Abstract
The richness and structure of symbiont assemblages are shaped by many factors acting at different spatial and temporal scales. Among them, host phylogeny and geographic distance play essential roles. To explore drivers of richness and structure of symbiont assemblages, feather mites and seabirds are an attractive model due to their peculiar traits. Feather mites are permanent ectosymbionts and considered highly host-specific with limited dispersal abilities. Seabirds harbour species-rich feather mite communities and their colonial breeding provides opportunities for symbionts to exploit several host species. To unravel the richness and test the influence of host phylogeny and geographic distance on mite communities, we collected feather mites from 11 seabird species breeding across the Atlantic Ocean and Mediterranean Sea. Using morphological criteria, we identified 33 mite species, of which 17 were new or recently described species. Based on community similarity analyses, mite communities were clearly structured by host genera, while the effect of geography within host genera or species was weak and sometimes negligible. We found a weak but significant effect of geographic distance on similarity patterns in mite communities for Cory’s shearwaters Calonectris borealis. Feather mite specificity mainly occurred at the host-genus rather than at host-species level, suggesting that previously inferred host species-specificity may have resulted from poorly sampling closely related host species. Overall, our results show that host phylogeny plays a greater role than geography in determining the composition and structure of mite assemblages and pinpoints the importance of sampling mites from closely-related host species before describing mite specificity patterns.
Similar content being viewed by others
Introduction
The composition and structure of symbiont assemblages (parasites, commensals or mutualists) can be shaped by a wide array of variables including symbiont intrinsic factors (i.e., host specificity)1,2, extrinsic host features (i.e., phylogeny, morphological characteristics, behaviour)3,4,5 or ecological factors (i.e., host diet, host habitat, geographic distance between sampling localities)6,7,8,9. For example, we would expect phylogenetically related hosts to harbour similar symbiont assemblages that were acquired through co-speciation or host-switching. In the latter case, similarity would be explained by the fact that related hosts have more characteristics in common than unrelated species, such as similar living conditions for symbionts or similar defense systems5. This similarity has been found in symbionts of freshwater fishes, rodents and primates5,9,10. However, in other studies host phylogeny failed to fully explain similarity in symbiont communities among hosts, indicating that other extrinsic factors, such as environmental conditions, host ecology and geographic distance could also play a role11,12. Among studies evaluating similarity of symbiont richness to date, most have focused on endoparasites, with less attention given to ectoparasites/ectosymbionts.
Geography may also play an important role in defining symbiont community richness, with an expectation of decreasing similarity with increasing distance. The predicted decay in similarity has been reported for various parasite groups from a wide range of host taxa including fishes6,8, birds13,14, mammals15,16 and invertebrates17. Several mechanisms have been proposed to explain this pattern, such as environmental structure (i.e., decreasing similarity in environmental features with increasing distance), spatial configuration of the landscape (i.e., major geographic barriers limiting dispersal and leading to reduced community similarity) and limited dispersal potential of the species18.
Feather mites (Astigmata: Pterolichoidea and Analgoidea) are the most common and diverse group of permanent ectosymbionts associated with birds19,20. Most species live on the external surface of feathers, with specific adaptations to feather morphology20,21. Almost all avian orders have their own specific feather mite fauna, inhabiting one or a very few closely-related host species22,23. However, some mite species live on different host genera and even on different host families or orders20,23. In addition, only a small part of feather mite diversity has been described so far22. Thus, it is important to characterize the entire mite community of interest before drawing conclusions on host-specificity.
Co-speciation has been considered to be the main process driving feather mite diversification and community structuring, under the assumption that feather mites are highly specialized, host-specific symbionts23,24. Surprisingly, molecular studies have shown that host-switching, rather than co-speciation, may be the main process defining evolutionary patterns of divergence, even for these symbionts with limited dispersal potential23,24. Many studies on feather mite-bird systems have examined the prevalence/abundance patterns of these ectosymbionts on passerine hosts in relation to different factors. Intraspecific and interspecific studies have shown that feather mite abundance correlates with various host traits (i.e., body size, body mass, body condition, size of the uropygial gland, plumage coloration, sociality;25,26,27) and/or with environmental variables (i.e., salt concentration in the air;28). Mite prevalence has also been shown to positively correlate with winter sociality (i.e., birds living in flocks during the non-breeding season;29), but was unrelated to bird size, body mass or migratory behaviour. Taken together, these results suggest that feather mite abundance may be mostly shaped by habitat-associated conditions (i.e., host body environment, air temperature, season, humidity), whereas prevalence is more linked to mite transmission opportunities (i.e., winter sociality, phoresis)30,31. Given the diversity of results from molecular and ecological data to date, the main factors driving overall community structure of feather mites remain difficult to discern.
Seabirds, particularly Procellariiformes, harbour diverse ectosymbiont communities. They are known to be exploited by hard and soft ticks, blood-feeding mites, feather mites, fleas, lice and hippoboscid flies20,32. In addition, seabirds are highly mobile pelagic species, with a global distribution, and most of them breed sympatrically in large, mixed species colonies on isolated islands, features that should promote ectosymbiont dispersal within and among host species. However, they also show strong interannual fidelity and natal philopatry to their breeding sites, which means that most contact transmission may take place among related individuals33,34. Feather mite species richness seems to be particularly high on these birds35,36,37,38,39, with Microspalax, Zachvatkinia and Brephosceles being the most common and diverse genera35,40,41.
The Mediterranean Sea and Northeast Atlantic Ocean are key breeding areas for a number of procellariiform species of the genera Calonectris, Puffinus, Bulweria, Pterodroma, Pelagodroma and Hydrobates, including many closely-related and regionally endemic species, all of them harboring rich feather mite communities42. Indeed, six new mite species were described based on the feather mite surveys used for the present study43,44,45. In addition, feather mites inhabiting sympatric seabird species in the Cape Verde archipelago were found to exhibit strong host-associated structure42. These different attributes render this group of birds ideal for studying the factors structuring ectosymbiont communities.
In this context, the aim of the present study was to examine the influence of host phylogeny and geography on the composition and structure of feather mite communities found on the procellariform seabirds of the Mediterranean Sea and the archipelagos of the north-east Atlantic Ocean. Based on our current knowledge of feather mites and their avian hosts, we expected to find an increase in similarity with host relatedness given that feather mites are permanent ectosymbionts and are considered to transmit primarily from parents to offspring. Second, we expected to find a decrease in mite community similarity with increasing geographic distance between breeding colonies based on the fact that nearby colonies are more ecologically similar and/or because feather mite dispersal is more likely among neighbouring birds.
Materials and methods
Seabird sampling
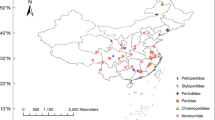
In total, we collected and examined feather mites from 964 sampled seabirds, covering 11 host species and 28 geographic locations. Host birds included 11 procellariiform species, nine belonging to Procellariidae (Scopoli´s shearwater, Calonectris diomedea; Cory’s shearwater, Calonectris borealis; Cape Verde shearwater, Calonectris edwardsii; Manx shearwater, Puffinus puffinus; Mediterranean shearwater, Puffinus yelkouan; Macaronesian shearwater, Puffinus baroli; Boyd’s shearwater, Puffinus boydi; Bulwer’s petrel, Bulweria bulwerii and Cape Verde petrel, Pterodroma feae), and two belonging to Hydrobatidae (Band-rumped storm-petrel, Hydrobates castro and European storm-petrel, Hydrobates pelagicus). The 28 sampled sites included eight breeding colonies across the Mediterranean Sea and 20 across the north-east Atlantic Ocean (Fig. 1, Supplementary Fig. S1 online). Certain colonies were found in an area, defined here as a group of colonies within an archipelago (e.g., Balearic Islands, Azores Islands, Canary Islands and Cape Verde Islands). Colonies were also defined at a regional scale, a region being a larger geographic zone that includes several colonies and areas (e.g., Western Mediterranean—WM, Eastern Mediterranean—EM, Northern NE Atlantic—NNEA, Central NE Atlantic—CNEA and Southern NE Atlantic—SNEA). Bird capture and mite sampling were performed in accordance with good animal practices, as defined by the current European legislation and under permission from corresponding governmental authorities (Cabildo Insular de Gran Canaria y Lanzarote, Gobierno de Canarias, Secretaria Regional do Ambiente da Região Autónoma dos Açores, Parque Nacional do Madeira, Direcção Geral do Ambiente from Cape Verde, Govern de les Illes Balears, Junta de Andalucia, Región de Murcia—Consejeria de Agricultura y Agua). It also followed the recommendations of the ARRIVE guidelines (https://arriveguidelines.org). Birds were conscious during sampling and immediately released after handling with no apparent detrimental effects. Birds were not anesthetized and/or unconscious on any occasion. From 2003 to 2012, we collected feather mites from adult birds using the dust-ruffling method46 for all species, except for Cory´s and Scopoli’s shearwaters due to their large body size. For these two host species, we collected mites by visual inspection of primary and body feather barbs. Nearly all individuals of these two species harboured at least one mite species. However, as visual detection is more restrictive than dust-ruffling, the comparison of species prevalence between the two methods should be treated with caution. All samples were stored in absolute ethanol at − 20 °C for subsequent morphological identifications.
Map of the study area showing location of the 28 sample sites for feather mites on procellariiform seabird species across the north-east Atlantic Ocean (grey circles) and Mediterranean Sea (black circles). Abbreviations of the sample sites are shown in Supplementary Table S1.
Feather mite identification and deposition of mite specimens
Mites were cleared in lactic acid for 24 h, mounted on microscope slides in PVA medium and examined using a Leica DM 5000B light microscope. Mites were identified using specific keys for avenzoariids35,36, xolalgids47 and alloptids40,41,48. Feather mite specimens were deposited in the Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia and in Centro de Recursos de Biodiversidad Animal, University of Barcelona, Spain.
Feather mite community construction and analyses
Here we examined the community structure of feather mite assemblages based on the presence-absence of feather mite species on each examined bird, and tested the influence of host phylogeny and geography on their composition and structure. For each host species, colonies belonging to the same archipelago were grouped to minimise differences in sample sizes among colonies (see columns “Colony” and “Area” in Supplementary Fig. S1 online). The number of European storm-petrels sampled was low and therefore, could not be included in these analyses.
We first described mite communities at different spatial scales. The terms for mite populations and communities follow Bush et al.49. Prevalence was calculated as the number of birds with a particular mite species divided by the number of examined birds. Overall mite prevalence was calculated for each host species and each colony/area using the statistical package Quantitative Parasitology 3.050. Whittaker’s beta diversity, which describes the change in community composition at different scales, was calculated for each host species and each colony/area using PAST (version 2.17c)51. To evaluate the completeness of mite sampling, species accumulation curves were generated for each procellariiform species, pooling data from the different colonies/areas. Species accumulation curves represent the observed mite species number (Sobs) and estimate the mite species number which would be collected as the number of samples approaches the population size. These analyses were conducted with PERMANOVA + for PRIMER v652.
The influence of geography on the composition and structure of feather mite assemblages was tested at different spatial scales: large spatial scale (among the five regions mentioned above) and small spatial scale (among colonies within an area, e.g., Cape Verde Islands), while the influence of host taxonomy was tested at different host taxonomic levels: among the five host genera (Calonectris, Puffinus, Bulweria, Pterodroma and Hydrobates); among host species within a given genus (e.g., for Calonectris and Puffinus genera); and among individuals within a given host species (e.g., for Scopoli’s and the Cory’s shearwaters).
Similarity in mite community composition was examined at the infracommunity level. A mite infracommunity was defined as all symbionts of all species found on an individual host, whereas a component community includes all infracommunities within the same host population49. Mite infracommunities were used as replicate samples for community similarity analyses. For these analyses, we added a dummy species with value 1 to all host individuals to include those seabirds harbouring no feather mites.
We used metaMDS function of the vegan v2.5–4 R package to perform a non-metric multidimensional scaling (NMDS) to obtain an ordination of mite infracommunities for different host species and at different spatial scales (colony, area and region)53. This analysis was assuming Jaccard dissimilarities that were obtained with the vegdist function (binary = T). To visualize the ordinations, we used the phyloseq, ggplot2, and wesanderson R packages54,55,56. To assess the effects of host species and spatial scale on the composition and structure of mite communities, we conducted a permutational multivariate analysis of variance (PERMANOVA) with either host species, genus, or spatial scale as a fixed factor. For the factors host species and genus, we also performed an additional analysis in which we included the date of origin of each genus (estimated divergence time for that genus) to better account for the phylogenetic relatedness of the taxa. For example, when analyzing the effect of the host genus, we used the approximate date of origin of each genus as a fixed factor rather than genus identity. In addition, when analyzing the effect of the host species in the case of Cape Verde Islands, in PERMANOVA models, the date of origin of each genus was included as the first factor followed by host species identity. The date of origin of each genus (MYA) was obtained from TimeTree using the evolutionary timeline tool57. PERMANOVAs were then performed using the adonis2 function from vegan on Jaccard distance matrices with 999 iterations. When a significant interaction between factors was detected, separate PERMANOVAs for each factor were performed, including post-hoc pairwise contrasts, (R package pairwiseAdonis v0.0.1), with a Bonferroni correction for multiple tests58. Finally, we used the vegan meandist function to obtain pairwise dissimilarities between samples.
To investigate the relationship between host phylogenetic relatedness and mite community similarity, we ran a mantel test, using the mantel function from vegan, and matrices of host phylogenetic distances (patristic) and mite dissimilarity (Jaccard index). The phylogenetic information for the hosts was obtained from BirdTree. Specifically, we downloaded 1,000 trees from the Ericson backbone tree and then summarized them by computing a single ultrametric 50% majority-rule consensus tree using SumTree v 4.1.0 in DendroPy v4.1.0, following Rubolini et al.59 (Supplementary Fig. S1 online). Three species (Cory´s shearwater, Macaronesian shearwater and Boyd’s shearwater) were included as polytomies (i.e., multiple branching from a single node) at the genus level as there was no phylogenetic information for them. We used the cophenetic.phylo function from ape to obtain the host's patristic distances matrix (Supplementary Table S2 online). Furthermore, given variation in sampling effort among bird species, we used rarefied mite dissimilarity matrices based on the bird species with the lowest number of mite samples (i.e., 31 samples) using the rrarefy function from vegan (Supplementary Table S3 online). Finally, in order to assess the significance of the correlation between pairwise variation in mite assemblages and geographical distance between sampling colonies/areas, we carried out a Mantel-type test (999 permutations) and calculated Spearman’s correlation coefficients using the RELATE routine in the PRIMER v6 package. All these analyses were based on host species that had been sampled in more than one colony/area with at least one infested host.
Results
Species identification and number of communities
On the 11 studied procellariiform species, we found 33 species of feather mites belonging to three families and eight genera: Zachvatkinia, Rhinozachvatkinia, Promegninia (Avenzoariidae), Microspalax, Brephosceles, Plicatalloptes (Alloptidae), Ingrassia and Opetiopoda (Xolalgidae) (Supplementary Tables S4, S5 online). Among the mite species found, 11 were undescribed species and six were recently described43,44,45.
Nine different mite species were collected from the Scopoli’s shearwaters and eight from the Cape Verde shearwaters and the Bulwer´s petrels (Table 2). For most host species, the species accumulation curves of mite species reached a plateau, except for the Cape Verde shearwaters and the Cape Verde petrels, indicating that mite species richness may be slightly underestimated for these hosts (see Supplementary Fig. S2 online).
At the host genus level, each avian genus generally harboured distinct feather mite species. However, three mite species (M. brevipes, B. puffini and Plicatalloptes sp.1) were found on both Calonectris and Puffinus shearwaters. Half of the feather mite genera (Zachvatkinia, Microspalax, Brephosceles and Ingrassia) co-occurred in multiple species of procellariiforms, three genera (Rhinozachvatkinia, Promegninia and Plicatalloptes) were restricted to two host genera, whereas Opetiopoda inhabited only one host species. Furthermore, each host genus/species carried only one mite species of a given genus, except Microspalax and Brephosceles. Thus, two different species of Microspalax inhabited Cory´s shearwaters, whereas two or more Brephosceles species co-occurred on the same host genus/species and even on the same individual host.
Similarity patterns and factors shaping feather mite community structure
From the 964 seabirds sampled in this study, 684 were infested with at least one mite species. The host species with highest overall mite prevalence were Scopoli’s and Cory’s shearwaters (100%), Cape Verde shearwaters (92.2%) and Manx shearwaters (90.6%), whereas those with lowest prevalence were Band-rumped storm-petrels and Cape Verde petrels (44% and 36.4%, respectively) (Table 1). The global prevalence of each feather mite species inhabiting procellariform birds, calculated across the host species harbouring a given mite species, ranged between 1.3% for M. pterodromae and 69.5% for Z. ovata (Table 2).
The non-metric multidimensional scaling (NMDS) ordination based on the relative similarities of feather mite infracommunities showed clear separation among the five seabird genera (Calonectris, Puffinus, Bulweria, Pterodroma and Hydrobates; Fig. 2a) with some overlap between Calonectris and Puffinus samples. In contrast, little separation was found among the five sampled regions (Fig. 2b). The PERMANOVA, used to assess the influence of host and region on the composition and structure of mite infracommunities, revealed a significant interaction between both factors (F = 9.15; P = 0.001). The separate PERMANOVAs for each factor indicated significant differences in feather mite community composition among both the five host genera (F = 103; P = 0.001) and the five geographic regions (F = 32.4; P = 0.001), but host genus explained more of the observed variation (30.1%) compared to geographic region (12.2%). An additional PERMANOVA analysis accounting for differences in the evolutionary age of each genus (i.e., using the date of origin of the genus as a factor instead of the genus identity) revealed similar results, with this factor explaining the 17.78% of the variance (F = 200, P = 0.001). Pairwise contrasts between all genera and regions indicated significant differences in mite community structure (host genera: F = 24.4–222, P = 0.01; regions: F = 2.02–80.4, P < 0.05), except for comparisons between the regions Western Mediterranean—Eastern Mediterranean and Central NE Atlantic—Eastern Mediterranean (P > 0.05; Fig. 2b).
Non-metric multidimensional scaling ordination plot based on Jaccard similarities in mite species occurrence (data non-transformed, stress = 0.136) for (a) feather mite infracommunities of five procellariiform genera and (b) feather mite infracommunities of these five host genera presented as pooled data from the five sampled regions. Abbreviations of the geographical regions are: Western Mediterranean—WM, Eastern Mediterranean—EM, Northern NE Atlantic—NNEA, Central NE Atlantic—CNEA and Southern NE Atlantic—SNEA.
At a smaller geographic scale, the non-metric multidimensional scaling (NMDS) ordination on mite infracommunities of the five host species breeding in the Cape Verde Islands showed a clear separation by host species, regardless of colony location (Fig. 3). Similarly, we found that closely related hosts had similar mite communities (rho = 0.802, P = 0.002). The PERMANOVA revealed an interaction between host species and sampling colony, (F = 4.17; P = 0. 001); a re-analysis with each separate factor indicated significant differences in mite community composition among the five host species (F = 50.7; P = 0.001) and among the five colonies (Raso, Curral Velho, Fogo, Ilheu Cima and Ilheu Grande) (F = 11.3; P = 0.001). Host species again explained a higher percentage of the observed variation compared to geography (28.14% compared to 8%). The same result was found after accounting for differences in the evolutionary age of each genus (16.3% variance; F = 39.1; P = 0.001). All pairwise contrasts for both factors were significant (host species: F = 23.6–107, P = 0.01; colony: F = 3.63–20.8, P < 0.05). The highest similarity in mite community composition was found between Cape Verde petrels and Band-rumped storm-petrels (63.4%) and the lowest between Cape Verde shearwaters and Bulwer’s petrels (25.4%).
Non-metric multidimensional scaling ordination plot based on Jaccard similarities (data non-transformed, stress = 0.103) of mite infracommunities for five procellariiform species breeding in different colonies of the Cape Verde Islands. Each host species and sampled colony is represented by its own colours and icons, respectively. Abbreviations of the colonies are: Curral Velho—CVE, Fogo—FOG, Ilheu Cima—ICI, Ilheu Grande—IGR, Raso—RAS.
Regarding differences in feather mite infracommunities among host species within Puffinus and Calonectris genera, visual inspection of the NMDS ordination did not suggest clear structure of mite infracommunities by host species (Fig. 4). However, the PERMANOVA indicated significant differences in feather mite infracommunity composition among species within each genus (Puffinus species F = 3.17; P = 0.001; Fig. 4a and Calonectris species F = 15.1; P = 0.001; Fig. 4b). Pairwise comparisons revealed significant differences for both Calonectris (all comparisons: F = 7.27–24.8, P < 0.003) and Puffinus species (only for Mediterranean and Boyd's and Manx shearwaters: F = 1.44–5.22, P < 0.05). Average similarities among individuals of the same Puffinus species ranged from 44.3% for the Manx shearwaters to 57.8% for the Mediterranean shearwaters, whereas the highest similarity between two host species was 50.7% (Macaronesian and Mediterranean shearwaters). Calculated similarities among individuals within the three Calonectris species ranged from 44.8% (Cape Verde shearwaters) to 54.9% (Scopoli’s shearwaters), whereas the highest similarity between two host species was 50.8% (Cory’s and Scopoli´s shearwaters).
Non-metric multidimensional scaling ordination plot based on Jaccard similarities in mite species occurrence (data non-transformed, stress = 0.138) for (a) mite infracommunities of four species of Puffinus and (b) three species of Calonectris. Although the graph suggests no clear structure of mite infracommunities by host species, the PERMANOVA indicated significant differences in feather mite infracommunity composition in both genera. Data were pooled when host species were sampled in different areas (see Table 1).
Detailed analyses of the influence of geography on the feather mite communities within and among host populations in the two wide-spread Calonectris species, the Scopoli´s and the Cory´s shearwaters, showed noticeable variability in the structure of mite assemblages. The non-metric multidimensional scaling (NMDS) indicated a lack of structuring of mite infracommunities by colony/area for the Scopoli’s shearwaters (Fig. 5a), a result further supported by the PERMANOVA (F = 1.28; P = 0.232). The pairwise contrast between colonies/areas also revealed no differences for this host species. Similarities in infracommunities within colonies/areas were always higher than 50.0%, while similarities between colonies/areas ranged from 44.2% (Murcia–Zembra) to 70.2% (Creta–Hyeres). In the case of Cory’s shearwaters, the NMDS ordination showed some geographic separation of mite communities (Fig. 5b), and the PERMANOVA indicated significant geographic structuring (F = 12.8; P = 0.001), with colony explaining 20% of the variation. Mite community structure differed significantly between the five sampled areas (F = 1.55–30.1, P < 0.05), except for comparisons between Canary Islands, Almeria and Berlengas. Similarities in infracommunities within colonies/areas ranged between 45.1% (Berlengas) and 63.0% (Azores Islands), while average similarities between colonies/areas ranged from 39.6% (Madeira – Berlengas) to 59.3% (Almeria–Canary Islands). Geographic distance among colonies/areas correlated positively with the similarity of the mite communities on Cory’s shearwaters, although the correlation coefficient was relatively low (RELATE: rho = 0.176, P = 0.0001).
Non-metric multidimensional scaling ordination plot based on Jaccard similarities (data non-transformed, stress = 0.100) for mite infracommunities of (a) Scopoli´s shearwaters and (b) Cory’s shearwaters across their corresponding sampling colonies/areas. No clear separation of mite infracommunities between colonies was observed for both host species. Abbreviations of the geographical sampling sites/areas are: Balearic Islands—BI, Crete—CRE, Hyeres—HYE, Murcia—MUR, Zembra—ZEM.
Discussion
In this study we used a large survey of seabird feather mite assemblages to examine the relative roles of host phylogeny and geography in shaping the communities of these symbionts. We found that similarity in mite assemblages was determined to a great extent by host phylogenetic relatedness, with weak or negligible community structure among host species from the same host genera or among host individuals from the same host species sampled across distant localities.
The 11 procellariiform species breeding in the north-east Atlantic Ocean and the Mediterranean Sea harboured at least 33 species belonging to eight mite genera, half of which were either undescribed or recently described species. Species belonging to the genera Brephosceles, Zachvatkinia, Microspalax and Ingrassia were the most frequently reported from these seabird hosts. At least three feather mite species were found per seabird species, with the highest mite species richness reported for Calonectris shearwaters and Bulwer´s petrels. Species accumulation curves of mite richness reached the asymptote in most host species, indicating that we detected all common mite species harboured by these birds. However, this was not the case for two seabird species (Cape Verde shearwater and Cape Verde petrel), suggesting that further sampling may be needed to reveal the entire feather mite richness in these hosts. These findings highlight the still incomplete knowledge we currently have of seabird acarofauna.
To identify factors driving community structure of feather mites on procellariiform birds, we examined the presence/absence of mite species at different host taxonomic and geographic scales. Based on community similarity analyses, we found high specificity at the host genus level at both small (within the Cape Verde Islands) and large (among the five defined regions) geographic scales. Within the Cape Verde Islands, feather mite communities were clearly structured by host genus. That is, even when seabird species of different genera breed sympatrically in close physical contact (i.e., sharing the same breeding habitat or even burrow), mite assemblages differed. This result is in line with the genetic analyses performed on these feather mite communities42. However, at this spatial scale, the different seabird genera were represented by single species, such that host specificity of feather mites at host species level cannot be ruled out. At a larger geographic scale, three out of five studied seabird genera (Calonectris, Puffinus and Hydrobates) were represented by several host species sampled across several archipelagos. In this case, different host species from the same genus showed similar mite assemblages, and each genus harboured essentially distinct feather mite species, confirming that feather mite communities are essentially structured at the host genus level. Despite this general pattern, two sister genera of shearwaters, Calonectris and Puffinus, with nine and six mite species respectively, shared three mite species (Brephosceles puffini, Microspalax brevipes and Plicatalloptes sp.1), indicating that some mite species can inhabit closely-related host genera23. These results suggest that the high degree of specificity at the host species level advocated in some previous studies19,23 may be inflated by incomplete sampling of closely related species. Indeed, some of the mite species detected in the present study were first records for the examined host species, but were previously found in other closely related host species. One should therefore be careful about concluding on the degree of feather mite specificity before all closely related host species have been thoroughly sampled.
At a lower taxonomic level, that is at the host species level (i.e., among host species within Calonectris and Puffinus genera), we also found small but significant dissimilarities in feather mite communities. The Cory´s, Cape Verde and Scopoli’s shearwaters hosted nine, eight and five mite species, respectively, of which, a set of five mite species was shared among all Calonectris species and a distinct set of three mite species was shared only by Cory’s and Cape Verde shearwaters. The three Calonectris species have parapatric distributions. Direct contact between individuals of Cory’s and Scopoli’s shearwaters is largely limited to the Chafarinas Islands, a breeding locality situated on the boundary of their geographic ranges (nominally the Strait of Gibraltar), with some occasional reports of shearwaters visiting and even breeding on other localities within the breeding range of the other species60. Neither of these species shares a breeding colony with the Cape Verde shearwaters, although some individuals of the latter species are occasionally found visiting breeding colonies of Cory’s shearwaters in the Canary Islands61. Similarly, in the closely related Puffinus species, two species, Manx and Boyd’s shearwaters, share the same set of six mite species, whereas the other two, Macaronesian and Yelkouan shearwaters, host only a subset of three species. These results show that even when contact zones still exist between recently diverged host species, geography can limit mite transmission and lead to some, although weak, structuring of their assemblages. In contrast, among the ten mite species found to inhabit the two Hydrobates species, the Band-rumped and the European storm-petrels, only one mite species (B. lanceolatus) was shared. The two Hydrobates species are more phylogenetically distant than the three Calonectris species or the four Puffinus species62,63, suggesting a decrease in the similarity of mite communities with host phylogenetic distance5,7,9.
After controlling for host effects, some geographic differences in feather mite communities were detected among the five defined regions. However, this geographic pattern may largely reflect the parapatric distribution of host species from the same genus rather than geography per se. Indeed, no geographic structure of feather mite communities was observed for Scopoli’s shearwaters in the Mediterranean Sea, and only a weak signal was found among localities for the widely distributed Cory’s shearwaters. These findings contrast results from other systems6,8,16,17, where parasite community similarity decreased with geographic distance. However, these studies mainly involved endoparasites from freshwater and terrestrial environments, and the decay in similarity was primarily linked to differences in host traits (e.g., vagility, body weight or trophic level) and/or environmental gradients. Our host-symbiont system clearly differs from other biological models by two main factors: feather mite life histories and extreme host vagility. These factors could explain the weak influence of geographic distances on seabird feather mite community structure. Feather mites spend their entire life cycle on the bird’s body and may therefore, be highly tolerant to rapid changes in environmental conditions across the geographic range of the host species19,20. In addition, the extreme vagility of seabirds can promote feather mite dispersal among host populations even at large spatial scales, thus weakening differentiation of feather mite assemblages with distance. Our results are in accordance with a recent study, which also reported strong host-associated structuring and no geographic signature of feather mite assemblages across avian taxa, even at a continental scale23.
In this study, we examined differences in mite community assemblages based on presence/absence data and did not consider the relative abundance of the different mite species. It is possible that community structure may differ in this respect among geographic locations or at lower host taxonomic levels. Studying feather mite communities in this way is nonetheless difficult without a strong standardization of the methods used for collecting mites. Indeed, even with the dust-ruffling method, differences in weather conditions during field sampling can modify the efficiency of the collection method (i.e., wind). In conclusion, among the highly diverse feather mite community harboured by procellariiform seabirds we found that feather mite communities showed a high degree of specificity at the level of the host genus, at both small and large geographical scales; each seabird genus harbouring essentially distinct feather mite communities, with some exceptions in phylogenetically related seabird genera Calonectris and Puffinus. These results suggest that specificity at the host species level advocated in previous studies could have resulted from incomplete sampling of closely related host species. Thus, a thorough sampling of feather mites from all closely related host species is required when concluding on the degree of host specificity of these ectosymbionts. Geographic structure of feather mite communities was weak at best, likely due to seabird vagility that provides dispersal opportunities for feather mites. Geographic distance showed little association with mite community similarity, and its effect was negligible compared to other factors, suggesting that symbionts confined to the host body are highly tolerant to environmental gradients. Overall, our results highlight the key importance of host phylogeny in explaining major patterns of community structure in ectosymbionts, but supports host genus-specific mite assemblages rather than species-specific. However, molecular studies of mite communities may reveal potential barriers to feather mite dispersal that are not observed by species presence/absence data alone.
Data availability
The dataset generated and analyzed during the current study is available from the corresponding author on reasonable request. Slide mounted mites are available at Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia and in Centro de Recursos de Biodiversidad Animal, University of Barcelona, Spain.
References
Poulin, R., Krasnov, B. R. & Mouillot, D. Host specificity in phylogenetic and geographic space. Trends Parasitol. 27, 355–361. https://doi.org/10.1016/j.pt.2011.05.003 (2011).
Duron, O. & Hurst, G. D. D. Arthropods and inherited bacteria: from counting the symbionts to understanding how symbionts count. BMC Biol. 11, 45. https://doi.org/10.1186/1741-7007-11-45 (2013).
Clayton, D. H. & Johnson, K. P. Linking coevolutionary history to ecological process: Doves and lice. Evolution 57, 2335–2341 (2003).
Felsõ, B. & Rózsa, L. Reduced taxonomic richness of lice (Insecta: Phthiraptera) in diving birds. J. Parasitol. 92, 867–869. https://doi.org/10.1645/GE-849.1 (2006).
Poulin, R. Decay of similarity with host phylogenetic distance in parasite faunas. Parasitology 137, 733–741. https://doi.org/10.1017/S0031182009991491 (2010).
Poulin, R. The decay of similarity with geographical distance in parasite communities of vertebrate hosts. J. Biogeogr. 30, 1609–1615. https://doi.org/10.1046/j.1365-2699.2003.00949.x (2003).
Poulin, R. Relative infection levels and taxonomic distances among the host species used by a parasite: Insights into parasite specialization. Parasitology 130, 109–115. https://doi.org/10.1017/S0031182004006304 (2005).
Pérez-del-Olmo, A. et al. Not everything is everywhere: The distance decay of similarity in a marine host–parasite system. J. Biogeogr. 36, 200–209. https://doi.org/10.1111/j.1365-2699.2008.02000.x (2009).
Krasnov, B. R. et al. Similarity in ectoparasite faunas of Palaearctic rodents as a function of host phylogenetic, geographic or environmental distances: Which matters the most?. Int. J. Parasitol. 40, 807–817. https://doi.org/10.1016/j.ijpara.2009.12.002 (2010).
Cooper, N. et al. Phylogenetic host specificity and understanding parasite sharing in primates. Ecol. Lett. https://doi.org/10.1111/j.1461-0248.2012.01858.x (2012).
Poulin, R. Phylogeny, ecology and the richness of parasite communities in vertebrates. Ecol. Monogr. 65, 283–302. https://doi.org/10.2307/2937061 (1995).
Luque, J. L., Mouillot, D. & Poulin, R. Parasite biodiversity and its determinants in coastal marine teleost fishes of Brazil. Parasitology 128, 671–682. https://doi.org/10.1017/S0031182004005050 (2004).
Gómez-Díaz, E., Navarro, J. & González-Solís, J. Ectoparasite community structure on three closely related seabird hosts: A multiscale approach combining ecological and genetic data. Ecography 31, 477–489. https://doi.org/10.1111/j.0906-7590.2008.05330.x (2008).
Locke, S. A. et al. The decay of parasite community similarity in ring-billed gulls Larus delawarensis and other hosts. Ecography 35, 530–538. https://doi.org/10.1111/j.1600-0587.2011.07244.x (2012).
Krasnov, B. R. et al. Spatial variation in species diversity and composition of flea assemblages in small mammalian hosts: Geographical distance or faunal similarity?. J. Biogeogr. 32, 633–644. https://doi.org/10.1111/j.1365-2699.2004.01206.x (2005).
Vinarski, M. V. et al. Decay of similarity of gamasid mite assemblages parasitic on Palaearctic small mammals: Geographic distance, host species composition or environment?. J. Biogeogr. 34, 1691–1700. https://doi.org/10.1111/j.1365-2699.2007.01735.x (2007).
Thieltges, D. W. et al. Distance decay of similarity among parasite communities of three marine invertebrate hosts. Oecologia 160, 163. https://doi.org/10.1007/s00442-009-1276-2 (2009).
Soininen, J., McDonald, R. & Hillebrand, H. The distance decay of similarity in ecological communities. Ecography 30, 3–12. https://doi.org/10.1111/j.0906-7590.2007.04817.x (2007).
Dabert, J. & Mironov, S. V. Origin and evolution of feather mites (Astigmata). Exp. Appl. Acarol. 23, 437–454. https://doi.org/10.1023/A:1006180705101 (1999).
Proctor, H. C. Feather mites (Acari: Astigmata): Ecology, behavior and evolution. Annu. Rev. Entomol. 48, 185–209. https://doi.org/10.1146/annurev.ento.48.091801.112725 (2003).
Stefan, L. M. et al. Niche partitioning of feather mites within a seabird host, Calonectris borealis. PLoS ONE 10, e0144728. https://doi.org/10.1371/journal.pone.0144728 (2015).
Gaud, J. & Atyeo, W.T. Feather mites of the World (Acarina, Astigmata): The supraspecific taxa. Annales du Musée Royal de l’Afrique Centrale, Sciences Zoologiques 277, 1–193 (Pt. 1, text), 1–436 (Pt. 2, illustrations) (1996).
Doña, J. et al. Host specificity, infrequent major host switching and the diversification of highly host-specific symbionts: The case of vane-dwelling feather mites. Global Ecol. Biogeogr. 27, 188–198. https://doi.org/10.1111/geb.12680 (2017).
Doña, J., Serrano, D., Mironov, S., Montesinos-Navarro, A. & Jovani, R. Unexpected bird–feather mite associations revealed by DNA metabarcoding uncovers a dynamic ecoevolutionary scenario. Mol. Ecol. 28, 379–390. https://doi.org/10.1111/mec.14968 (2019).
Blanco, G. & Frías, O. Symbiotic feather mites synchronize dispersal and population growth with host sociality and migratory disposition. Ecography 24, 113–120. https://doi.org/10.1034/j.1600-0587.2001.240201.x (2001).
Figuerola, J., Domènech, J. & Senar, J. C. Plumage colour is related to ectosymbiont load during moult in the serin, Serinus serinus: an experimental study. Anim. Behav. 65, 551–557. https://doi.org/10.1006/anbe.2003.2027 (2003).
Galván, I. et al. Feather mites (Acari: Astigmata) and body condition of their avian hosts: A large correlative study. J. Avian Biol. 43, 273–279. https://doi.org/10.1111/j.1600-048X.2012.05686.x (2012).
Dowling, D. K., Richardson, D. S., Blaakmeer, K. & Komdeur, J. Feather mite loads influenced by salt exposure, age and reproductive stage in the Seychelles Warbler Acrocephalus sechellensis. J. Avian Biol. 32, 364–369. https://doi.org/10.1111/j.0908-8857.2001.320412.x (2001).
Figuerola, J. Ecological correlates of feather mite prevalence in passerines. J. Avian Biol. 31, 489–494. https://doi.org/10.1034/j.1600-048X.2000.310408.x (2000).
Harbison, C. W. & Clayton, D. H. Community interactions govern host-switching with implications for host-parasite coevolutionary history. Proc. Natl. Acad. Sci. USA 108, 9525–9529. https://doi.org/10.1073/pnas.1102129108 (2011).
Diaz-Real, J. et al. Repeatability of feather mite prevalence and intensity in passerine birds. PLoS ONE 9, e107341. https://doi.org/10.1371/journal.pone.0107341 (2014).
Khan, J. S. et al. Parasites of seabirds: A survey of effects and ecological implications. Adv. Mar. Biol. 82, 1–50. https://doi.org/10.1016/bs.amb.2019.02.001 (2019).
Brooke, M. Albatrosses and Petrels Across the World (Oxford University Press, 2004).
McCoy, K. D. et al. The role of seabirds of the Iles Eparses as reservoirs and disseminators of parasites and pathogens. Acta Oecol. 72, 98–109. https://doi.org/10.1016/j.actao.2015.12.013 (2016).
Mironov, S. V. A brief review of the feather mites of the genus Zachvatkinia in the USSR (Analgoidea, Avenzoariidae). Parazitol. Sbornik 36, 91–115 (1989).
Mironov, S. V. A new subgenus and three new species of the feather mite genus Zachvatkinia from Procellariiformes. Parazitologiya 23, 309–319 (1989).
Mironov, S. V. A new feather mite of the genus Promegninia Gaud and Atyeo, 1967 (Acariformes: Avenzoariidae) from the gray-headed albatross Thalassarche chrysostoma (Procellariiformes: Diomedeidae). Acarina 22, 127–132 (2014).
Mironov, S. V., González-Solís, J., Mihalca, A. D. & Stefan, L. M. Feather mites of the genus Brephosceles Hull, 1934 (Acariformes: Alloptidae) from the European storm petrel Hydrobates pelagicus (Procellariiformes: Hydrobatidae). Syst. Appl. Acarol. 27, 1273–1294. https://doi.org/10.11158/saa.27.7.2 (2022).
Doña, J. et al. Global associations between birds and vane-dwelling feather mites. Ecology 97, 3242. https://doi.org/10.1002/ecy.1528 (2016).
Peterson, P. C. A revision of the feather mite genus Brephosceles (Proctophyllodidae: Alloptinae). Bull. Univ. Nebraska State Museum 9, 89–172 (1971).
Atyeo, W. T. & Gaud, J. Microspalacinae, a new subfamily of the feather mite family Alloptidae Gaud (Acarina, Analgoidea). Folia Parasitol. 38, 327–343 (1991).
Stefan, L. M. et al. “More than meets the eye”: Cryptic diversity and contrasting patterns of host-specificity in feather mites inhabiting seabirds. Front. Ecol. Evol. 6, 97. https://doi.org/10.3389/fevo.2018.00097 (2018).
Stefan, L. M., Gómez-Díaz, E. & Mironov, S. Three new species of the feather mite subfamily Ingrassiinae (Acariformes: Xolalgidae) from shearwaters and petrels (Procellariiformes: Procellariidae). Zootaxa 3682, 105–120. https://doi.org/10.11646/zootaxa.3682.1.4 (2013).
Stefan, L. M., McCoy, K. D. & Mironov, S. V. A new species of the feather mite genus Rhinozachvatkinia (Acari: Avenzoariidae) from Calonectris shearwaters (Procellariiformes: Procellariidae): Integrating morphological descriptions with DNA barcode data. Folia Parasitol. 61, 90–96. https://doi.org/10.14411/fp.2014.009 (2014).
Mironov, S. V., Stefan, L. M. & González-Solís, J. New species of the feather mite genus Promegninia Gaud & Atyeo (Acari: Avenzoariidae) from petrels and shearwaters (Procellariiformes: Procellariidae). Syst. Parasitol. 90, 91–103. https://doi.org/10.1007/s11230-014-9532-1 (2015).
Walther, B. A. & Clayton, D. H. Dust-ruffling: A simple method for quantifying ectoparasite loads of live birds. J. Field Ornithol. 68, 509–518 (1997).
Mironov, S. V. & Palma, R. L. Two new feather mite species (Acari: Analgoidea) from the Tuamotu Sandpiper Aechmorhynchus parvirostris (Charadriiformes: Scolopacidae). Tuhinga 17, 49–59 (2006).
Mironov, S. V. On a validity of the genus Plicatalloptes (Acarina: Analgoidea: Alloptidae). Parasitologiya 30, 216–222 (1996).
Bush, A. O., Aho, J. M. & Kennedy, C. R. Parasitology meets ecology on its own terms: Margolis et al revisited. J. Parasitol. 83, 575–583. https://doi.org/10.1007/BF02270711 (1997).
Rόzsa, L., Reiczigel, J. & Majoros, G. Quantifying parasites in samples of hosts. J. Parasitol. 86, 228–232. https://doi.org/10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2 (2000).
Hammer, Ø., Harper, D. A. T. & Ryan, P. D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 9 (2001).
Anderson, M. J., Gorley, R. N. & Clarke, R. K. Permanova+ for Primer: Guide to Software and Statistical Methods (Primer-E, 2008).
Oksanen, J. et al. vegan: Community Ecology Package. R package version 2.5–4. https://CRAN.R-project.org/package=vegan (2019).
McMurdie, P. J. & Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217. https://doi.org/10.1371/journal.pone.0061217 (2013).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
Ram, K. & Wickham, H. wesanderson: A Wes Anderson Palette Generator. R package version 0.3.6. https://CRAN.R-project.org/package=wesanderson (2018).
Kumar, S., Stecher, G., Suleski, M. & Hedges, S. B. TimeTree: A resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 34, 1812–1819. https://doi.org/10.1093/molbev/msx116 (2017).
Martinez Arbizu, P. pairwiseAdonis: Pairwise multilevel comparison using adonis. R package version 0.0, 1 (2017).
Rubolini, D., Liker, A., Garamszegi, L. Z., Møller, A. P. & Saino, N. Using the BirdTree.org website to obtain robust phylogenies for avian comparative studies: A primer. Curr. Zool. 61, 959–965. https://doi.org/10.1093/czoolo/61.6.959 (2015).
Gómez-Díaz, E., González-Solís, J. & Peinado, M. A. Population structure in a highly pelagic seabird, the Cory’s shearwater Calonectris diomedea: An examination of genetics, morphology and ecology. Mar. Ecol. Prog. Ser. 382, 197–209. https://doi.org/10.3354/meps07974 (2009).
González-Solís, J. & Zango, L. Cape Verde Shearwater at Montaña Clara Island, N of Lanzarote, Canary Islands on June 2013. 3rd for Spain. Retrieved from http://www.rarebirdspain.net/arbsf072.htm (2013).
Pyle, P., Welch, A. J. & Fleischer, R. C. A new species of shearwater (Puffinus) recorded from Midway Atoll, northwestern Hawaiian Islands. Condor 113, 518–527. https://doi.org/10.1525/cond.2011.100117 (2011).
Wallace, S. J. et al. A phylogenetic test of sympatric speciation in the Hydrobatinae (Aves: Procellariiformes). Mol. Phylogenet. Evol. 107, 39–47. https://doi.org/10.1016/j.ympev.2016.09.025 (2017).
Acknowledgements
We would like to thank all the people who collected feather mite samples for us from all breeding colonies during 2003–2012.
Funding
This work was supported by the APIF postgraduate project from the University of Barcelona, by a Grant of the Romanian Ministry of Research, Innovation and Digitization, CNCS/CCCDI-UEFISCDI, project number PN-III-P1-1.1-PD-2019-0611, within PNCDI III and by an Institutional Performance Project for Excellence Financing in RDI, contract no. 2PFE/2021 for L.M.S. Financial support was also provided by REN2002-01164/GLO, CGL2006-01315/BOS, CGL2009-11278/BOS and CGL2013-42585-P from the Spanish Government, Fondos FEDER and BIOCON04/099 from Fundación Banco Bilbao Vizcaya Argentaria.
Author information
Authors and Affiliations
Contributions
J.G.S., L.M.S., E.G.D. and K.D.M. conceived and designed the study. E.G.D. and J.G.S. conducted fieldwork and collected data. L.M.S. performed the laboratory work. S.V.M. collaborated in morphological identifications. W.I. and J.D. analyzed the data with input from J.G.S., K.D.M. and E.G.D. L.M.S., W.I., J.D. and J.G.S. wrote the manuscript with input from K.D.M., E.G.D. J.G.S. and S.V.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stefan, L.M., Isbert, W., Gómez-Díaz, E. et al. Diversity and structure of feather mite communities on seabirds from the north–east Atlantic and Mediterranean Sea. Sci Rep 13, 4793 (2023). https://doi.org/10.1038/s41598-023-30858-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30858-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.