Abstract
We compared the impact of treatment strategies on postoperative complications and prognosis between robot-assisted radical prostatectomy (RARP) plus extended pelvic lymph-node dissection (ePLND) and RARP plus neoadjuvant chemohormonal therapy (NCHT) without ePLND. We retrospectively evaluated 452 patients with high-risk prostate cancer (defined as any one of prostate-specific antigen ≥ 20 ng/mL, Gleason score 8–10, or cT2c–3) who were treated with RARP between January 2012 and February 2021. The patients were divided into two groups: RARP with ePLND (ePLND group) and NCHT plus RARP without ePLND (NCHT group). We compared the complication rate (Clavien–Dindo classification), biochemical recurrence-free survival, and castration-resistant prostate cancer (CRPC)-free survival between the groups. We performed multivariable Cox regression analysis using inverse probability weighting (IPTW) methods to assess the impact of the different treatments on prognosis. There were 150 and 302 patients in the ePLND and NCHT groups, respectively. The postoperative complication rate was significantly higher in the ePLND group than in the NCHT group (P < 0.001). IPTW-adjusted biochemical recurrence-free survival and CRPC-free survival were significantly higher in the NCHT group than in the ePLND group (hazard ratio [HR] 0.29, P < 0.001, and HR 0.29, P = 0.010, respectively). NCHT plus RARP without ePLND may reduce the risk of postoperative complications compared with ePLND during RARP. The impact of treatment strategies on oncological outcomes needs further studies.
Similar content being viewed by others
Introduction
Prostate cancer (PC) is the most common malignancy in men in Western countries and in Japan. Although radical prostatectomy (RP) is one of the standards of care in localized PC1, the optimal treatment for high-risk PC remains unclear. The current European Association of Urology (EAU) guidelines recommend extended pelvic lymph-node dissection (ePLND) for intermediate- and high-risk disease for optimal staging2, while those of the American Urological Association (AUA) do not make any recommendation for ePLND because the evidence supporting its therapeutic benefit is lacking3. Recently, two randomized controlled trials compared extended and limited PLND. They found that ePLND did not improve biochemical recurrence-free survival (BCR-FS) compared with limited PLND4,5. Furthermore, ePLND may increase postoperative complications. Therefore, the indications for ePLND, the optimal extent of lymph-node dissection, and the balance between the benefits and harms of treatment must be carefully considered6,7.
Neoadjuvant hormonal therapy (NHT) followed by RP is an alternative option that uses androgen deprivation therapy (ADT) or ADT plus bicalutamide to treat high-risk PC. However, there is insufficient evidence to assess the possible effect of NHT in cases of high-risk PC8,9,10. Our previous study on patients with high-risk PC demonstrated that neoadjuvant chemohormonal therapy (NCHT) plus open RP using ADT and low-dose estramustine phosphate (EMP) significantly improved BCR-FS compared with patients treated with open RP and ePLND11. Thus, an alternative option might be NCHT using ADT plus a low dose of EMP. However, the oncological outcomes of robot-assisted radical prostatectomy (RARP) plus ePLND versus NCHT plus RARP without ePLND have yet to be compared. To this end, we compared the impact of treatment strategies on postoperative complications and oncological outcomes between ePLND (ePLND group) and NCHT without ePLND (NCHT group) in patients with high-risk PC treated with RARP.
Results
Baseline characteristics
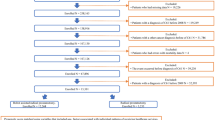
We used the Ageo-Hirosaki database to retrospectively identify 1997 patients treated with RARP, of whom 919 were identified as PC patients with high-risk disease. After applying the exclusion criteria, there were 150 and 302 patients in the ePLND and NCHT groups, respectively (Fig. 1a). The median age and follow-up periods were 69 (IQR 65, 72) years old and 62 (IQR 41, 88) months, respectively, in this cohort. There was a significant difference between the ePLND and NCHT groups in PSA, Gleason score, and clinical T stage at baseline (Table 1). The median duration of NCHT in the NCHT group was 8.7 (IQR 7.1, 10) months. In the ePLND group, 33 (22%) patients underwent NHT with either ADT alone or ADT plus bicalutamide (Table 1). The median risk of lymph-node invasion in the NCHT and ePLND groups was 16% (IQR 8%, 38%) and 41% (IQR 20%, 70%) respectively (P < 0.001). as determined by the Briganti nomogram12.
Patient selection and postoperative complications. (a) Patient selection. (b) Comparison of postoperative complications. ADT androgen deprivation therapy, EAU European Association of Urology, EMP estramustine phosphate, ePLND extended pelvic lymph-node dissection, GS Gleason score, NCHT neoadjuvant chemohormonal therapy, PSA prostate-specific antigen, RARP robot-assisted radical prostatectomy.
Surgical and pathological outcomes
The median operation time and blood loss were significantly different between the ePLND group (289 min and 150 g, respectively) and the NCHT group (169 min and 25 g, respectively). Additionally, the pathological tumor stage was also significantly different between the groups (Table 1). The median number of removed lymph-nodes and the rate of positive nodes in patients in the NCHT group who underwent limited PLND (n = 135/302, 44.7%) were 4 (IQR 3, 7) and 0.74% (n = 1/135), respectively. The median number of removed lymph-nodes and the rate of positive nodes in the ePLND group (n = 150) were 23 (IQR 17, 28) and 28.7% (n = 43/150), respectively.
The rate of postoperative complications of any grade and the rate of major complications in the ePLND group was significantly higher (36.0% and 7.3%, respectively) compared with the NCHT group (11.5% and 1.7%, respectively) (Fig. 1b, Table 2). The multivariable logistic regression analysis for any grade complications shows that ePLND is the independent factor for increased risk of postoperative major complications (OR 5.06, P < 0.001, Table 3).
Oncological outcomes
The inverse probability weighting (IPTW)-adjusted analysis showed that BCR-FS was significantly lower in the ePLND group than that in the NCHT group (HR 0.29, P < 0.001, Fig. 2a). Similarly, the IPTW-adjusted castration-resistant prostate cancer-free survival (CRPC-FS) was significantly lower in the ePLND group than in the NCHT group (HR 0.29, P = 0.017, Fig. 2b).
Comparison of oncological outcomes between ePLND during RARP and NCHT plus RARP without ePLND. (a) The Cox regression analysis using inverse probability weighting (IPTW) methods of biochemical recurrence-free survival (BCR-FS). (b) The Cox regression analysis using IPTW methods of castration-resistant prostate cancer-free survival (CRPC-FS). (c) Unadjusted analysis of biochemical recurrence-free survival (BCR-FS). (d) Unadjusted analysis of castration-resistant prostate cancer-free survival (CRPC-FS). ePLND extended pelvic lymph-node dissection, GS Gleason score, NCHT neoadjuvant chemohormonal therapy, PSA prostate-specific antigen, RARP robot-assisted radical prostatectomy.
The unadjusted BCR-FS rate was significantly higher in the patients with NCHT than those in the ePLND alone (P < 0.001, Fig. 2c). The unadjusted CRPC-FS rate was significantly higher in the patients with NCHT than those in the ePLND alone (P = 0.002, Fig. 2d). Oncological outcomes between treatment with ePLND alone (n = 117) and neoadjuvant ADT therapy plus ePLND (n = 33) were not significantly different for BCR-FS (Fig. 2c, P = 0.099) and CRPC-FS (Fig. 2d, P = 0.524).
EMP-related toxicities
We evaluated 302 patients who were treated with neoadjuvant EMP + ADT in this study. Of those, we observed 58 (19.2%) patients with EMP-related toxicities. The major toxicity was low grade (grade 1–2) liver dysfunction (7.0%) followed by gastrointestinal symptoms (6.3%). We had severe cardiovascular events (suspicion of angina pectoris) in 1 patient (0.3%) that required short-term hospitalization (Table 4).
Furthermore, we additionally evaluated all patients (n = 533) who were treated with neoadjuvant EMP + ADT in our database. Of those, we observed 105 (19.7%) patients with EMP-related toxicities. The major toxicity was low grade (grade 1–2) gastrointestinal symptoms (7.3%) followed by liver dysfunction (6.2%). We had severe cardiovascular events in 3 patients (0.6%) that required short-term hospitalization (Table S1).
Discussion
We compared the impact on prognosis between ePLND and NCHT (ADT + EMP) without ePLND in high-risk PC treated with RARP. NCHT without ePLND was significantly associated with a lower rate of postoperative complications (difference of 24.5% across all grades), prolonged BCR-FS, and prolonged CRPC-FS compared with ePLND. However, the analyses of CRPC progression were underpowered because of the small number of events (21 and 8 events, respectively). These limitations were imposed by the retrospective nature of our study. Furthermore, it is well-known that the role of NCHT in the context of PC is still highly debated and many prospective studies have failed to demonstrate its efficacy on oncological outcomes. Thus, our data should serve as a basis to perform a clinical trial and needs to be validated by prospective studies.
There have been numerous attempts to treat cancer using lymph-node dissection. However, several randomized controlled trials did not show any therapeutic benefits of PLND in bladder, esophageal, gastric, pancreatic, lung, breast, and ovarian cancers, except for colorectal cancer13,14,15,16,17,18,19,20. The benefit of PLND during RP had been discussed in retrospective studies, and it was reported that the diagnostic benefit of ePLND is its ability to identify twice as many nodal metastases as limited PLND and may help to cure nodal micrometastases21,22,23,24,25. The 2021 EAU guidelines strongly recommend ePLND for intermediate-risk disease with an estimated risk for positive lymph-nodes ≥ 5% and all high-risk diseases for optimal nodal staging2. Conversely, the 2017 AUA guidelines do not recommend ePLND because of the lack of evidence supporting its therapeutic benefit3. A recent systematic review including 44 retrospective studies (n = 275,269) found no significant difference in survival benefit between any form of PLND and no PLND26. Furthermore, two randomized controlled trials did not demonstrate survival benefits of ePLND compared with limited PLND4,5. Currently, there is no level 1 evidence confirming the survival benefit of ePLND. It is hoped that an ongoing trial comparing ePLND with no PLND (NCT03921996) will provide information on whether or not PLND can be omitted during RP.
It is unclear whether extended PLND provides an oncologic benefit over limited PLND4,5. A previous randomized study including 1440 patients (NCT01407263) suggested that extended PLND (the median number of nodes removed: 14, IQR 10–20) did not improve biochemical recurrence-free survival over limited PLND (the median number of nodes removed: 12, IQR 8–17) for men with clinically localized prostate cancer5. However, the number of lymph-nodes removed between the two groups in this study may be too close for a proper comparison. Furthermore, little is known about the significance of PLND in the case of neoadjuvant therapy. Some of the patients in the NCHT group underwent limited PLND in this study. Based on the results of our previous study27, we included both localized and no dissection in the neoadjuvant group. However, the mixture of limited and no PLND in the neoadjuvant group is a limitation of this study. The appropriate extent of dissection in neoadjuvant therapy needs further investigation.
Although there is insufficient evidence to show a survival benefit of NHT in the high-risk PC population8,9,10, a strategy of intensive therapy using second-generation androgen receptor axis-targeting agents (ARATs) might be reasonable for high-risk nonmetastatic PC instead of ePLND. A recent phase III randomized trial (STAMPEDE) showed a survival benefit of the combination of radiotherapy (99% and 71% of cN0 and cN1 patients received local radiotherapy, respectively) and ARATs (abiraterone acetate with or without enzalutamide) in men with nonmetastatic PC28. However, ARATs are not approved for nonmetastatic PC in Japan at present. Accordingly, our NCHT strategy for high-risk PC may be a promising option because of its shorter operating time and lower amount of blood loss, low rate of postoperative complications, and its contribution to survival. However, the toxicity of EMPs must be noted, particularly because it can cause liver dysfunction and gastrointestinal symptoms. We experienced discontinuations due to the grade 1 or 2 toxicity of EMP (313.4 mg/day) in 3.2% of cases (17/533) in the entire cohort. Although no thrombosis was observed in this study, thrombotic events with estrogenic agents are definitely a concern, even at half doses. Therefore, not all high-risk patients are candidates for NCHT using EMP because of the intensive efficacy of estrogen and nitrogen mustard. The ongoing phase III, randomized, double-blind, placebo-controlled, multicenter PROTEUS trial (NCT03767244)29 might change the treatment paradigm of high-risk PC because it is evaluating the efficacy (dual primary endpoints of pathological complete response rate and metastasis-free survival) and safety of apalutamide plus ADT compared with placebo plus ADT before and after RP in patients with localized or locally advanced high-risk PC. However, the results will only be available in 2027.
The present study has several limitations. First, the limited sample size, retrospective design, and background differences between groups prevent us from drawing a definitive conclusion. Second, the follow-up period was shorter in the ePLND group. Third, our results cannot be generalized to non-Asian populations because of racial differences. Finally, a direct comparison of NCHT with ePLND on oncological outcomes is inconclusive based on retrospective data. The statistical methodology (IPTW) could not resolve all unmeasured confounders and residual confounding may still play a role. Our data should serve as a basis to perform a clinical trial.
In conclusion, NCHT plus RARP without ePLND may reduce the risk of postoperative complications in comparison with ePLND during RARP. The impact of treatment strategies on oncological outcomes needs further prospective studies.
Methods
Ethics statement
This study was performed in accordance with the ethical standards established by the Declaration of Helsinki and was approved by the Ethics Review Board of Hirosaki University Graduate School of Medicine and all hospitals (authorization number: 2021-2419).
Study design and participants
We used the Hirosaki and Ageo database to retrospectively evaluate 1997 patients with PC who underwent RARP at Hirosaki University Hospital and Ageo Central General Hospital between January 2012 and February 2021. Information on the patients’ background; disease status; and surgical, pathological, and oncological outcomes were obtained from their medical records. All tumors were staged according to the 2017 American Joint Committee on Cancer staging manual30. The inclusion criteria were as follows: (1) patients with a high-risk disease defined as any one of prostate-specific antigen (PSA) ≥ 20 ng/mL, a Gleason score 8–10, or cT2c–4; (2) clinically negative lymph-node metastases (cN0); and (3) patients who underwent ePLND without NCHT or NCHT without ePLND. The indication, duration, and type of NHT or NCHT depended on institutional protocols. The risk of lymph-node invasion was evaluated using the Briganti nomogram with a cutoff value of ≥ 5% in patients with ePLND12. The exclusion criteria were as follows: (1) patients who underwent open RP, (2) metastatic disease, (3) missing data on initial PSA level, biopsy Gleason score, and cT stage, and (4) missing survival data and/or follow-up duration.
Treatment procedures
The RARP and ePLND were performed by expert surgeons. The ePLND template included the obturator, external iliac, and internal iliac regions bilaterally. The ePLND group included some patients treated with NHT plus ePLND for very high-risk disease. The patients in the NCHT group received ADT (luteinizing hormone-releasing hormone agonist or gonadotropin-releasing hormone antagonist) plus low-dose EMP (313.4 mg/day) for 6–9 months before RARP, as previously described11. The patients in the NCHT group underwent limited PLND (removal of the bilateral obturator node chains) or no PLND.
Follow-up protocol
After surgery, the serum levels of PSA and testosterone were tested for all patients every 3 months. Adjuvant ADT or radiation therapy was not routinely administered. The date of BCR was defined as the date when the serum PSA level exceeded 0.2 ng/mL. If the PSA level did not decrease to < 0.2 ng/mL after the surgery, the date of RARP was defined as the date of BCR. Castration-resistant prostate cancer (CRPC) was defined by the Prostate Cancer Working Group 2 or identification of clinical progression by attending physician. CRPC-free survival (CRPC-FS) was defined from the time of surgery to the CRPC progression or any cause of death.
Outcomes
We divided the patients into two groups: RARP with ePLND (ePLND group) and NCHT plus RARP without ePLND (NCHT group). We compared the complication rate (Clavien–Dindo classification), BCR-FS and CRPC-FS between the groups. We also assessed the impact of treatment on prognosis using multivariable Cox regression analysis via inverse probability weighting (IPTW) methods. Overall survival was not compared between the groups due to the very small events (n = 8).
EMP-related toxicities
We evaluated EMP-related toxicities to estimate the balance between benefit and harm. We included all patients who were treated with neoadjuvant EMP + ADT in our database (n = 533). Toxicity was evaluated using Common Terminology Criteria for Adverse Events ver. 5.0.
Statistical analysis
Statistical analyses were performed using the Microsoft Excel (Microsoft Corp., Redmond, WA, USA), Bell curve in Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan) and GraphPad Prism 7.00 (GraphPad Software, San Diego, CA, USA) and R v4.0.2 (The R Foundation for Statistical Computing, Vienna, Austria) software. Categorical variables were compared using Fisher exact test or χ2 test. Quantitative variables were expressed as medians with interquartile ranges (IQRs). The differences between the groups were compared using Student t-test or Mann–Whitney U test. Odds ratio (OR) with 95% confidence interval (95% CI) were obtained from the multivariable logistic regression analysis for any grade postoperative complications. BCR-FS and CRPC-FS were compared using the Kaplan–Meier method. Hazard ratios (HRs) with 95%CI were obtained from the multivariable Cox regression analysis using PTW methods. The variables included in the IPTW-adjusted model were age (continuous), PSA (continuous), GS (6–10), cT stage (1–3), and % of positive core (7–100%). P values < 0.05 were considered statistically significant.
Ethical approval
This retrospective, multicenter study was performed per the ethical standards of the Declaration of Helsinki and was approved by the Ethics Review Board of Hirosaki University School of Medicine (authorization No. 2021-2419) and all hospitals.
Patient consent statement
An informed consent was obtained from all participants via written, verbal, and/or disclosure of study information.
Data availability
Data are available for bona fide researchers who request it from the authors.
References
Hyndman, M. E., Mullins, J. K. & Pavlovich, C. P. Pelvic node dissection in prostate cancer: Extended, limited, or not at all? Curr. Opin. Urol. 20, 211–217. https://doi.org/10.1097/MOU.0b013e328338405d (2010).
Mottet, N. et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 79, 243–262. https://doi.org/10.1016/j.eururo.2020.09.042 (2021).
Sanda, M. G. et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part II: Recommended approaches and details of specific care options. J. Urol. 199, 990–997. https://doi.org/10.1016/j.juro.2018.01.002 (2018).
Lestingi, J. F. P. et al. Extended versus limited pelvic lymph node dissection during radical prostatectomy for intermediate- and high-risk prostate cancer: Early oncological outcomes from a randomized phase 3 trial. Eur. Urol. 79, 595–604. https://doi.org/10.1016/j.eururo.2020.11.040 (2021).
Touijer, K. A. et al. Limited versus extended pelvic lymph node dissection for prostate cancer: A randomized clinical trial. Eur. Urol. Oncol. 4, 532–539. https://doi.org/10.1016/j.euo.2021.03.006 (2021).
Fujimoto, N., Shiota, M., Tomisaki, I., Minato, A. & Yahara, K. Reconsideration on clinical benefit of pelvic lymph node dissection during radical prostatectomy for clinically localized prostate cancer. Urol. Int. 103, 125–136. https://doi.org/10.1159/000497280 (2019).
Cacciamani, G. E. et al. Impact of pelvic lymph node dissection and its extent on perioperative morbidity in patients undergoing radical prostatectomy for prostate cancer: A comprehensive systematic review and meta-analysis. Eur. Urol. Oncol. 4, 134–149. https://doi.org/10.1016/j.euo.2021.02.001 (2021).
Tosco, L. et al. The survival impact of neoadjuvant hormonal therapy before radical prostatectomy for treatment of high-risk prostate cancer. Prostate Cancer Prostatic Dis. https://doi.org/10.1038/pcan.2017.29 (2017).
Gleave, M. E. et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: Biochemical and pathological effects. J. Urol. 166, 500–506 (2001).
Soloway, M. S. et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxM0 prostate cancer: 5-year results. J. Urol. 167, 112–116. https://doi.org/10.1016/S0022-5347(05)65393-1 (2002).
Narita, T. et al. The impact of extended lymph node dissection versus neoadjuvant therapy with limited lymph node dissection on biochemical recurrence in high-risk prostate cancer patients treated with radical prostatectomy: A multi-institutional analysis. Med. Oncol. 34, 1. https://doi.org/10.1007/s12032-016-0859-0 (2017).
Briganti, A. et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: The essential importance of percentage of positive cores. Eur. Urol. 61, 480–487. https://doi.org/10.1016/j.eururo.2011.10.044 (2012).
Gschwend, J. E. et al. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: Survival results from a prospective, randomized trial. Eur. Urol. 75, 604–611. https://doi.org/10.1016/j.eururo.2018.09.047 (2019).
Hulscher, J. B. et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N. Engl. J. Med. 347, 1662–1669. https://doi.org/10.1056/NEJMoa022343 (2002).
Bonenkamp, J. J. et al. Extended lymph-node dissection for gastric cancer. N. Engl. J. Med. 340, 908–914. https://doi.org/10.1056/nejm199903253401202 (1999).
Riall, T. S. et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma—Part 3: Update on 5-year survival. J. Gastrointest. Surg. 9, 1191–1204. https://doi.org/10.1016/j.gassur.2005.08.034 (2005).
Izbicki, J. R. et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: Results of a prospective randomized trial. Ann. Surg. 227, 138–144. https://doi.org/10.1097/00000658-199801000-00020 (1998).
Fisher, B. et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N. Engl. J. Med. 347, 567–575. https://doi.org/10.1056/NEJMoa020128 (2002).
Harter, P. et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N. Engl. J. Med. 380, 822–832. https://doi.org/10.1056/NEJMoa1808424 (2019).
Tsukamoto, S. et al. Long-term follow-up of the randomized trial of mesorectal excision with or without lateral lymph node dissection in rectal cancer (JCOG0212). Br. J. Surg. 107, 586–594. https://doi.org/10.1002/bjs.11513 (2020).
Touijer, K. et al. Standard versus limited pelvic lymph node dissection for prostate cancer in patients with a predicted probability of nodal metastasis greater than 1%. J. Urol. 178, 120–124. https://doi.org/10.1016/j.juro.2007.03.018 (2007).
Allaf, M. E., Palapattu, G. S., Trock, B. J., Carter, H. B. & Walsh, P. C. Anatomical extent of lymph node dissection: Impact on men with clinically localized prostate cancer. J. Urol. 172, 1840–1844. https://doi.org/10.1097/01.ju.0000140912.45821.1d (2004).
Blas, L. et al. Validation of models predicting lymph node involvement probability in patients with prostate cancer. Int. J. Urol. https://doi.org/10.1111/iju.14802 (2022).
Claps, F. et al. Indocyanine green guidance improves the efficiency of extended pelvic lymph node dissection during laparoscopic radical prostatectomy. Int. J. Urol. 28, 566–572. https://doi.org/10.1111/iju.14513 (2021).
Shimbo, M., Endo, F., Matsushita, K. & Hattori, K. Impact of indocyanine green-guided extended pelvic lymph node dissection during robot-assisted radical prostatectomy. Int. J. Urol. 27, 845–850. https://doi.org/10.1111/iju.14306 (2020).
Fossati, N. et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: A systematic review. Eur. Urol. 72, 84–109. https://doi.org/10.1016/j.eururo.2016.12.003 (2017).
Iwamura, H. et al. Significance of pelvic lymph node dissection during radical prostatectomy in high-risk prostate cancer patients receiving neoadjuvant chemohormonal therapy. Sci. Rep. 12, 9675. https://doi.org/10.1038/s41598-022-13651-x (2022).
Attard, G. et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: A meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet 399, 447–460. https://doi.org/10.1016/s0140-6736(21)02437-5 (2022).
Taplin, M.-E. et al. PROTEUS: A randomized, double-blind, placebo (PBO)-controlled, phase 3 trial of apalutamide (APA) plus androgen deprivation therapy (ADT) versus PBO plus ADT prior to radical prostatectomy (RP) in patients with localized high-risk or locally advanced prostate cancer (PC). J. Clin. Oncol. 37, 5100. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS5100 (2019).
Paner, G. P. et al. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur. Urol. 73, 560–569. https://doi.org/10.1016/j.eururo.2017.12.018 (2018).
Acknowledgements
The authors thank Yuki Fujita, Yukie Nishizawa, Satomi Sakamoto, and Atsuko Sakuraba for their invaluable support with data collection.
Funding
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grants 19H05556 (C.O.) and 20K09517 (S.H.).
Author information
Authors and Affiliations
Contributions
S.H. had full access to all study data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: S.H. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: S.H. and T.O. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: S.H. Administrative, technical, or material support: all authors. Study supervision: C.O.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oishi, T., Hatakeyama, S., Tabata, R. et al. Comparison of neoadjuvant chemohormonal therapy vs. extended pelvic lymph-node dissection in high-risk prostate cancer treated with robot-assisted radical prostatectomy. Sci Rep 13, 3436 (2023). https://doi.org/10.1038/s41598-023-30627-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30627-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.