Abstract
This study aimed to demonstrate the effectiveness of nonemergent extracranial-to-intracranial bypass (EIB) in symptomatic chronic large artery atherosclerotic stenosis or occlusive disease (LAA) through quantitative analysis of computed tomography perfusion (CTP) parameters using RAPID software. We retrospectively analyzed 86 patients who underwent nonemergent EIB due to symptomatic chronic LAA. CTP data obtained preoperatively, immediately postoperatively (PostOp0), and 6 months postoperatively (PostOp6M) after EIB were quantitatively analyzed through RAPID software, and their association with intraoperative bypass flow (BF) was assessed. The clinical outcomes, including neurologic state, incidence of recurrent infarction and complications, were also analyzed. The time-to-maximum (Tmax) > 8 s, > 6 s and > 4 s volumes decreased significantly at PostOp0 and up through PostOp6M (preoperative, 5, 51, and 223 ml (median), respectively; PostOp0, 0, 20.25, and 143 ml, respectively; PostOp6M, 0, 7.5, and 148.5 ml, respectively; p < 0.001, p < 0.001, and p < 0.001, respectively). The postoperative improvement in the Tmax > 6 s and > 4 s volumes was significantly correlated with the BF at PostOp0 and PostOp6M (PostOp0, r = 0.367 (p = 0.001) and r = 0.275 (p = 0.015), respectively; PostOp6M r = 0.511 (p < 0.001) and r = 0.391 (p = 0.001), respectively). The incidence of recurrent cerebral infarction was 4.7%, and there were no major complications that produced permanent neurological impairment. Nonemergent EIB under strict operation indications can be a feasible treatment for symptomatic, hemodynamically compromised LAA patients.
Similar content being viewed by others
Introduction
Although large artery atherosclerotic steno-occlusive disease (LAA) is a risk factor for primary or recurrent ischemic stroke, there is no clear treatment for prevention except medication1,2. Recently, endovascular treatment for LAA has been attempted via widening of the stenotic lesion, but intracranial arterial stenting does not fully overcome the periprocedural complications despite the development of various instruments, including improved catheters and stents3,4,5. On the other hand, although early randomized controlled trials (RCTs), such as the EC-IC Bypass Trial (EIBT) and the Carotid Occlusion Surgery Study (COSS), rejected extracranial-to-intracranial (EC-IC) bypass surgery6,7,8, its effectiveness has been re-evaluated, given recent indications of concerns with those studies9,10,11,12,13. In particular, many recent studies have shown that emergent EC-IC bypass (EIB) is effective in reducing stroke progression and recurrence in patients with acute ischemic stroke (AIS)14,15,16,17,18,19.
Furthermore, some studies have been conducted on nonemergent EIB surgery for preventing primary or recurrent stroke in hemodynamically compromised symptomatic LAA20,21,22. However, in most of these studies, either the number of subjects was small or quantitative analyses of perfusion changes, which could serve as objective evidence for the effectiveness of EIB, were not performed.
Therefore, we quantitatively analyzed pre- to postoperative changes in cerebral perfusion using computed tomography perfusion (CTP) with RAPID software and assessed their associations with clinical outcomes to determine the efficacy and safety of EIB for hemodynamically compromised symptomatic LAA.
Materials and methods
Patient enrollment
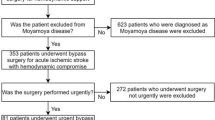
The medical data of patients who underwent nonemergent EIB for LAA between January 2006 and January 2020 were retrospectively reviewed under the approval of the Seoul National University Bundang Hospital institutional review board (IRB number: B-2103/673-103). The requirement to obtain informed consent from the patients has been waived by the Seoul National University Bundang Hospital institutional review board, and all methods were performed in accordance with the relevant guidelines and regulations. During the research period, we performed a total of 1,091 EIB procedures. A total of 738 patients who underwent EIB due to moyamoya disease or intracranial aneurysm or who underwent urgent or emergent bypass for AIS in the acute period or for acute ischemic symptoms such as transient ischemic attack (TIA) with an onset within 4 weeks were excluded. Among the remaining 272 patients, 186 whose perfusion data could not be reconstructed with RAPID software or who did not undergo pre- and immediate postoperative CTP scans were additionally excluded. Finally, 86 patients were analyzed in this study (Fig. 1).
Operation indication
We performed nonemergent superficial temporal artery-to-middle cerebral artery (STA-MCA) bypass in patients with symptomatic hemodynamically compromised LAA who met all three of the following indications: 1) LAA at the intracranial artery or extracranial internal carotid artery (ICA), as diagnosed by computed tomography angiography (CTA), magnetic resonance imaging angiography (MRA) or transfemoral cerebral angiogram (TFCA); 2) moderate or severe perfusion delay, as confirmed by CTP; and 3) recurrent or aggravated TIA symptoms despite administration of the best medical treatment for at least 4 weeks. The definition of large artery included internal carotid artery, proximal middle cerebral artery (M1, M2), proximal anterior cerebral artery (A1, A2), vertebral artery, basilar artery, and proximal posterior cerebral artery (P1)23. Mild, moderate, and severe perfusion delay were defined as hypoperfusion with time-to-maximum (Tmax) > 4 s (s), Tmax > 6 s, and Tmax > 8 s, respectively24. For patients with AIS who had passed the acute phase without worsening of symptoms, EIB was performed if symptoms and perfusion do not improve or worsen despite medical treatment for at least 4 weeks to wait for collateral development25,26,27.
Surgical procedure
The parietal or frontal branch of the patient's STA was dissected under general anesthesia. Craniotomy was performed around Chater's point where the angular, posterior temporal, or supramarginal arteries meet so that blood flow could be supplied to areas with severe perfusion delay while avoiding the eloquent area. The STA and distal MCA (M4) branches were anastomosed with 8–10 stiches with prolene 10–0 sutures. After anastomosis, the STA flow was measured using an ultrasonic flow meter. Because surgery was performed on patients whose symptoms persisted despite medical treatment, the operation was performed while maintaining aspirin monotherapy, which was restarted immediately after surgery. Intra-/post-operative blood pressure (BP) was maintained within 10% of the mean preoperative BP, and was generally controlled within the range of systolic BP 120–150 mmHg to prevent hypoperfusion or hyperperfusion.
Radiologic analysis
All patients underwent CTP to evaluate the perfusion status within 1 week before surgery. To confirm short- and long-term improvement in perfusion, a follow-up perfusion study was performed within 48 h immediately after EIB and 6 months postoperatively, respectively. We quantitatively evaluated the CTP data of patients in two ways: one via comparison of preoperative and immediate postoperative perfusion data to ensure that the perfusion changes were due to the operation, and the other via the comparison of the data across the three time periods, i.e., preoperative, immediate postoperative, and 6 months postoperative, to confirm the long-term change in the perfusion status after bypass surgery. The CTP scan was taken using a 256 slice CT scanner (Brilliance, Philips Medical Systems, Best, The Netherlands) with a 40 mL bolus contrast injection and a 60- to 70 s CT scan of 8 cm of brain tissue. Image data is sent to RAPID software (version 5.0.4, iSchemaView, Menlo Park, California, USA) after capturing the entire passage of contrast through the brain with the scan cycles every 1–3 s. The Tmax > 10 s, Tmax > 8 s, Tmax > 6 s, and Tmax > 4 s of the time-to-peak (TTP) were analyzed using RAPID. Cerebral blood flow (CBF) < 30%, which is known to represent the ischemic core volume, and the difference between Tmax > 6 s and CBF < 30%, which is known to indicate salvageable tissue, that is, the mismatch volume or penumbra, were also compared.
All patients underwent postoperative diffusion MRI between 1 and 3 days after bypass, and additional MRI scan were performed when neurological symptoms occurred. One week after surgery, TFCA was performed to confirm whether the bypass pedicle was intact.
Clinical outcomes
To evaluate the effectiveness of EIB, the occurrence of primary or recurrent infarction and TIA was checked. Infarction was defined as having neurologic symptoms and diffusion restriction at the site at which it was localized on diffusion MRI, and a scatter diffusion restriction point less than 3 mm on postoperative diffusion MRI without any neurological symptoms was defined as nonsignificant silent infarction, which was expected to be caused by transient hemodynamic instability or microembolism formed during anastomosis28. In addition to infarction, we also analyzed the changes in TIA symptoms after surgery. The above points were analyzed by dividing the patients into those who had a previous infarction on the lesion side before surgery and those without a previous infarction.
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics 25 (SPSS, Chicago, Illinois). Continuous variables are expressed as the median (interquartile range). The Wilcoxon matched-pairs signed-rank test was performed to compare perfusion data between two time points, and the Friedman test was performed to compare the data across three time points. Pearson correlation analysis was performed to evaluate whether the intraoperative post-anastomosis BF and the degree of improvement in perfusion before and after EIB was significant considering that Pearson's coefficient of correlation (r) 0.3–0.5 is a low positive correlation, 0.5–0.7 is a moderate positive correlation, and 0.7–0.9 is a high positive correlation29. Improvement in both pre- and immediate postoperative perfusion status and preoperative and postoperative 6 month perfusion status were evaluated. Generally, a p value < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 86 patients who underwent nonemergent EIB for LAA were included in this study. The median age was 62 (51–68.3) years old, and 65 (75.6%) patients were male. Among the 86 patients, 61 (70.9%) patients had a history of previous AIS due to LAA, and 40 (46.5%) patients showed recurrent or aggravated TIA. A median of 3.0 (1.2–7.5) months transpired from the time of diagnosis of the first AIS or the onset of the first TIA symptoms to the time of surgery, and the follow-up period after surgery was 46.3 (33.5–67.4) months, i.e., patients with a follow-up period of at least approximately 3 years were analyzed. Fifty-four patients (62.8%) and 32 patients (37.2%) underwent EIB due to arterial occlusion and arterial stenosis, respectively. Additionally, the locations of the lesion and the preoperative modified Rankin Scale scores are shown in Table 1.
Radiologic analysis
Quantitative assessment of immediate postoperative perfusion status
All 86 patients underwent TFCA within 1 week after EIB, and bypass occlusion was confirmed in 2 (2.3%) patients. We analyzed the CTP data reconstructed with RAPID software of all 86 patients. When comparing the group with previous infarction and the group without previous infarction, there was no significant difference in the preoperative Tmax > 10 s, Tmax > 8 s, Tmax > 6 s, Tmax > 4 s volumes and mismatch. However, the immediate postoperative Tmax > 8 s (p = 0.013), Tmax > 6 s (p = 0.008), Tmax > 4 s volumes (p = 0.028) and mismatch (p = 0.011) were significantly lower in the group without previous infarction (Table 1).
Before and immediately after surgery, Tmax > 10 s showed no significant difference (0 ml (0–5) vs. 0 ml (0–3), respectively, p = 0.510). Tmax > 8 s showed a significant difference (3.5 ml (0–41.25) vs. 0 ml (0–9)) before and after surgery, respectively (p = 0.019). Tmax > 6 s (48 ml (5.75–139.75) to 16 ml (0–70)) and Tmax > 4 s (216 ml (116.5–315.25) to 141.5 ml (83.75–266)) were also significantly different, with p values of 0.001 and < 0.001, respectively. CBF < 30% did not show a significant difference from 0 ml (0–0) before surgery to 0 ml (0–0) after surgery (p = 0.177). The mismatch volume, that is, the penumbra volume, calculated as the difference between Tmax > 6 s and CBF < 30%, showed a statistically significant improvement from 48 ml (5–139.75) before surgery to 12.5 ml (0–66) after surgery (p < 0.001) (Table 2).
Quantitative assessment of long-term postoperative perfusion status
Of the patients analyzed above, the 6-month postoperative perfusion data of 74 patients could also be analyzed. Tmax > 10 s before surgery, immediately after surgery, and 6 months after surgery showed a significant decreasing trend, from 0 ml (0–5.25) to 0 ml (0–3), and 0 ml (0–0), respectively (p = 0.002). Tmax > 8 s continued to show a significant decrease from 5 ml (0–43) to 0 ml (0–12.25) and 0 ml (0–4.25) (p < 0.001), and Tmax > 6 s also showed a significant decreasing trend from 51 ml (8.25–145.25) to 20.25 ml (0–73.5) and 7.5 ml (0–42.25) (p < 0.001). Tmax > 4 s also showed significant changes, from 223 ml (116.5–321.75) to 143 ml (86.5–266) and 148.5 ml (52.25–323) (p < 0.001). However, CBF < 30% did not show a significant change, from 0 ml (0–0) to 0 ml (0–0) and 0 ml (0–0) (p = 0.250). Finally, the penumbra volume calculated by the abovementioned method showed statistically significant changes, from 51 ml (5.75–145.25) to 19 ml (0–68.25) and 7 ml (0–42.25) (p < 0.001). The Wilcoxon matched-pairs signed-rank test was performed to determine whether there was a significant difference in perfusion status before and immediately after surgery, immediately after surgery and 6 months after surgery, and before and 6 months after surgery; the corresponding p values are shown in Fig. 2 and Table 2.
(A) The value of Tmax > 10 s continuously decreased as follows: preoperatively, 0 ml (0–5.25); immediately postoperatively, 0 ml (0–3); and 6 months postoperatively, 0 ml (0–0) (p = 0.002). (B) The value of Tmax > 8 s continuously decreased as follows: preoperatively, 5 ml (0–43); immediately postoperatively, 0 ml (0–12.24); and 6 months postoperatively, 0 ml (0–4.25) (p < 0.001). (C) The value of Tmax > 6 s continuously decreased as follows: preoperatively, 51 ml (8.25–145.25); immediately postoperatively, 20.25 ml (0–73.5); and 6 months postoperatively, 7.5 ml (0–42.25) (p < 0.001). (D) The value of Tmax > 4 s continuously decreased as follows: preoperatively, 223 ml (116.5–321.75); immediately postoperatively, 143 ml (86.5–266); and 6 months postoperatively, 148.5 ml (52.25–323) (p < 0.001).
Clinical outcomes
During the 3-year mean observational period for the 86 patients, only 4 (4.7%) patients with recurrent cerebral infarction were identified, and there was no primary infarction. In 2 (2.3%) of 4 patients with recurrent infarction, infarction occurred on the 5th and 7th days after bypass, and 1 of those 2 patients showed bypass occlusion. The other 2 (2.3%) of 4 patients with recurrent infarction presented with infarction on the 63rd and 352th days after bypass, respectively (Table 3). Insignificant silent infarction occurred in 5 patients (5.8%), 2 of whom had previous infarction and 3 of whom did not. Seven patients (8.1%) showed TIA symptoms without infarction, but all TIA symptoms disappeared within 1 month after EIB. It is noteworthy that 2 out of 4 patients who developed cerebral infarction were patients with bypass failure. That is, among 84 patients with an intact bypass pedicle, the occurrence of cerebral infarction was 2.4% (2/84).
Operation site infections occurred in 4 (4.7%) patients. After antibiotic treatment, 3 patients improved, and 1 patient underwent empyema removal. Three (3.5%) patients had a seizure event within 3 days after surgery, but the condition did not persist thereafter. Four (4.7%) patients had chronic subdural hematoma at the operation site; 3 patients improved after burr-hole drainage, and 1 patient improved after conservative treatment. Overall, there were no major complications that left permanent neurological impairment (Table 4).
Correlation between BF and perfusion parameter
Additional statistical analysis was performed to check whether there was a significant correlation between the BF as measured on STA after anastomosis and the degree of improvement in perfusion after surgery. First, Pearson correlation analysis was performed to determine whether the amount of change in the perfusion parameters before and immediately after surgery was related to BF. The amount of change in Tmax > 10 s, Tmax > 8 s, and CBF < 30% were not statistically significant (p = 0.242, 0.067, and 0.892, respectively) with a Pearson's r value of 0.135, 0.210, and 0.016, respectively. The changes in Tmax > 4 s were identified as having a p-value of 0.015, but Pearson's r value was 0.275, showing no positive correlation. However, the changes in Tmax > 6 s and penumbra volume showed a low positive correlation with Pearson's r values of 0.367 and 0.376, respectively (p = 0.001 and < 0.001, respectively) (Fig. 3). We then performed Pearson correlation analysis to evaluate the correlation between the BF and long-term change of perfusion parameters measured in the 6th month postoperatively. CBF < 30% still did not show a significant correlation, with Pearson's r at 0.006 (p = 0.959). Although the changes in Tmax > 10 s showed significant value (p = 0.041), Pearson's r showed no positive correlation (r = 0.252). However, the changes in Tmax > 8 s and Tmax > 4 s showed significant low positive correlations with Pearson's r values of 0.399 and 0.391, respectively (p < 0.001 and 0.001, respectively). The changes in Tmax > 6 s (r = 0.511; p < 0.001) and the penumbra volume (r = 0.537; p < 0.001) showed a significant moderate positive correlation, respectively (Fig. 4).
(A: The amount of change in Tmax > 6 s pre- and immediately postoperatively was correlated with the amount of STA flow measured during surgery (Pearson's r = 0.367, p = 0.001). (B) The amount of change in Tmax > 4 s pre- and immediately postoperatively was correlated with the amount of STA flow measured during surgery (Pearson's r = 0.275, p = 0.015).
(A) The amount of change in Tmax > 10 s pre- and 6 months postoperatively was correlated with the amount of STA flow measured during surgery (Pearson's r = 0.252, p = 0.041). (B) The amount of change in Tmax > 8 s pre- and 6 months postoperatively was correlated with the amount of STA flow measured during surgery (Pearson's r = 0.399, p < 0.001). (C) The amount of change in Tmax > 6 s pre- and 6 months postoperatively was correlated with the amount of STA flow measured during surgery (Pearson's r = 0.533, p < 0.001). (D) The amount of change in Tmax > 4 s pre- and 6 months postoperatively was correlated with the amount of STA flow measured during surgery (Pearson's r = 0.391, p = 0.001).
Discussion
Ischemic stroke is one of the leading causes of death worldwide, and stroke recurrence worsens the prognosis of patients and increases both the patient's and society’s costs. Numerous studies have been conducted on the recurrence rate of ischemic stroke and treatment for the prevention of recurrence2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33. In particular, the incidence of recurrent ischemic stroke at 5 years in patients classified as LAA based on the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification was 8.2–16.8%30. However, a clear treatment for preventing recurrence of ischemic stroke due to LAA has yet to be established. As previously mentioned, several attempts to improve the disease through intracranial stent insertion in patients with intracranial stenosis have not been as successful as expected3,4,5. Conversely, there are some reports that nonemergent EIB, whose effectiveness had previously been questioned, is helpful in limited indications20,21,22,34.
Nonemergent EIB for patients with symptomatic and hemodynamic compromised LAA
Two representative RCTs studied the effectiveness of nonemergent bypass for hemodynamically compromised LAA35,36. The EIBT analyzed the efficacy and safety of EIB in patients with TIA or stroke that had occurred within 3 months before entry into the study35. Perioperative ischemic events occurred in 12.2%, and major infarction occurred in 4.5%35. Furthermore, 18% and 20% of single strokes occurred in the medical treatment group and the EIB group, respectively, and two or more strokes occurred in 10% and 11% of each group over an average of 55.8 months, which demonstrated that EIB had no benefit in preventing stroke35. Another study was the COSS, which also compared a medical treatment group and EIB group, which consisted of ICA-occluded patients with TIA or ischemic stroke that had occurred within 120 days36. In that study, the 30-day rates for ipsilateral ischemic stroke were 14.3% in the EIB group and 2.0% in the medical treatment group, and the 2-year rates for the primary endpoint were 21.0% and 22.7%, respectively, indicating that EIB had no preventive effectiveness36. However, the problem with these two studies was that the perioperative morbidity was high, 12.2% and 14.3%, respectively36. Recently, as bypass technology has been developed and become widespread, several studies on EIB have reported good results with low perioperative morbidity15,16,17,18,19,20,21,22,34,39,40,41,42,43,44. Nevertheless, since most studies are on emergent EIB in AIS, they are of limited use in demonstrating the effectiveness and safety of nonemergent bypass for symptomatic and hemodynamically compromised LAA. We performed elective EIB for highly selected patients with symptomatic and hemodynamically compromised LAA, and cerebral infarction occurred in only 4.7% of patients over a mean period of 3 years without major complications associated with surgery, which is remarkably lower than the natural course of hemodynamically compromised LAA reported previously2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33.
Following quantitative analysis with RAPID software, we showed that cerebral perfusion could be improved and hemodynamic instability could be alleviated through EIB. Perfusion parameters before, immediately after surgery, and long-term indexes up to 6 months after surgery were compared sequentially, and the results demonstrated that most of the parameters improved after EIB. In particular, using imaging evidence, we established that EIB can prevent cerebral infarction, confirming that the volume of Tmax > 6 s, an index of penumbra45, is significantly improved. The recurrent TIA symptoms in all patients resolved within 1 month, and in patients with previous infarction, based only on if the operation was successful, symptoms disappeared in 97.6%; the other 2 of the 84 (2.4%) patients developed recurrent infarction. When nonemergent EC-IC bypass was performed on patients with symptomatic hemodynamically compromised chronic LAA under our indications, the surgical success rate was 97.7% (84/86), and the probability of symptom improvement was 97.6% (82/84).
Comparison of perfusion parameters with or without previous infarction
When we compared patients with and without previous infarction, there were no significant differences in other characteristics, including preoperative perfusion parameters and intraoperative STA flow, with the exception of the preoperative mRS score. However, postoperative perfusion parameters were significantly better in the non-infarction group than in the previous infarction group. Although there is no known evidence for this, in the previous infarction group, it was assumed that the area where the infarction had already occurred would not have effectively increased perfusion. Additionally, since the previous-infarction group was more likely to have more severe arterial atherosclerosis, we also hypothesized that the intracranial arterial network, such as the leptomeningeal collateral flow, would be relatively poor, so less improvement in perfusion status may occur. Based on this, it can be hypothesized that in patients with symptomatic LAA, EIB before infarction may be more helpful in improving perfusion parameters.
Relationship between BF and perfusion change
According to the results of this study, STA BF as measured with a flowmeter was positively correlated with the degree of perfusion improvement. Amin-Hanjani et al. demonstrated that the lower the cerebrovascular reserve capacity (CVRC), the higher BF and cut flow index (CFI; post-anastomosis BF (ml/min)/preoperative cut off flow (ml/min))46. Similarly, Inoue et al. demonstrated that the STA mean flow was significantly associated with regional cerebral blood flow when cerebral hemodynamics were evaluated by STA duplex ultrasonography47. Since CVRC is one of the factors that can affect BF, the lower the CVRC, the higher the BF, which can lead to more improved perfusion, that was assumed to be reflected in the results of this study. As a factor that can affect BF other than CVRC, it is advantageous to use a method that increases BF, and it may be helpful to select and design the STA and anastomosis site based on Poiseulle's law, which states that flow is proportional to the fourth power of diameter and inversely proportional to length. Accordingly, it is advantageous to select an STA or recipient artery with a larger diameter, although caution is required because there is a risk of hemorrhagic cerebral hyperperfusion syndrome if the STA is significantly thicker than the recipient artery44,45,46,47. Nakamizo et al. reported that the STA diameter and mean STA flow were significantly associated with CVRC, with an STA diameter cutoff value of 1.8 mm48. In addition, long-slit arteriotomy and fish-mouth cutting of the donor artery to enlarge the anastomosis site may be helpful49,50. Furthermore, according to Poiseulle's law, to reduce resistance, the length of the STA should be minimized to within the distance from the recipient artery as much as possible, which will contribute to increasing BF.
Limitations
There are several limitations in this study. First, this study was conducted retrospectively. Additionally, it is a single-arm study without a control group. Therefore, in particular, there may be controversy in the indication of EIB for a small number of preoperative poor conditioned patients. To overcome these limitations, the effectiveness of EIB was evaluated quantitatively using CTP, and we compared the results indirectly with the results of other studies that demonstrated the natural course of symptomatic LAA. Second, we did not measure CVRC, one of the previously known indications of EIB. However, this study only included patients with symptomatic moderate to severe perfusion delay, and it is indirectly possible to predict a decrease in CVRC because they are all worse than stage I hemodynamic failure51. In addition, the area of Tmax > 6 s RAPID CTP is defined as penumbra in DIFUSE3, DAWN, and SWIFT PRIME trial, so it is considered reasonable enough as an indication of bypass52,53,54. Additionally, since this study was conducted in a single tertiary general hospital that has much experiences with bypass surgeries and postoperative care, there may be limitations in applying this result to all hospitals. However, as the bypass technique has recently improved and become more common, this treatment can be considered sufficient for other institutions.
Conclusion
EIB in patients with symptomatic hemodynamically compromised LAA can contribute to lowering the incidence of recurrent infarction and improving perfusion parameters, which are positively related to intraoperative BF (Supplementary File 1).
Data availability
We provided all raw data used in this study as supplementary file.
Abbreviations
- LAA:
-
Large artery atherosclerotic steno-occlusive disease
- EC-IC:
-
Extracranial-to-intracranial
- EIB:
-
EC-IC bypass
- CTP:
-
Computed tomography perfusion
- Tmax:
-
Time-to-maximum
- STA-MCA:
-
Superficial temporal artery-to-middle cerebral artery
- TTP:
-
Time-to-peak
- CBF:
-
Cerebral blood flow
- mRS:
-
Modified Rankin Scale
- BF:
-
Bypass flow
References
Cole, J. W. Large Artery atherosclerotic occlusive disease. CONTINUUM Lifelong Learn. Neurol. 23(1), 133 (2017).
Flach, C., Muruet, W., Wolfe, C. D. A., Bhalla, A. & Douiri, A. Risk and secondary prevention of stroke recurrence: A population-base cohort study. Stroke 51, 2435–2444 (2020).
Kim, D. J. Intracranial stenting; The current landscape. Neurointervention 16, 2–5 (2021).
Abualhasan, A. et al. Intracranial stenting: is it still an option for treatment of patients with intracranial atherosclerosis?. Front. Neurol. https://doi.org/10.3389/fneur.2019.01248 (2019).
Siddiq, F., Adil, M. M., Norby, K. & Qureshi, A. I. Intracranial stent placement for symptomatic intracranial stenosis as part of a clinical trial versus outside a clinical trial. Stroke 44(12), 3571–3572 (2013).
Barnett, H. J. M. et al. Further conclusions from the extracranial–intracranial bypass trial. Surg. Neurol. 26, 227–235 (1986).
Farber, S., Diamond, L. K., Mercer, R. D., Sylvester, R. F. & Wolff, J. A. Failure of extracranial–intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. Nejm 313, 1191–1200 (1985).
Powers, W. J. et al. Extracranial–intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: The carotid occlusion surgery study randomized trial. JAMA J. Am. Med. Assoc. 306, 1983–1992 (2011).
Ausman, J. I. & Diaz, F. G. Critique of the extracranial–intracranial bypass study. Surg. Neurol. 26, 218–221 (1986).
Sundt, T. M. Jr. Was the international randomized trial of extracranial–intracranial arterial bypass representative of the population at risk?. N. Engl. J. Med. 316(13), 814–816 (1987).
Goldring, S., Zervas, N. & Langfitt, T. The extracranial–intracranial bypass study. N. Engl. J. Med. 316(13), 817–820 (1987).
Carlson, A. P., Yonas, H., Chang, Y. F. & Nemoto, E. M. Failure of cerebral hemodynamic selection in general or of specific positron emission tomography methodology? Carotid Occlusion Surgery Study (COSS). Stroke 42, 3637–3639 (2011).
Amin-Hanjani, S. et al. Extracranial–intracranial bypass for stroke - Is this the end of the line or a bump in the road?. Neurosurgery 71, 557–561 (2012).
Hwang, G. et al. Superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke and stroke in progress. Neurosurgery 68, 723–729 (2011).
Burkhardt, J. K. et al. Emergency extracranial–intracranial bypass to revascularize salvageable brain tissue in acute ischemic stroke patients. World Neurosurg. 109, e476–e485 (2018).
Park, H. S. et al. Patient selection and clinical efficacy of urgent superficial temporal artery-middle cerebral artery bypass in acute ischemic stroke using advanced magnetic resonance imaging techniques. Oper. Neurosurg. 13, 552–559 (2017).
Nussbaum, E. S., Janjua, T. M., Defillo, A., Lowary, J. L. & Nussbaum, L. A. Emergency extracranial–intracranial bypass surgery for acute ischemic stroke. J. Neurosurg. 112, 666–673 (2010).
Kim, J. H. et al. Efficacy and safety of timely urgent superficial temporal artery-to-middle cerebral artery bypass surgery in patients with acute ischemic stroke: A single-institutional prospective study and a pooled analysis. Cerebrovasc. Dis. 50(1), 34–45 (2021).
Jo, H. et al. Quantitative radiological analysis and clinical outcomes of urgent EC-IC bypass for hemodynamic compromised patients with acute ischemic stroke. Sci. Rep. 12, 8816 (2022).
Low, S. W. et al. Improvement in cerebral hemodynamic parameters and outcomes after superficial temporal artery-middle cerebral artery bypass in patients with severe stenoocclusive disease of the intracranial internal carotid or middle cerebral arteries. J. Neurosurg. 123, 662–669 (2015).
Gunawardena, M., Rogers, J. M., Stoodley, M. A. & Morgan, M. K. Revascularization surgery for symptomatic non-moyamoya intracranial arterial stenosis or occlusion. J. Neurosurg. 132, 415–420 (2020).
Yamashita, T. et al. The effect of EC-IC bypass surgery on resting cerebral blood flow and cerebrovascular reserve capacity studied with stable Xe-CT and acetazolamide test. Neuroradiology 33, 217–222 (1991).
Rennert, R. C. et al. Epidemiology, natural history, and clinical presentation of large vessel ischemic stroke. Neurosurgery 85, S4–S8 (2019).
Pang, C. H. et al. Prediction of hemorrhagic cerebral hyperperfusion syndrome after direct bypass surgery in adult nonhemorrhagic moyamoya disease: Combining quantitative parameters on RAPID perfusion CT with clinically related factors. J. Neurosurg. https://doi.org/10.3171/2022.5.jns212838 (2022).
Iwasawa, E., Ichijo, M., Ishibashi, S. & Yokota, T. Acute development of collateral circulation and therapeutic prospects in ischemic stroke. Neural Regen. Res. 11, 368 (2016).
Liu, J. et al. Vascular remodeling after ischemic stroke: Mechanisms and therapeutic potentials. Prog. Neurobiol. 115, 138–156 (2014).
Ergul, A., Alhusban, A. & Fagan, S. C. Angiogenesis: A harmonized target for recovery after stroke. Stroke 43, 2270–2274 (2012).
Bang, O. Y. Silent brain infarction: A quiet predictor of future stroke. Precis. Future Med. 2, 167–174 (2018).
Mukaka, M. M. Statistics corner: A guide to appropriate use of Correlation coefficient in medical research. Malawi Med. J. 24, 69–71 (2012).
Kolominsky-Rabas, P. L., Weber, M., Gefeller, O., Neundoerfer, B. & Heuschmann, P. U. Epidemiology of ischemic stroke subtypes according to TOAST criteria: Incidence, recurrence, and long-term survival in ischemic stroke subtypes: A population-based study. Stroke 32(12), 2735–2740 (2001).
Soda, T. et al. Stroke recurrence within the first year following cerebral infarction - Tottori University Lacunar Infarction Prognosis Study (TULIPS). Acta Neurol. Scand. 110, 343–349 (2004).
Khanevski, A. N. et al. Recurrent ischemic stroke: Incidence, predictors, and impact on mortality. Acta Neurol. Scand. 140, 3–8 (2019).
Buenaflor, F. G. Recurrence rate of ischemic stroke_ A single center experience. J. Neurolog. Sci. 381, 399 (2017).
Komatani, H., Okamoto, Y., Aoki, T., Noguchi, K. & Morioka, M. Long-term prognosis after extracranial–intracranial bypass surgery for symptomatic cerebrovascular occlusive disease. Kurume Med. J. 64, 1–4 (2017).
Goldring, S., Zervas, N. & Langfit, T. The extracranial–intracranial bypass study. A report of the committee appointed by the American Association of Neurological Surgeons to examine the study. Nejm 316, 817–820 (1987).
Martin, B. E. Coss. ACM SIGMOD Record 23, 479 (1994).
Schmiedek, P., Piepgras, A., Leinsinger, G., Kirsch, C. M. & Einhäupl, K. Improvement of cerebrovascular reserve capacity by EC-IC arterial bypass surgery in patients with ICA occlusion and hemodynamic cerebral ischemia. J. Neurosurg. 81(2), 236–244 (1994).
Sugiyama, T. et al. Reappraisal of microsurgical revascularization for anterior circulation ischemia in patients with progressive stroke. World Neurosurg. 84, 1579–1588 (2015).
Lee, S. B. et al. Early superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke. Clin. Neurol. Neurosurg. 115, 1238–1244 (2013).
Takeuchi, S. et al. Emergency superficial temporal artery to middle cerebral artery bypass after intravenous administration of tissue plasminogen activator for stroke. Turk. Neurosurg. 25, 633–637 (2015).
Kanematsu, R., Kimura, T., Ichikawa, Y. & Inoue, T. Safety of urgent STA-MCA anastomosis after intravenous rt-PA treatment: A report of five cases and literature review. Acta Neurochir. (Wien) 160, 1721–1727 (2018).
Inoue, A. et al. Efficacy of early superficial temporal artery-middle cerebral artery double anastomoses for atherosclerotic occlusion in patients with progressing stroke. J. Stroke Cerebrovasc. Dis. 26, 741–748 (2017).
von Weitzel-Mudersbach, P., Andersen, G. & Rosenbaum, S. Low morbidity after extracranial–intracranial bypass operation. The danish extracranial–intracranial bypass study: A nationwide survey. Cerebrovasc. Dis. 45, 252–257 (2018).
Horiuchi, T. et al. Emergency EC-IC bypass for symptomatic atherosclerotic ischemic stroke. Neurosurg. Rev. 36, 559–565 (2013).
Laughlin, B. B., Chan, A., Tai, W. A. & Moftakhar, P. RAPID automated CT perfusion in clinical practice. Neuroimaging 38–55 (2019).
Amin-Hanjani, S. et al. The cut flow index: An intraoperative predictor of the success of extracranial–intracranial bypass for occlusive cerebrovascular disease. Neurosurgery 56, 75–85 (2005).
Inoue, T. & Fujimoto, S. Prediction of cerebral blood flow restoration after extracranial–intracranial bypass surgery using superficial temporal artery duplex ultrasonography (STDU). Acta Neurochir. 94, 159–63 (2005).
Nakamizo, A. et al. Postoperative evaluation of changes in extracranial–intracranial bypass graft using superficial temporal artery duplex ultrasonography. Am. J. Neuroradiol. 30, 900–905 (2009).
el Rifaï, S., Boudard, J., Haïun, M., Obert, L. & Pauchot, J. Tips and tricks for end-to-side anastomosis arteriotomies. Hand Surg. Rehabil. 35, 85–94 (2016).
Tan, B. K., Wong, C. H., Chew, W. & Hong, S. W. Use of the slit arteriotomy for end-to-side arterial anastomosis in free-tissue transfers to the extremities. J. Plast. Reconstr. Aesthet. Surg. 62, 1519–1523 (2009).
Grubb, R. L. et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 280, 1055–1060 (1998).
de Havenon, A. et al. Results from DEFUSE 3: Good collaterals are associated with reduced ischemic core growth but not neurologic outcome. Stroke 50, 632–638 (2019).
Nogueira, R. G. et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N. Engl. J. Med. 378, 11–21 (2018).
Saver, J. L. et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 372, 2285–2295 (2015).
Funding
This work was supported by a 2020 Seoul National University Bundang Hospital research grant.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by H.J. The first draft of the manuscript was written by H.J., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jo, H., Lee, S.U., Jeong, HG. et al. Long-term outcomes and quantitative radiologic analysis of extracranial–intracranial bypass for hemodynamically compromised chronic large artery occlusive disease. Sci Rep 13, 3717 (2023). https://doi.org/10.1038/s41598-023-30874-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30874-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.