkers, an additional aim was to generate robust point estimates for NTRK fusion prevalence in specific cancer type populations through a meta-analysis of rates deemed optimal for pooling. These results hope to inform the significant public health challenge of how to screen a solid-tumour population for NTRK fusions and predict rates of patients potentially eligible for treatment with TRK inhibitors.
Methods
We performed a systematic review adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines19 with checklist available in Appendix supplementary table S1 and a protocol was registered on the PROSPERO database on 30/04/2021.
Search strategy
A combined search of Medline and Embase via Ovid was conducted on 31/03/2021 that involved two components. The principal search was structured to identify articles that mention ‘NTRK’ or ‘TRK’ and terms related to ‘cancer’ and ‘fusions’. To address the increasing number of cohort studies reporting results of genomic testing including targetable fusions, without explicitly referencing ‘NTRK’ as a keyword, we added a supplemental search with keyword ‘genomic’ in conjunction with the most relevant cancer types for this review to identify additional large-scale ‘genomic landscape’ studies. The supplemental search was restricted from 1/1/2020 to 31/3/2021. All identified reviews in the principal search were scanned for relevant citations and this was considered likely to identify relevant ‘genomic landscape’ studies prior to 2020. The Cochrane library was also broadly searched for the keyword ‘NTRK’. Duplications, case reports and abstracts were removed. The review workflow is outlined in Fig. 1 and the detailed search strategy is available in Appendix, supplementary tables S2 and S3.
Screening and selection criteria
Title and abstract screening was performed by one reviewer (SOH) with independent second review of all records by one of the review team (FF, YJK, JS). Full text review was performed independently by two reviewers (SOH, FF) to ensure studies met selection criteria. In both stages, discrepancies were resolved through discussion. Studies testing for NTRK fusions in solid tumours were included if they reported at least one cohort above the minimum sample size for inclusion. Studies from 2010 or earlier were excluded due to outdated test methods and small cohorts limited to thyroid cancer. Studies where NTRK fusion status was known before testing or where the cancer type was defined by NTRK status were excluded. A detailed table of exclusion and inclusion criteria are available in Appendix, supplementary table S4.
Data collection
Study characteristics including population demographics, country of origin, study design and assay were extracted for each included study. Fusion prevalence, including relative frequency of NTRK1/2/3 were extracted for specific cancer type cohorts reported within an eligible study. Numerators and denominators were extracted to match unique patients, not samples. Only cancer types with cohorts above an absolute minimum sample size (50 common cancer type, 20 rare cancers) were extracted. Pan Cancer estimates were extracted where studies reported rates in cohorts in over ten cancer types. Specific cancer type prevalence rates were extracted as reported, with morphology as detailed as per the article, including those rates within Pan Cancer cohorts if fusion rates were reported at both Pan Cancer and cancer type levels. These cancer type estimates were allocated to broad tumour group categories for collation. Paediatric cohorts were extracted separately wherever possible. Cohorts defined entirely by a molecular subtype of a cancer were extracted as a unique cancer type to explore potential enrichment for NTRK fusions. Further information on data extraction and categorisation is available in Appendix section 4. Data extraction and supplementary tables S5, S6 and S7.
Critical appraisal
A checklist for assessing potential sources of bias and study quality in a cancer profiling context was developed through adapting items from two published checklists identified in a recent systematic review of tools for evaluation of prevalence studies and their ability to assess three domains of external validity, internal validity and statistical/reporting quality20. We utilised the Joanna Briggs Critical Appraisal Checklist for Prevalence Studies21 and the Risk of Bias in Prevalence Studies Tool22. We contextualised items to reflect corresponding practical scenarios of cancer genomic studies as opposed to the more conventional epidemiological survey nature of studies that these tools were designed to evaluate. Several items were synonymous and consolidated into an 11-item appraisal tool for NTRK fusion prevalence studies (Section 5. Study appraisal and supplementary table S8, Appendix). With regards to external validity, studies were appraised for their design and recruitment in terms of representing a national cancer type population. Further selection bias issues that would limit generalisability of an estimate such as referral or restrictive eligibility for testing versus a series or registry-based cohort were also assessed. Factors potentially influencing the internal validity or accuracy of NTRK fusion rates were assessed including the failure rate of testing, the definition of NTRK fusions (only looking for NTRK1 fusions versus all three NTRK genes), and the consistency and accuracy (in terms of existing sensitivity and specificity data) of testing platforms used. Finally, the statistical and reporting quality was considered including the sample size for each rate being sufficiently powered to detect a rare biomarker, clarity in the timeframe of recruitment and the fraction reported positive, and reporting of key clinical demographics (e.g., age, sex, histology) for a tested cohort. Appraisal was performed once per study except for sample size assessment (Item 10) which was performed for each extracted specific cancer rate within a study as this was cancer type dependent. Items had binary ratings (Ideal vs Not Ideal) except for testing methods and sample size which could be classified further in to ‘Ideal’, ‘Okay’ and ‘Poor’ rankings, with thresholds detailed in Appendix, supplementary table S9.
Data extraction and critical appraisal were performed independently by two reviewers (SOH and FF) to confirm consistency and discrepancies resolved through discussion.
Data synthesis
To capture the broad evidence base and provide robust summary estimates, the synthesis of data was done in two stages: a broad narrative summary and a meta-analysis of high-quality estimates. All included studies with prevalence data extracted were combined into a narrative summary of estimates for the Pan Cancer category and specific cancer cohorts which were collated within tumour groups to explore relative enrichment/variation of fusions in specific cancer types. This narrative summary allowed for summation of key study characteristics while facilitating visual exploration of variation in prevalence estimates within cancer groups and specific types.
In addition to the narrative summary, we aimed to derive robust NTRK fusion population prevalence estimates for each unique cancer type using only those rates that met additional synthesis criteria. Specific cancer cohort rates within each tumour group were further collated into ‘unique’ cancer type categories where the name was considered a synonymous type (e.g., Breast Cancer and Breast Carcinoma). Rates for a unique cancer type with multiple estimates were only eligible for meta-analysis if each estimate was from a unique cohort (not overlap), explicitly reported NTRK fusion rates, and did not have a ‘poor’ ranking for the methods and sample size ranking as per quality assessment. These two items from the assessment were used for criteria as they provided a quantifiable classification to address two key factors that could lead to inaccuracy (particularly underestimation) of NTRK fusion prevalence in a cancer type. We used a generalised linear mixed model with random effects to calculate pooled prevalence if more than one rate met the synthesis criteria per cancer type23. Where only one estimate met criteria, this is the prevalence point estimate presented. In both cases these estimates are considered optimal and highlighted in bold in Table 1. For cancer types where no rates were eligible for pooling but fusions were identified, then the study with largest sample size is presented as a less robust estimate (not in bold) to demonstrate the presence of NTRK fusions in that cancer type. Cancer types with no rates meeting synthesis criteria and no fusions identified are reported separately in Appendix supplementary table S10, as these studies reported a prevalence rate of 0% but there is less confidence these are reliable null findings. 95% confidence intervals for single study rates were calculated using Clopper Pearson exact method, with calculations and meta-analysis performed using R package ‘metaprop’. Pooling was not considered for estimates for Pan Cancer cohorts, paediatric cancers or for cancer types in the ‘Other’ category due to the heterogeneity in cancer type distribution expected across these cohorts. A schema of data synthesis is available in the Appendix supplementary figure S1.
Results
160 studies were included in the systematic review after full text screening. Between 01/01/2011 and 31/03/2021, no studies met the inclusion criteria for the year 2011, and most studies (62%) were published from 2019 onwards (Fig. 2). 31% of studies (n = 51) were from the United States of America, followed by China and International studies (both 14%). Studies were more commonly retrospective (70%), and approximately one third of studies involved analysis of existing data from genomic data repositories, as opposed to retrospective testing of samples from an identified clinical patient cohort. Most studies (62%) identified NTRK fusions through RNA and DNA targeted panels, with DNA panel being the most utilised (n = 45). Overall, 69% of studies performed at least one next generation sequencing assay (n = 114), while the use of histology-based assays remained constant over the period considered and was often used as a primary identification method of fusions, followed by orthogonal validation (n = 26).
NTRK Fusion prevalence rates were extracted for 15 Pan Cancer (including 5 Paediatric) cohorts and additionally across 429 specific cancer type cohorts that were allocated to one of fourteen tumour groups. Lung, paediatric, colorectal, and thyroid cancers represented the most analysed cancer types, with respectively 36, 24, 19, and 19 studies, accounting for 61% of all included studies (Fig. 2).
Narrative summary
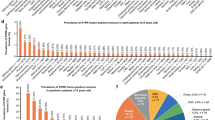
The details of the Pan Cancer prevalence estimates are displayed in Figs. 3 and 4. For Adult or mixed age cohorts, these were all below 1%, ranging from 0.03 to 0.70%. Higher rates were seen with more comprehensive testing methods either using an RNA NGS assay or whole genome sequencing (WGS), and lower rates seen for studies using immunohistochemistry (IHC) or circulating tumour DNA (ctDNA) (Fig. 3). Paediatric Pan Cancer cohorts had higher and more variable rates than the adult cohorts, ranging from 0.44 to 3.33%, likely reflecting the greater representation of mid to high NTRK fusion prevalence cancer types in paediatric cancers compared to adult (Fig. 4).
Across the 14 tumour groups, the largest number of cancer specific rates (429 in total) were extracted for paediatric cancers (14.69%), lung cancers (13.75%) and thyroid cancers (10.49%). Narrative summary tables for the specific cancer cohort prevalence point estimates are presented for each tumour group in Appendix, supplementary tables S11–S24. High NTRK fusion prevalence rates were confirmed in previously identified rare cancers of infantile fibrosarcoma (70.37%), secretory carcinoma of the breast (90.91%) and secretory carcinoma of the salivary gland (range 83.33–89.66%). Additional cancer types with substantial (> 10%) NTRK fusion prevalence were largely paediatric cancers; (congenital mesoblastic nephroma (18.75–22.73%), differentiated or papillary thyroid cancer (11.5–26.09%), infantile high-grade glioma (27.59%) and rare skin neoplasms such as spitzoid tumours (12.50–16.43%) and spindle cell nevus of reed (56.52%)). 184 (43.0%) specific cancer type cohorts included had zero NTRK fusions identified which may be an indication that these cancer types should not be prioritised for testing but given the rarity of NTRK fusions there is also potential that these studies lack sufficient sample size and sensitive methodology to detect fusions at such a low rate.
The relative proportion of NTRK1, NTRK2 and NTRK3 fusions varied across Pan Cancer cohorts but NTRK3 fusions were generally the most frequent. This could indicate a higher representation of the canonical (E26 transformation-specific transcription factor 6) ETV6-NTRK3 fusion in several cancer types but could also reflect testing platforms and referral bias affecting the cancer distribution for each study. Low rates of NTRK2 fusions identified may be due to the difficulty in identifying fusion breakpoints in NTRK2 compared to NTRK1, particularly using targeted DNA panels6,10. 200 of 245 (81.63%) specific cancer cohorts that reported fusions specified the NTRK gene involved. Of these, NTRK1 and NTRK3 fusions were commonly reported (137, 68.50%) and (128, 64.00%) but NTRK2 fusions were only identified in (46, 23.00%) cohorts. Ten cohorts listed NTRK2 fusions exclusively which were either brain/CNS tumours or lung adenocarcinoma.
Meta-analysis for population prevalence estimates
To arrive at population-point estimates for NTRK fusion prevalence, (332) specific cancer cohort rates were consolidated into 135 unique cancer types and assessed for suitability for meta-analysis. Rates for 85 types are presented in Table 1, with 50 cancer types listed separately in Appendix supplementary table S10 as none of these were eligible for meta-analysis and no fusions were identified. Studies included in the meta-analysis are highlighted in blue in supplementary tables S11–S24 of the Appendix. A total of 48 (35.56%) cancer types had rates considered eligible for pooling and just 15 (11.11%) had multiple cohorts eligible. Point estimates for major cancer types such as Colorectal Cancer, Non-Small Cell Lung Cancer, Melanoma, Breast, Prostate and Pancreatic Cancer were very low, ranging between 0.10–0.25%, confirming the rarity of the fusions in broad unselected cancer populations. Higher rates were seen in Thyroid and Sarcomas, but still rare at around 1%. Enrichment was seen in molecular subtypes such as driver WT (wild type) lung and MSI-H/dMMR (microsatellite instability-high/deficient in mismatch repair) colorectal cancers, and in both cancers post-treatment cohorts revealed NTRK fusions as resistant mutations at relatively higher prevalence. For all cancer types with multiple cohorts eligible for pooling, non-zero prevalence of NTRK fusions were found. This suggests, despite their rarity, the evidence supports the wide range of cancer types that potentially harbour NTRK fusions at very low frequencies. A comparison of the point estimates in this study, a previous meta-analysis and the Foundation Medicine cohort are presented for common cancer types in the Appendix supplementary table S25 (and demonstrate consistency for these broad groups).
Critical appraisal
Of the 444 rates extracted (15 Pan and 429 specific cancer), over half (261, 58.78%) had sample sizes deemed insufficient to identify NTRK fusions at the expected prevalence with 95% confidence as per item 11 of the critical appraisal tool (Appendix, supplementary table S8), with only (83, 18.69%) having ideal sample size. The methods used to detect NTRK fusions were assessed at the study level, with over 70% studies using methods considered ideal (RNA based NGS assays or WGS, 39.62%) or okay (DNA NGS assays, 32.08%), but substantial variation in specific testing methods was evident within each of these categories. With regards to generalising prevalence rates for population estimates, a minority of studies were considered as nationally representative cohorts (10.37%), largely due to studies being single site or limited in geographic coverage. Additionally, 54.88% studies had risk of external validity bias through systematically selected cohorts (as opposed to consecutive series) due to referral-based recruitment and restricted study eligibility criteria such as patient prognosis or minimal sample requirements for testing. Further to the assessment of testing methods used, the internal validity of each study’s rates was assessed for underestimation of NTRK fusion prevalence with 20.12% of studies having greater than a 5% failure rate in cohort testing and 28.30% of studies explicitly looking for NTRK fusions in less than the three NTRK genes or assessing limited fusion partners. Notably, only 60.37% of studies reported the demographics of the tested cohort in terms of age, sex, stage, morphology, and ethnicity, illustrating a reporting gap for 40% of studies with unclear representativeness of basic cohort demographics. The least reported demographic item was ethnicity, which was not included in 59.15% of studies. Results of the customised study appraisal tool are available in supplementary table S26 in the Appendix.
Discussion
This review of the current available evidence for NTRK fusion prevalence supports the notion that they are extremely rare and widely distributed across solid tumours. The Pan Cancer estimates for adult cohorts all sit comfortably below the commonly cited ‘1%’ prevalence approximation, with prevalence for paediatric cohorts also rare but slightly higher. Variation in these rates could be due to the different sensitivities of testing methods used, such as the low rates seen for cohorts using IHC or ctDNA relative to more comprehensive and reliable NGS on tissue samples. However, variation is also likely due to the relative distributions of cancer types included in these studies, and potential enrichment for high prevalence NTRK fusion cancers such as infantile fibrosarcoma and gliomas in some paediatric cohorts.
Our meta-analysis results indicate that the prevalence of NTRK fusions in common cancer types is also very low, with many close to 0.20% and upper bound confidence intervals below 0.50%. These results have utilised more recent data sources to provide a comprehensive evidence map of prevalence data and validate common cancer type rates described in Forsythe et al.18. There is also consistency with our rates and the predominant data source of Foundation Core, which through substantially larger cohorts, has considerable influence on our pooled prevalence.
With confidence that these rates are representative of the current literature through broad review, NTRK fusion prevalence estimates and the associated confidence intervals can be used in a public health context to predict targeted therapy uptake and inform health technology assessments for specific cancer types with the likely impact of drug uptake costs across a cancer population being minimal. The real challenge for integration of targeted therapies for rare biomarkers is the efficiency of testing and identifying positive patients. Our results indicate a thousand patients with common cancer types would need testing to identify two or three patients with NTRK fusions. Therefore, this supports the rationale of broad NGS testing (ideally a combined DNA/RNA platform) for advanced cancer patients with greater capability for detecting the broad range of NTRK fusion types, but also a spectrum of other targetable and potentially rare fusions and mutations. Additionally, highlighting subpopulations enriched for NTRK fusions allows for a more cost-effective approach to screening and the detail in this review provides quantitative evidence to support this approach which has been suggested in literature11,24. Prioritised testing for rare histology cancers with higher prevalence as listed in the results offers efficiency, but our evidence also indicates enrichment in cancer types based on molecular status such as lung cancers negative for other drivers, microsatellite instability high colorectal cancers and in some post-therapeutic cohorts with NTRK fusions emerging as potential biomarkers of drug resistance. However, large scale data indicate this is evidence of enrichment not exclusivity25,26, so although screening patients using sequential biomarker testing may be more cost effective in the specific context of rare NTRK fusions this may still miss positive patients, requiring more time and tumour tissue to arrive at a result compared to upfront comprehensive NGS testing which would be the preferred option from a patient perspective, ideally on fresh frozen tissue. Where this technology is not available routinely, NTRK FISH or pan-TRK IHC can be broad practical diagnostic options provided sufficient archival tissue is available and could be considered for not only high prevalence cancers or enriched molecular sub-types (such as MSI-H colorectal cancer), but potentially any solid cancer in clinical need of treatment. Given the diagnostic performance of these tests vary by cancer and fusion type, awareness of the evolving literature on validation of NTRK fusion diagnostics is critical for pathologists using these methodologies7,8 and could further inform optimal population screening algorithms along with accurate prevalence estimates.
More broadly, the results of this review demonstrate the challenge in consolidating evidence for biomarker epidemiology in the context of a rare biomarker. Thorough assessment using a structured tool allowed for comparison and detailed scoping of evidence quality, and this methodology could be adapted for other biomarkers in cancer genomic studies to derive robust prevalence estimates. Despite a large number of included studies, almost 60% of rates extracted had insufficient sample size. This mandates larger scale profiling studies and consolidation of genomic data, but standardisation of factors such as disease ontology, and transparency in methods and demographic reporting is critical to data synthesis being meaningful. Rare targets such as NTRK, NRG1 (neuregulin 1) and RET (rearranged during transfection) fusions are becoming increasingly relevant for clinical management Pan-Cancer and efforts to unify biomarker epidemiology data is critical from a public health perspective to understand the potential impact of novel targeted therapies at a population level, and ensure these patients are identified efficiently.
This review aimed to minimise reporting bias through adding a supplemental genomic study search rather than just studies focused on NTRK fusion identification but there are certainly NGS profiling studies that would not be captured in this search strategy. Large institutional precision oncology programs may not publish results regularly and an inclusion of international genomic registry data would likely add depth to the evidence base for NTRK fusion prevalence. Our decision to synthesise studies using DNA NGS panels rather than restricting to preferred RNA based or WGS methods only means pooled estimates may underestimate the true prevalence, but far fewer studies met this criterion and estimates at least reflect a prevalence that may be identified with population level DNA panel testing which are currently much more accessible at scale through commercial platforms.
Conclusion
This review provides comprehensive evidence on the prevalence of NTRK fusions in solid tumours available in the literature to date. Although rare, the range of cancer types with fusions is extensive, and detailed and accurate epidemiological data is critical to health service planning that supports efficient identification and treatment of patients with these rare, yet targetable Pan Cancer biomarkers.
Data availability
Data outlined in this review is available through the article and within the appendix.
Abbreviations
- NTRK:
-
Neurotrophic tyrosine receptor kinase
References
Yoshino, T. et al. JSCO-ESMO-ASCO-JSMO-TOS: International expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann. Oncol. 31(7), 861–872 (2020).
Drilon, A. et al. A phase II basket study of the oral TRK inhibitor LOXO-101 in adult subjects with NTRK fusion-positive tumors. Annals of Oncology. Conference: 41st European society for medical oncology congress, ESMO 2016. Conference start: 20161007. Conference end: 20161011. 27 (2016).
Demetri, G. D. et al. Efficacy and safety of entrectinib in patients with NTRK fusion-positive (NTRK-fp) tumors: Pooled analysis of STARTRK-2, STARTRK-1 and ALKA-372–001. Ann. Oncol. 29(Suppl 8), viii713 (2018).
Cooper, S. et al. How should we assess the clinical and cost effectiveness of histology independent cancer drugs?. BMJ 368, l6435 (2020).
Rosen, E. Y. et al. Trk fusions are enriched in cancers with uncommon histologies and the absence of canonical driver mutations. Clin. Cancer Res. 26(7), 1624–1632 (2020).
Hsiao, S. J. et al. Detection of tumor NTRK gene fusions to identify patients who may benefit from tyrosine kinase (TRK) inhibitor therapy. J. Mol. Diagn. 21(4), 553–571 (2019).
Solomon, J. P. et al. NTRK fusion detection across multiple assays and 33,997 cases: Diagnostic implications and pitfalls. Mod. Pathol. 33(1), 38–46 (2020).
Solomon, J. P. & Hechtman, J. F. Detection of NTRK fusions: Merits and limitations of current diagnostic platforms. Cancer Res. 79(13), 3163–3168 (2019).
Cocco, E., Scaltriti, M. & Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 15(12), 731–747 (2018).
Hechtman, J. F. NTRK insights: Best practices for pathologists. Mod. Pathol. 35, 298–305 (2022).
Solomon, J. P. et al. Identifying patients with NTRK fusion cancer. Ann. Oncol. 30(Suppl 8), VIII16–VIII22 (2019).
Marino, F. Z. et al. Ntrk fusions, from the diagnostic algorithm to innovative treatment in the era of precision medicine. Int. J. Mol. Sci. 21(10), 3718 (2020).
Naito, Y. et al. Japan society of clinical oncology/Japanese society of medical oncology-led clinical recommendations on the diagnosis and use of tropomyosin receptor kinase inhibitors in adult and pediatric patients with neurotrophic receptor tyrosine kinase fusion-positive advanced solid tumors, cooperated by the Japanese society of pediatric hematology/oncology. Int. J. Clin. Oncol. 25(3), 403–417 (2020).
Demetri, G. D. et al. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: Expert recommendations from the World Sarcoma Network. Ann. Oncol. 31(11), 1506–1517 (2020).
Westphalen, C. B. et al. Genomic context of NTRK1/2/3 fusion-positive tumours from a large real-world population. NPJ Precis. Oncol. 5(1), 69 (2021).
Adashek, J. J., Subbiah, V. & Kurzrock, R. From tissue-agnostic to N-of-one therapies: (R)evolution of the precision paradigm. Trends Cancer 7(1), 15–28 (2021).
Rohrberg, K. S. & Lassen, U. Detecting and targeting NTRK fusions in cancer in the era of tumor agnostic oncology. Drugs 81, 445–452 (2021).
Forsythe, A. et al. A systematic review and meta-analysis of neurotrophic tyrosine receptor kinase gene fusion frequencies in solid tumors. Ther. Adv. Med. Oncol. https://doi.org/10.1177/1758835920975613 (2020).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Migliavaca, C. B. et al. Quality assessment of prevalence studies: A systematic review. J. Clin. Epidemiol. 127, 59–68 (2020).
Munn, Z. et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. Based Healthc. 13(3), 147–153 (2015).
Hoy, D. et al. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 65(9), 934–939 (2012).
Schwarzer, G. & Rücker, G. Meta-analysis of proportions. Methods Mol. Biol. 2345, 159–172 (2022).
Wang, J. et al. Prevalence of recurrent oncogenic fusion in mismatch repair-deficient colorectal carcinoma with hypermethylated MLH1 and wild-type BRAF and KRAS. Mod. Pathol. 32(7), 1053–1064 (2019).
Westphalen, C. B. et al. Neurotrophic tropomyosin receptor kinase (NTRK) and nerve growth factor (NGF) are not expressed in Caucasian patients with biliary tract cancers: Pooled data from three independent cohorts. Clin. Transl. Oncol. 21(8), 1108–1111 (2019).
Lieu, C. H. et al. Comprehensive genomic landscapes in early and later onset colorectal cancer. Clin. Cancer Res. 25(19), 5852–5858 (2019).
Acknowledgements
We would like to acknowledge Ms. Suzanne Hughes and Mr. Geoff Links for their advice on the design and search strategy employed in this review.
Funding
Both the Predicting the population health economic impact of current and new cancer treatments project (PRIMCAT), grant number: MRF1199701) and Cancer-Patient Population Projections project (Cancer-PPP, grant number: MRF1200535) are funded through the Medical Research Future Fund (MRFF)—Preventive and Public Health Research Initiative—2019 Targeted Health System and Community Organization Research Grant Opportunity.
Author information
Authors and Affiliations
Contributions
S.O’.H., F.F., J.S., Y.J.K., and M.I.J. designed the study. S.O’.H. performed the literature search. S.O’.H., F.F., Y.J.K., J.S. contributed to the title and abstract screening. S.O’.H. and F.F. performed full-text review of articles. S.O’.H. and F.F. performed the data extraction, analysis, and visualisation. J.S., J.D., S.F., M.I.J. supervised the study. S.O’.H. drafted the manuscript. All authors contributed to interpretation of the results and critical review of the manuscript, approving the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
O’Haire, S., Franchini, F., Kang, YJ. et al. Systematic review of NTRK 1/2/3 fusion prevalence pan-cancer and across solid tumours. Sci Rep 13, 4116 (2023). https://doi.org/10.1038/s41598-023-31055-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31055-3
This article is cited by
-
Rezeptor-Tyrosinkinase-Fusionen in spindelzelligen Tumoren des Kindesalters
Die Pathologie (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.