Abstract
Cuticle pigmentation was shown to be associated with body temperature for several relatively large species of insects, but it was questioned for small insects. Here we used a thermal camera to assess the association between drosophilid cuticle pigmentation and body temperature increase when individuals are exposed to light. We compared mutants of large effects within species (Drosophila melanogaster ebony and yellow mutants). Then we analyzed the impact of naturally occurring pigmentation variation within species complexes (Drosophila americana/Drosophila novamexicana and Drosophila yakuba/Drosophila santomea). Finally we analyzed lines of D. melanogaster with moderate differences in pigmentation. We found significant differences in temperatures for each of the four pairs we analyzed. The temperature differences appeared to be proportional to the differently pigmented area: between Drosophila melanogaster ebony and yellow mutants or between Drosophila americana and Drosophila novamexicana, for which the whole body is differently pigmented, the temperature difference was around 0.6 °C ± 0.2 °C. By contrast, between D. yakuba and D. santomea or between Drosophila melanogaster Dark and Pale lines, for which only the posterior abdomen is differentially pigmented, we detected a temperature difference of about 0.14 °C ± 0.10 °C. This strongly suggests that cuticle pigmentation has ecological implications in drosophilids regarding adaptation to environmental temperature.
Similar content being viewed by others
Introduction
Drosophilid pigmentation has been used as a fruitful model to dissect the molecular bases of sexual dimorphism and morphological variation and evolution1,2,3,4. Indeed, it is a particularly rapidly evolving trait, such that different populations or closely related species can have dramatically different pigmentations5,6,7. In contrast, the ecological relevance of pigmentation is much less well known, and its effects on fitness are difficult to establish in the field, as this trait is pleiotropically linked to many other traits affecting fitness, such as life history (longevity, fecundity), cuticular hydrocarbons, and resistance against pathogens, parasites, UV or desiccation8,9,10,11,12,13,14. The direct influence of pigmentation, independent from other traits to which it may be correlated in the field, can instead be assessed by measuring its effect on aspects of performance (sensu Arnold 198315) related to specific hypotheses, in controlled environments. For instance, a common hypothesis is that drosophilid pigmentation plays a role in thermoregulation, and thus in their adaptation to environmental temperature16. Dark-colored flies may warm up more in the sun, while light-colored flies may avoid overheating. In agreement with this hypothesis, in Drosophila melanogaster, populations living at higher altitudes or higher latitudes are darker5,17,18,19,20 and abdominal pigmentation shows some phenotypic plasticity16: flies which develop at low temperature are darker, which is thought to be adaptive. The correlation between pigmentation and body temperature was shown in many ectotherms (thermal melanism)21 and even in distantly related organisms such as yeasts22. In insects, it has been demonstrated in species from several orders (Orthoptera, Hemiptera, Coleoptera, Lepidoptera)23,24,25,26,27,28. In the studied species, darker individuals absorb more heat. However, all these insect species have relatively large sizes. It was shown, using pairs of insects of comparable sizes and different pigmentations, that the association of pigmentation with the temperature of insects exposed to sunlight was clear for large insects but was extremely limited for small insects (around 3 mg)29. For such small body sizes and with the calorimetry tools available at the time, it was not possible to conclude on the existence of a relation between body temperature and pigmentation29. Drosophilids usually have a smaller weight (between 1 and 1.5 mg fresh weight for a Drosophila melanogaster female30) than the insects used in this previous study29, which makes the relation between drosophilid pigmentation and body temperature unclear. In this work, we tested the hypothesis that darker drosophilids can absorb more heat. We used a thermal camera equipped with a macro lens to monitor the body temperature of drosophilids exposed to a light source mimicking sunlight, to assess the statistical association between pigmentation and body temperature increase under light exposure in these organisms. Thermoregulation was treated as an element of performance related to pigmentation, and thus as a proxy for fitness. We tested pairs of Drosophila lines or species differing by their pigmentation over their whole body, or only over some portion of their abdomens. These differences in pigmentation have been previously described and their genetic bases characterized6,7,31,32,33,34,35,36. The choice of these pairs of lines or species was based on the existence of strong phenotypic differences within the same species (Drosophila melanogaster ebony and yellow mutants), natural genetic variation within the same species (Drosophila melanogaster Dark and Pale lines), or different pigmentation in very closely related species with otherwise similar morphology (Drosophila americana/Drosophila novamexicana and Drosophila yakuba/Drosophila santomea). We compared the evolution of body temperature between the darkest fly and the lightest fly using the thermal camera, which allowed us to visualize very small differences in temperature (as low as 0.05 °C).
Results
We divided the results into five sections. The first section compares mutants of large effects within species (Drosophila melanogaster ebony and yellow mutants). The second and the third sections concern naturally occurring variation within species complexes (suggestive of local adaptation), with either whole-body or anatomically restricted pigmentation differences (respectively Drosophila americana/Drosophila novamexicana and Drosophila yakuba/Drosophila santomea). The fourth section focuses on lines of D. melanogaster obtained by artificial selection with moderate differences in pigmentation.
In each section, we give detailed information on the lines or species used. Temperature measures are available in Tables S1-S8 (see Material and Methods for their treatment). The fifth section analyses the relationship between pigmentation difference and body temperature difference.
ebony and yellow Drosophila melanogaster
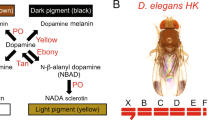
In order to compare Drosophila melanogaster individuals with very different pigmentations, we used loss of function alleles of ebony (ebony1, e1) and yellow (yellow1, y1). The e1 allele blocks the production of yellow NßAD sclerotin (Fig. 1), such that the fly cuticle is strongly melanized as more dopamine is available to produce black and brown melanins. Conversely, y1 flies cannot produce black melanin (Fig. 1) and their cuticle is pigmented only with brown melanin and yellow NßAD sclerotin.
Thus, despite belonging to the same species, e1 and y1 flies have dramatically different pigmentations (see Fig. 2a).
(a) Picture of ebony1 (left) and yellow1 (right) Drosophila melanogaster females. (b) Boxplots showing the normalized temperatures in °C for D. melanogaster ebony (E) and yellow (Y) mutant females. Pairs of individuals recorded simultaneously are indicated by lines. In all pairs, the ebony fly is hotter than the yellow fly. This is confirmed by a Wilcoxon rank signed test showing that E is significantly hotter than Y (p-value p = 0.00072, V = 120 being the value of the test statistic). ***p < 0.001.
A Wilcoxon signed rank test on paired samples was performed on the subset of data_norm corresponding to pairs of ebony1 and yellow1 flies to test whether flies with different genotypes (leading to different pigmentations) had different temperatures when exposed to light. More precisely, based on a sample of 15 pairs of ebony1 and yellow1 flies, we tested whether the empirical distribution of the difference in temperature increase between darker and lighter flies was symmetric about 0 (implying no statistical association between pigmentation and temperature increase). This H0 hypothesis was rejected, revealing a significant correlation between the genotype and the temperature increase of the flies (p < 0.001).
Figure 2b shows significant variation between the 15 experimental replicates, which may be due to individual variations of phenotypic expression or to other individual factors that were not controlled for as well as environmental differences between experiments. However, in each replicate, the y1 fly was constantly colder than the e1 fly. The average temperature difference between e1 and y1 females was 0.63 ± 0.19 °C.
Drosophila americana and Drosophila novamexicana
Drosophila americana and Drosophila novamexicana are sister species within the Drosophila virilis species group and diverged recently, about 300,000 to 500,000 years ago6. The body colour of Drosophila novamexicana has a derived yellow pigmentation, while the colour of other members of this group (including Drosophila americana) is dark brown6 (see Fig. 3a).
(a) Picture of D. americana (left) and D. novamexicana (right). (b) Boxplots showing the normalized temperatures in °C for D. americana (A) and D. novamexicana (NM) females. Pairs of individuals recorded simultaneously are indicated by lines. In all pairs, the D. americana individual is hotter than the D. novamexicana individual. This is confirmed by a Wilcoxon rank signed test showing that A is significantly hotter than NM (p-value p = 0.00024, V = 91 being the value of the test statistic). ***p < 0.001.
These species are native to North America. D. novamexicana is localized in the arid south-western regions of the USA and Mexico, whereas D. americana extends over a wide geographical and climatic range, from the western Great Plains to the east coast of North America37. The occurrence of D. novamexicana in an arid zone at one edge of the range of D. americana and its lighter pigmentation suggests that this species is specialized to this hotter habitat. In the laboratory, these species can mate and produce fertile offspring. Genetic mapping has shown that genomic regions containing the ebony and tan genes contributed to the pigmentation divergence between D. novamexicana and D. americana6 and further studies confirmed the role of both genes32,33.
Using the same type of statistical test (see Materials and Methods) with a sample of 13 pairs of D. novamexicana/D. americana flies, we obtained that the increase in body temperature after light exposure was significantly different between the 2 species (p < 0.001, see Fig. 3b). There is again a strong variation between replicates, but in each replicate the body temperature of the D. novamexicana fly was always lower than that of the D. americana fly (Fig. 3b). The average temperature difference between D. americana and D. novamexicana females was 0.61 ± 0.21 °C.
Drosophila yakuba and Drosophila santomea
This pair of closely related species belongs to the Drosophila melanogaster species group. They diverged between 500 000 years and 1 million years ago38. Drosophila yakuba is widely present on the African continent and on several African islands, whereas Drosophila santomea is endemic of the Island of Sao Tome, where it co-occurs with Drosophila yakuba39. They show contrasting pigmentation patterns: in both sexes, Drosophila santomea has a pure yellow body color, without the black pattern observed in Drosophila yakuba and other species of the Drosophila melanogaster group39. These two species have a reduced sexual dimorphism compared to the pigmentation of other species of the Drosophila melanogaster subgroup, where the last segments of the abdomen of females are less pigmented than those of males. The difference in pigmentation between these two species is however maximal in males, in which abdominal segments 5 and 6 are fully melanized in Drosophila yakuba, but homogeneously yellow in Drosophila santomea (see Fig. 4a). The difference in pigmentation between these species is much more localized than between the previous species.
(a) Picture of Drosophila yakuba (left) and Drosophila santomea (right) males. (b) Boxplots showing the normalized temperatures in °C for D. yakuba (Y) and D. santomea (S) males. Pairs of individuals recorded simultaneously are indicated by lines. In all pairs but one, the D. yakuba individual is hotter than the D. santomea individual. This is confirmed by a Wilcoxon rank signed test showing that Y is significantly hotter than S (p-value p = 0.0012, V = 3 being the value of the test statistic). **p < 0.01.
In the laboratory, these species can mate and produce fertile hybrid females, but sterile males (consistent with the classical pattern described as Haldane’s rule40). There is evidence from field studies and population genetics that hybridization occurs in the wild between these species on the island of Sao Tome41. Genetic analyzes indicated that at least 5 loci are responsible for the difference in pigmentation between D. yakuba and D. santomea: the pigmentation enzyme coding genes yellow (y), tan (t) and ebony (e) and the genes encoding the transcription factors Abdominal-B (Abd-B) and Pdm3 (pdm3)34. A recent study based on artificial introgression identified an additional locus involved, Grunge (Gug)35. Interestingly, long-term introgression experiments of pigmentation genes between Drosophilia santomea and Drosophilia yakuba revealed pigmentation-based assortative mating35, which suggests that pigmentation differences contribute to reproductive isolation between these species.
As for previous comparisons, we found that the empirical distribution of the difference in temperature increase between darker and lighter flies, based on a sample of 13 replicates, was not symmetric about 0 but put significantly more weight on positive values. That is, body temperatures were significantly different between the two species (p < 0.01). Despite the variations between replicates, in all replicates but one, the D. santomea individual was observed to be colder than the D. yakuba individual (Fig. 4b). The average temperature difference between D. yakuba and D. santomea males was 0.15 ± 0.13 °C.
Drosophila melanogaster Dark and Pale lines
These two lines were generated by artificial selection starting from a Drosophila melanogaster Canadian population that was polymorphic for female abdominal pigmentation31. Each line was isogenized through brother-sister crosses for 10 generations. The pigmentation difference between females of these two lines is located in the posterior abdomen (see Fig. 5a) and is mainly caused by allelic variation at the bric-à-brac locus encoding the transcription factors bab1 and bab231. Indeed, in the enhancer driving bab gene expression in posterior abdominal epidermis, there is a deletion removing two Abdominal-B binding sites in the Dark line which reduces the activity of the enhancer31.
(a) Picture of Drosophila melanogaster Dark (left) and Pale (right) females. (b) Boxplots showing the normalized temperatures in °C for D. melanogaster Dark (D) and Pale (P) females. Pairs of individuals recorded simultaneously are indicated by lines. In all pairs but one, the Dark individual is hotter than the Pale individual. This is confirmed by a Wilcoxon rank signed test showing that D is significantly hotter than P (p-value p = 0.00049, with V = 90 being the value of the test statistic). ***p < 0.001.
Again, body temperatures were significantly different between individuals of the 2 genotypes (p < 0.001) despite strong variation between replicates. Indeed, in all replicates but one the Pale fly was observed to be colder than the Dark fly (Fig. 5b). The average temperature difference between D. melanogaster Dark and Pale females was 0.14 ± 0.11 °C.
The temperature difference is related to the difference in pigmentation
In order to visualize the relation between pigmentation differences and temperature differences for the four pairs of fly comparisons, we plotted them on the same graph. For this, we measured pigmentation differences of 10 pairs of flies for each of the four comparisons (thorax and abdomen, see Material and Methods): ebony-yellow (Table S9), D. americana-D. novamexicana (Table S10), D. yakuba-D. santomea (Table S11) and D. melanogaster Dark-Pale (Table S12). The graph shows that the difference in temperature is related to the difference in pigmentation (Fig. 6). It is maximum (around 0.6 °C) for the most differently pigmented flies (ebony-yellow and D. americana-D. novamexicana), for which the whole body is differently pigmented, and smaller (around 0.14 °C) for the least differently pigmented ones (D. yakuba-D. santomea and D. melanogaster Dark-Pale) for which the pigmentation difference is localized to the posterior abdomen.
Discussion
Here, we showed that for the 4 pairs of Drosophila species or lines that we compared, the most pigmented Drosophila in each pair was warmer than the less pigmented one when exposed to a light source mimicking sunlight. The temperature difference appeared to be proportional to the differently pigmented area: between Drosophila melanogaster e1 and y1 mutants or between Drosophila americana and Drosophila novamexicana, for which the whole body is differently pigmented, the difference in temperatures was approximately 0.6 °C ± 0.2 °C. By contrast, between D. yakuba and D. santomea or between Drosophila melanogaster Dark and Pale lines, for which only the posterior abdomen is differentially pigmented, we detected a temperature difference of about 0.14 °C ± 0.10 °C. Thus, although the positive correlation between a darker pigmentation and the warming capacity was previously undetected for small insects29, using the thermal camera we could measure temperature differences between drosophilids of different pigmentation, even if they were of low magnitude. The fact that darker drosophilids absorb more heat is in line with results obtained with larger insects from various orders23,24,25,26,27,28,29. However, we cannot completely exclude that other parameters than pigmentation differing between the lines or species that we compared could be involved in the difference in body temperature. Indeed, except for the ebony/yellow pairs for which we controlled the genetic background (see method), we cannot exclude that uncontrolled genetic differences between the compared individuals affecting other traits than pigmentation have an impact on warming capacity.
Nevertheless, at the beginning of the experiment the two individuals have similar temperatures, the difference in temperature appears only after the light is switched on (Fig. 7). It strongly suggests that the difference in body warming can be attributed to difference in light absorption between the compared individuals. Furthermore, the fact that the difference in temperature increase is proportional to the proportion of the body which is differently pigmented agrees also with this interpretation.
Evolution of fly body temperatures in an experiment with D. melanogaster ebony and yellow mutants. (a) snapshot taken after the light was switched on (lowest temperature is blue, hottest temperature is white). (b) temperature curves of the two flies recorded during the whole course of the experiment.
These effects of pigmentation on body temperature are likely to have ecological impacts. Indeed, climatic factors such as temperature condition drosophilid species range42.
For example, the derived light pigmentation of D. novamexicana, which we showed to be correlated with body temperature, could have helped this species to adapt to the hot desert areas where it lives37. Furthermore, the darker pigmentation of D. melanogaster populations living at high altitude and latitude is very likely adaptive as mentioned in the introduction5,17,18,19,20. Indeed, darker D. melanogaster are more resistant to cold (shorter chill coma recovery time)43. That said, we do not pretend that drosophilid pigmentation has only a major impact on thermoregulation as it is known to be pleiotropically linked to many other traits affecting fitness, such as resistance to UV, as mentioned in the introduction. However, the role of pigmentation in adaptation to temperature or UV was recently tested using tropical populations of D. melanogaster collected over a wide geographical range and it was shown that pigmentation was better predicted by temperature-related climatic variables than UV44.
We showed that natural genetic variation for pigmentation within species was associated with differences in warming ability (D. melanogaster Dark and Pale line). For D. yakuba, D. santomea, D. novamexicana and D. americana, only one line per species was analyzed. However, in species such as Drosophila americana for example6,37, it was shown that there was genetic variation for pigmentation. It would then be interesting to investigate how such variation affects body temperature.
Our results strongly support the conclusion that thermal melanism applies to drosophilids. Thus, we expect that drosophilid pigmentation should vary with spatial and temporal gradients of temperature that influence natural selection in the field. It is already known that populations of D. melanogaster living at high altitude in Africa and India have darker abdomens5,17. Similarly, D. melanogatser thoracic pigmentation is darker at high altitudes and latitudes18,19,20. Furthermore, D. melanogaster developed at low temperature show a darker pigmentation, which is thought to be an adaptive trait16. Thus, it would be interesting to elaborate a model showing how genetic variation for pigmentation is modulated by spatial and temporal variations of temperature. This model would take into account that pigmentation is modulated both by genetic variation and by the temperature at which development takes place. It was shown that there is latitudinal and seasonal genetic variation in Drosophila melanogaster45,46,47. However, it is not known whether this variation involves allele frequencies of genes involved in abdominal pigmentation, although there is latitudinal variation for thoracic pigmentation18,19,20. A related and timely issue is whether global warming will affect the genetic variation for pigmentation in drosophilids, as it was shown to have an impact on the distribution of species of butterflies and dragonflies of particular pigmentation in Europe48 and on pigmentation variation in ladybirds49 and some species of leaf beetles50. Indeed, it was already shown that global warming had a detectable impact on genetic variation in particular species of drosophilids51.
Our demonstration that a darker pigmentation is positively correlated with a better ability to warm up in drosophilids opens the way for studies investigating the fitness consequences of this trait, and therefore how natural selection operates on it. In several insect species, the relation between pigmentation and body temperature has an impact on global activity24,25,26. Thus, it would be interesting to test whether we can detect an effect of pigmentation in drosophilids on activity, for example by measuring locomotion performance. More generally, the impact of body temperature on life-history components of fitness (such as age at maturity or fertility) is important to understand how selection operates on traits related to thermal regulation, such as pigmentation. This may also explain how and why anatomically localized pigmentation may be favored, if the temperature of some organs (such as gonads) is more determinant to fitness that others. For any such studies, the results we report here will provide a much-needed quantitative baseline for relating pigmentation to temperature, and thus connect to the abundant literature on thermal adaptation.
Materials and methods
Origin of the drosophilids
The Drosophila melanogaster alleles ebony1 (e1) and yellow1 (y1) were obtained from the Bloomington Drosophila Stock Center (Reference BL1658 and BL169). In order to be assessed in the same genetic background, they were introgressed for more than 8 generations in the w1118 stock.
Drosophila americana (line w11) and Drosophila novamexicana (line 15010-1031-04) were provided by Jorge Vieira (University of Porto, Portugal).
Drosophila yakuba was provided by the late Jean David (EGCE, Gif sur Yvette, France) and Drosophila santomea (line Cago 315) was provided by Virginie Courtier-Orgogozo (Institut Jacque Monod, Paris, France).
The Drosophila melanogaster lines Dark and Pale were generated by artificial selection starting from a population polymorphic for female abdominal pigmentation and were previously described31.
Flies were grown on standard medium at 25 °C.
Infrared thermography experiments
A FLIR thermal camera (FLIR A655sc) equipped with a macro lens (FLIR 2.9x) was used to image flies in the infrared spectrum for a given time interval.
During the experiment, flies were exposed to a source of light mimicking sunlight (25w, Repti Basking Spot Lamp, ZOO MED Europe).
The infrared thermography experiments were performed in an incubator maintaining a temperature of 16 °C (POL EKO ST3 BASIC SMART). This prevented temperature disturbances due to external events except the ignition of the lamp, and allowed the experiments to start at similar temperatures.
The software FLIR ResearchIR Max was used to acquire and treat infrared thermography images. We used the following parameters: Emissivity: 0.95; Distance: 0.1 m; Reflected Temp: 20 °C; Atmospheric Temp: 16 °C; Relative humidity: 50%; Transmission: 1; External optic: 16 °C; Transmission: 1.
Flies were anesthetized using vapors of flynap (50% triethylamine, 25% ethanol, 25% water). This facilitated imaging and blocked locomotor activity that could otherwise have impacted body temperature. For each experiment, a dark-colored fly and a light-colored fly were filmed simultaneously and side by side with the thermal camera in order to minimize acquisition biases. During each recording, flies were placed in the incubator, on a white paper, close to each other and equidistant from the camera and the lamp. These positions were chosen for the flies to be subjected to the same influence of the lamp when it was switched on. The recording of the thermal camera began when the average surface temperature measured by the camera was close to 16 °C. Each recording lasted 3min30s and contained 1245 images. Starting at timestamp 30 s after the beginning of the recording, we switched on the lamp until timestamp 2min30. The recording was stopped at 3 min, giving access to the temperature decrease dynamics. At the end of the first recording, the positions of the flies were reversed in order to minimize the potential non-homogeneity of the illumination of the lamp on the surface, thus preventing a position effect. The recording was then reproduced identically to the previous one in this new configuration. This experiment was repeated on several pairs of flies, and for several pairs of fly species or lines: we carried out 15 comparisons of Drosophila melanogaster ebony and yellow flies, 13 comparisons of Drosophila americana and Drosophila novamexicana flies, 13 comparisons of Drosophila yakuba and Drosophila santomea flies, and 13 comparisons of flies from the Drosophila melanogaster Dark and Pale lines.
We illustrate the type of data collected with the experiment on ebony1 and yellow1 D. melanogaster mutants shown in Fig. 7.
In this experiment, we see that the final body temperature of the ebony1 fly was observed to be higher than the temperature of the yellow1 fly (see Fig. 7a). When the lamp was switched on, the temperature of the two flies increased rapidly and a difference in temperatures between the two flies emerged after 15 s (Fig. 7b).
In order to further reduce any position effect and possible variations between experiments, we subtracted the average temperature of the paper surrounding each fly to the temperature of the fly. To do so, we drew ellipses in zones around each fly and called “temperature in the ellipse” the mean temperature in the area covered by the ellipse (see Fig. 8).
Principle of temperature normalization. In this screenshot of a video taken with the FLIR camera, Drosophila santomea is on the left and Drosophila yakuba is on the right. The flies are surrounded by eight ellipses numbered 1 to 8. The regions denoted by “Freehand” delimit the areas covered by the bodies of the two flies monitored during the experiment. In this example, ellipses 4 to 7 were used to normalize the body temperature of the fly to the left, while ellipses 1, 2, 3 and 8 were used to normalize the body temperature of the fly to the right.
We then averaged these temperatures over the three to five ellipses surrounding each fly. The average temperature of each fly was acquired by delimiting the body (abdomen + thorax) of the fly using the freehand tool included in the software FLIR ResearchIR Max.
More precisely, the normalizing procedure we used is the following. For every experiment i, the pair (data_norm)i of temperature differences between the flies and the background on which they laid is given by
where
-
darkesti is the average over the first and second recordings in experiment i of the mean temperature measured on the abdomen and thorax of the darkest fly in the time interval [1 min, 2 min];
-
lightesti is the average over the first and second recordings in experiment i of the mean temperature of the lightest fly in the time interval [1 min, 2 min];
-
(ellipse+)i is the average over the two recordings and over the ellipses that are closest to the darkest fly in experiment i of the spatial and temporal mean temperature in each of these ellipses (the mean temperature of an ellipse being computed from a recording as the average over the time interval [1 min, 2 min] of the average over all pixels inside the ellipse of the temperature measured in these pixels).
-
(ellipse−)i is constructed in the same way as (ellipse+)i but with the lightest fly.
The average over the first and second recordings of each pair of flies (after the positions of the flies were inverted) is taken in order to minimize the position effect. Moreover, the average temperature of the flies is computed over the time interval [1 min, 2 min] instead of the whole duration of the experiment [0 min, 3 min 30] to focus on the interval of time in which a relatively stable difference in temperatures between the two flies has established, after the initial increase and before switching off the lamp.
To test the hypothesis that the darkest fly becomes hotter that the lightest fly when both flies are exposed to light, we used a Wilcoxon signed rank test on the normalized paired measures of temperatures, for each of the following 4 groups of fly species or lines (with 13 to 15 pairs measured per group): ebony1 and yellow1 Drosophila melanogaster, Drosophila americana and Drosophila novamexicana, Drosophila yakuba and Drosophila santomea, and Drosophila melanogaster Dark and Pale lines. The use of this statistical test requires that the distribution of the difference between the first and the second coordinate of (data_norm)i (that is, the two standardized temperature measurements) within each group should be symmetrical about its mean (this mean need not be zero). We confirmed that this assumption was indeed satisfied with another (one-dimensional) Wilcoxon signed rank test applied to the empirical distribution of these differences across replicate pairs within each group. The histograms obtained are displayed in Figure S1.
Based on the p-values for the Wilcoxon rank signed test, which are all larger than 0.05, the distribution of the histograms of [darkesti − (ellipse+)i] − [lightesti − (ellipse−)i] could be considered to be approximately symmetric about their means for the 4 groups of species or lines. This allowed us to perform Wilcoxon signed rank tests on paired samples for the 4 series of measurements of pairs of flies with different pigmentations.
Measure of pigmentation differences
Photographs of flies were taken with a binocular equipped with Leica DC480 digital camera using Leica IM50 Image Manager Software. We took photos of pairs of flies corresponding to each of the four comparisons (10 pairs for each comparison). Using ImageJ, we decomposed each picture in hue, saturation and brightness and measured hue mean pixel intensity in thorax + abdomen of each fly. We then calculated the hue difference between the darkest and the lightest fly for each pair (Table S9-S12).
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Change history
21 March 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-31605-9
References
Massey, J. H. & Wittkopp, P. J. The genetic basis of pigmentation differences within and between Drosophila species. Curr. Top. Dev. Biol. 119, 27–61 (2016).
Yassin, A. et al. The pdm3 locus is a hotspot for recurrent evolution of female-limited color dimorphism in Drosophila. Curr. Biol. 26, 2412–2422 (2016).
Williams, T. M. et al. The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell 134, 610–623 (2008).
Bastide, H. et al. A genome-wide, fine-scale map of natural pigmentation variation in Drosophila melanogaster. PLoS Genet. 9, e1003534 (2013).
Pool, J. E. & Aquadro, C. F. The genetic basis of adaptive pigmentation variation in Drosophila melanogaster. Mol. Ecol. 16, 2844–2851 (2007).
Wittkopp, P. J. et al. Intraspecific polymorphism to interspecific divergence: genetics of pigmentation in Drosophila. Science 326, 540–544 (2009).
Jeong, S. et al. The evolution of gene regulation underlies a morphological difference between two Drosophila sister species. Cell 132, 783–793 (2008).
Rajpurohit, S. et al. Pigmentation and fitness trade-offs through the lens of artificial selection. Biol. Lett. 12, (2016).
Massey, J. H. et al. Pleiotropic effects of ebony and tan on pigmentation and cuticular hydrocarbon composition in Drosophila melanogaster. Front. Physiol. 10, 518 (2019).
Parkash, R., Rajpurohit, S. & Ramniwas, S. Impact of darker, intermediate and lighter phenotypes of body melanization on desiccation resistance in Drosophila melanogaster. J. Insect Sci. 9, 1–10 (2009).
Dombeck, I. & Jaenike, J. Ecological genetics of abdominal pigmentation in Drosophila falleni: A pleiotropic link to nematode parasitism. Evolution 58, 587–596 (2004).
Kutch, I. C., Sevgili, H., Wittman, T. & Fedorka, K. M. Thermoregulatory strategy may shape immune investment in Drosophila melanogaster. J. Exp. Biol. 217, 3664–3669 (2014).
Wittkopp, P. J. & Beldade, P. Development and evolution of insect pigmentation: Genetic mechanisms and the potential consequences of pleiotropy. Semin. Cell Dev. Biol. 20, 65–71 (2009).
Bastide, H., Yassin, A., Johanning, E. J. & Pool, J. E. Pigmentation in Drosophila melanogaster reaches its maximum in Ethiopia and correlates most strongly with ultra-violet radiation in sub-Saharan Africa. BMC Evol. Biol. 14, 179 (2014).
Arnold, S. J. Morphology, performance and fitness. Am. Zool. 23, 347–361 (1983).
Gibert, P., Moreteau, B. & David, J. R. Developmental constraints on an adaptive plasticity: Reaction norms of pigmentation in adult segments of Drosophila melanogaster. Evol. Dev. 2, 249–260 (2000).
Parkash, R., Rajpurohit, S. & Ramniwas, S. Changes in body melanisation and desiccation resistance in highland vs. lowland populations of D. melanogaster. J. Insect Physiol. 54, 1050–1056 (2008).
Telonis-Scott, M., Hoffmann, A. A. & Sgro, C. M. The molecular genetics of clinal variation: A case study of ebony and thoracic trident pigmentation in Drosophila melanogaster from eastern Australia. Mol. Ecol. 20, 2100–2110 (2011).
Munjal, A. K. et al. Thoracic trident pigmentation in Drosophila melanogaster: latitudinal and altitudinal clines in Indian populations. Genet. Sel. Evol. 29, 601–610 (1997).
David, J. R., Capy, P., Payant, V. & Tsakas, S. Thoracic trident pigmentation in Drosophila melanogaster: Differentiation of geographical populations. Genet. Sel. Evol. 17, 211–224 (1985).
Clusella Trullas, S., van Wyk, J. H. & Spotila, J. R. Thermal melanism in ectotherms. J. Therm. Biol. 32, 235–245 (2007).
Cordero, R. J. B. et al. Impact of yeast pigmentation on heat capture and latitudinal distribution. Curr. Biol. 28, 2657-2664.e3 (2018).
Sibilia, C. D. et al. Thermal Physiology and Developmental Plasticity of Pigmentation in the Harlequin Bug (Hemiptera: Pentatomidae). J. Insect Sci. 18, (2018).
Jong, P., Gussekloo, S. & Brakefield, P. Differences in thermal balance, body temperature and activity between non-melanic and melanic two-spot ladybird beetles (Adalia bipunctata) under controlled conditions. J. Exp. Biol. 199, 2655–2666 (1996).
Zverev, V., Kozlov, M. V., Forsman, A. & Zvereva, E. L. Ambient temperatures differently influence colour morphs of the leaf beetle Chrysomela lapponica: Roles of thermal melanism and developmental plasticity. J. Therm. Biol 74, 100–109 (2018).
Watt, W. B. Adaptive significance of pigment polymorphisms in Colias butterflies, II. Thermoregulation and photoperiodically controlled melanin variation in Colias eurytheme. Proc. Natl. Acad. Sci. USA 63, 767–74 (1969).
Kuyucu, A. C., Sahin, M. K. & Caglar, S. S. The relation between melanism and thermal biology in a colour polymorphic bush cricket, Isophya rizeensis. J. Therm. Biol. 71, 212–220 (2018).
Köhler, G. & Schielzeth, H. Green-brown polymorphism in alpine grasshoppers affects body temperature. Ecol. Evol. 10, 441–450 (2020).
Willmer, P. G. & Unwin, D. M. Field analyses of insect heat budgets: Reflectance, size and heating rates. Oecologia 50, 250–255 (1981).
Pecsenye, K., Bokor, K., Lefkovitch, L. P., Giles, B. E. & Saura, A. Enzymatic responses of Drosophila melanogaster to long- and short-term exposures to ethanol. Mol. Gen. Genet. 255, 258–268 (1997).
De Castro, S., Peronnet, F., Gilles, J.-F., Mouchel-Vielh, E. & Gibert, J.-M. bric à brac (bab), a central player in the gene regulatory network that mediates thermal plasticity of pigmentation in Drosophila melanogaster. PLoS Genet. 14, e1007573 (2018).
Cooley, A. M., Shefner, L., McLaughlin, W. N., Stewart, E. E. & Wittkopp, P. J. The ontogeny of color: Developmental origins of divergent pigmentation in Drosophila americana and D. novamexicana. Evol. Dev. 14, 317–25 (2012).
John, A. V., Sramkoski, L. L., Walker, E. A., Cooley, A. M. & Wittkopp, P. J. Sensitivity of allelic divergence to genomic position: Lessons from the Drosophila tan Gene. G3 (Bethesda) (2016) doi:https://doi.org/10.1534/g3.116.032029.
Liu, Y. et al. Changes throughout a genetic network mask the contribution of hox gene evolution. Curr. Biol. 29, 2157-2166.e6 (2019).
David, J. R. et al. Evolution of assortative mating following selective introgression of pigmentation genes between two Drosophila species. Ecol. Evol. 12, e8821 (2022).
Wittkopp, P. J., True, J. R. & Carroll, S. B. Reciprocal functions of the Drosophila yellow and ebony proteins in the development and evolution of pigment patterns. Development 129, 1849–1858 (2002).
Davis, J. S. & Moyle, L. C. Desiccation resistance and pigmentation variation reflects bioclimatic differences in the Drosophila americana species complex. BMC Evol. Biol. 19, 204 (2019).
Nagy, O. et al. Correlated evolution of two copulatory organs via a single cis-regulatory nucleotide change. Curr. Biol. 28, 3450-3457.e13 (2018).
Lachaise, D. et al. Evolutionary novelties in islands: Drosophila santomea, a new melanogaster sister species from São Tomé. Proc. Biol. Sci. 267, 1487–1495 (2000).
Haldane, J. B. S. Sex ratio and unisexual sterility in hybrid animals. J. Gen. 12, 101–109 (1922).
Turissini, D. A. & Matute, D. R. Fine scale mapping of genomic introgressions within the Drosophila yakuba clade. PLoS Genet. 13, e1006971 (2017).
Hoffmann, A. A. Physiological climatic limits in Drosophila: Patterns and implications. J. Exp. Biol. 213, 870–880 (2010).
Sunaga, S., Akiyama, N., Miyagi, R. & Takahashi, A. Factors underlying natural variation in body pigmentation of Drosophila melanogaster. Genes Genet. Syst. 91, 127–137 (2016).
Rajpurohit, S. & Schmidt, P. S. Latitudinal pigmentation variation contradicts ultraviolet radiation exposure: A case study in Tropical Indian Drosophila melanogaster. Front. Physiol. 10, 84 (2019).
Bergland, A. O., Behrman, E. L., O’Brien, K. R., Schmidt, P. S. & Petrov, D. A. Genomic evidence of rapid and stable adaptive oscillations over seasonal time scales in Drosophila. PLoS Genet. 10, e1004775 (2014).
Rudman, S. M. et al. Direct observation of adaptive tracking on ecological time scales in Drosophila. Science 375, eabj7484 (2022).
Fabian, D. K. et al. Genome-wide patterns of latitudinal differentiation among populations of Drosophila melanogaster from North America. Mol. Ecol. 21, 4748–4769 (2012).
Zeuss, D., Brandl, R., Brändle, M., Rahbek, C. & Brunzel, S. Global warming favours light-coloured insects in Europe. Nat. Commun. 5, 3874 (2014).
Brakefield, P. M. & de Jong, P. W. A steep cline in ladybird melanism has decayed over 25 years: A genetic response to climate change?. Heredity (Edinb) 107, 574–578 (2011).
Zvereva, E. L., Hunter, M. D., Zverev, V., Kruglova, O. Y. & Kozlov, M. V. Climate warming leads to decline in frequencies of melanic individuals in subarctic leaf beetle populations. Sci. Total Environ. 673, 237–244 (2019).
Balanyá, J., Oller, J. M., Huey, R. B., Gilchrist, G. W. & Serra, L. Global genetic change tracks global climate warming in Drosophila subobscura. Science 313, 1773–1775 (2006).
Acknowledgements
We thank Virginie Courtier-Orgogozo, the late Jean David and Jorge Vieira for drosophila stocks. We thank CNRS Mission for Transversal and Interdisciplinary Initiatives (MITI) for funding this research and LF PhD.
Funding
The project was financed by CNRS Mission for Transversal and Interdisciplinary Initiatives (MITI). LF PhD is funded by MITI. LF, SM, MR and AV acknowledge partial funding by the chair program Mathematical Modelling and Biodiversity (Ecole Polytechnique, Museum National d’Histoire Naturelle, Veolia Environment, Fondation X).
Author information
Authors and Affiliations
Contributions
Conception and design of the work: J.M.G.; acquisition of data: L.F. and J.M.G.; analysis and interpretation of data L.F., J.M.G., A.V., L.M.C., S.M., M.R. and P.C.; drafting of the manuscript: L.F., A.V. and J.M.G., with comments and suggestions from L.M.C., S.M., M.R. and P.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of the Article contained an error in the Author Information section, where the authors Laurent Freoa and Luis‑Miguel Chevin were incorrectly tagged as authors who jointly supervised the work.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Freoa, L., Chevin, LM., Christol, P. et al. Drosophilids with darker cuticle have higher body temperature under light. Sci Rep 13, 3513 (2023). https://doi.org/10.1038/s41598-023-30652-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30652-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.