Abstract
Nitroepoxides were introduced as efficient substrates for the one-pot three-component synthesis of 2-iminothiazoles under catalyst-free conditions. Reaction of amines, isothiocyanates, and nitroepoxides in THF at 10–15 °C afforded corresponding 2-iminothiazoles in high to excellent yields. The reaction proceeds via the in situ formation of thiourea from an amine and an isothiocyanate, followed by nitroepoxide ring opening with the sulfur of thiourea, cyclization reaction, and dehydration cascade. The structures of products were confirmed by IR, NMR, HRMS analyses and X-ray crystallography.
Similar content being viewed by others
Introduction
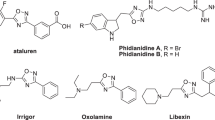
Nitrogen and sulfur containing heterocyclic compounds such as thiazoles are of great practical and fundamental interest. Development of novel synthetic strategies for the synthesis of thiazole derivatives with various substitution pattern have received considerable attention in the field of pharmaceutical and biomedical chemistry1,2,3,4,5. Particularly, the 2-imino-1,3-thiazolines are available in many biologically active compounds with antimicrobial, anticancer, anti-inflammatory, antihistaminic, antihypertensive, hypnotic, and anticonvulsant activities6,7,8,9,10,11,12,13. In addition, they were applied for the identification of human cells with positive myeloperoxidase reactivity14. For example 4-methyl-3-H-thiazoline derivative, PS-028, is a potent and selective GPIIb/IIIa receptor antagonist (Fig. 1)15,16. Also, KHG22394 containing 2-imino-1,3-thiazoline moiety is known as a melanin production inhibitor in a dose-dependent manner while not directly inhibit tyrosinase as a rate-limiting melanogenic enzyme and also applied for the production of skin whitening cream (Fig. 1)17. Another example with thiazoline core is Pifithrin, which is widely used as a p53 transcription inhibitor in cells and could restrict cellular apoptosis (Fig. 1)18,19. Furthermore, thiazolines have been found applications as acaricides, insecticides, and plant growth regulators in agriculture20,21.
Nitroepoxides, which can be simply prepared from nitroalkenes by epoxidation reaction with H2O2/NaOH22, are efficient intermediates in synthetic organic chemistry and were applied extensively as synthons of vicinal double electrophilic compounds such as α-diones and α-haloketones for the synthesis of valuable heterocyclic compounds23. Although the synthesis of nitroepoxides and their ring opening with heteroatom-centered nucleophiles such as sodium phenoxides, sodium thiophenolates, and amines came back to around 50 years ago24, but research on these compounds was accelerated in recent 15 years. Diversities of mono nucleophiles such as potassium xanthates, dithiocarbamic acids, sodium aryl sulfonates and sodium bisulfite were added to nitroepoxides to obtain the corresponding α-heterosubstituted ketones23,25. In addition, bis-nucleophiles such as N-alkyl(thio)ethanolamine26, amidines27, diamines28, S-alkyl dithiocarbamates23 and etc.29,30,31 have been utilized in the reaction with nitroepoxides for the synthesis of valuable heterocyclic compounds including imidazoles, 1,3-thiazoles, benzodiazepines, thiazoline-2-thiones, and pyrazines.
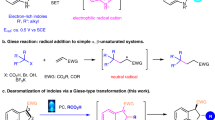
Multicomponent reactions (MCRs) provide simple access to complex molecules in a one-pot. MCRs have several advantages such as generation of several bonds in a single operation, avoiding the need for isolation and purification of reaction intermediates, saving in time, materials, solvents and energy32,33,34,35,36,37,38. For this purpose, multicomponent reaction of isothiocyanates with amines in the presence of an electrophile is an efficient strategy for the synthesis of 2-imino-1,3-thiazolines. In this context, Yavari et al. developed a synthetic route for the synthesis of functionalized 2-imino-1,3-thiazoles using tetramethylguanidine, isothiocyanates, and 2-chloro-1,3-dicarbonyls (Scheme 1a)39. Meshram and co-workers investigated the one-pot three-component reaction of amines, phenyl isothiocyanates and β-nitroacrylates in [Hbim]BF4 ionic liquid (Scheme 1b)40. Furthermore, Raja et al. reported the reaction of aryl isothiocyanates, phenacyl bromides, and aryl amines in ethanol as green reaction media in 2016 (Scheme 1c)41. Chen and coworkers described the synthesis of 2-imino-1,3-thiazolidines and 2-imino-1,3-thiazolines through the reaction of ionic liquid attached 2-aminobenzimidazoles with isothiocyanates and 1,2-dichloroethane, followed by ionic cleavage with methanolysis reaction and oxidation with Mn(OAc)3 (Scheme 1d)42. In 2017, a one-pot three-component route for the synthesis of 2-aminothiazoles was developed by Guo et al. via the reaction of nitroepoxides with cyanamide and sodium sulfide. The scope of this reaction is limited to the use of nitroepoxides and other components cannot be changed (Scheme 1e)43. Beside multicomponent reactions, condensation reaction of N,Nʹ–disubstituted thiourea with α-haloketones and ring transformation of 2-(thiocyanomethyl) aziridines were also developed for the synthesis of 2-imino-1,3-thiazolines44,45,46,47.
Experimental section
General
All chemicals and solvents were obtained from commercial sources and used as received. The 1H and 13C NMR spectra of products were recorded on a Bruker AMX 300 MHz spectrometers referenced to internal Me4Si at 0.00 ppm. Reaction monitoring was carried out by thin-layer chromatography using TLC silica gel 60 F254 plates. HRMS (High Resolution Mass Spectra) was measured on a THERMO SCIENTIFIC Advantage and a THERMO SCIENTIFIC Exactive instrument equipped with an APCI source in the positive-ion mode. Mass analysis was performed using Agilent Technology (HP); 5973 Network Mass Selective Detector equipped with EI mode and ionization energy of 70 eV. The temperatures of the electronic-impact ion source and MS quadrupole were 230 °C. IR spectra were recorded on a Perkin-Elmer Spectrum RXI FT-IR Spectrometer. Nitroepoxides were prepared according to the literature procedures22,48.
General procedure for the synthesis of 4a-s
In a test tube equipped with magnetic stirrer bar, an isothiocyanate (1.0 mmol, 1 equiv), an amine (1.2 equiv) and THF (4 mL) were mixed for 1 h at room temperature. Progress of the reaction was monitored by TLC. After complete consumption of the isothiocyanate, the reaction temperature was decreased to 10–15 °C and a nitroepoxide (1 equiv) was added and the mixture was stirred at 10–15 °C for 6 h. The solvent was removed under reduced pressure to afford yellow viscous oil. Purification was carried out by recrystallization in a minimum amount of MeOH or by column chromatography using silica gel and n-hexane/EtOAc (7/3) (for compounds 4f., 4 h, and 4i). The structures of products were confirmed by IR, 1H NMR, 13C NMR, Mass/HRMS analyses and X-ray crystallography. Detailed information is available in the “Supporting Information” file.
Results and discussion
In continuation of our interest on the synthesis of N,S-heterocycles and the chemistry of nitroepoxides49,50,51,52, herein we report a novel catalyst-free one-pot three-component route for the synthesis of 2-imino-1,3-thiazolines via nitroepoxide ring opening reaction (Scheme 1f). Reaction of ethylamine 1 with phenyl isothiocyanate 2 and nitroepoxide 3a was considered as a model reaction for optimization of the reaction conditions. Mixing an equimolar amount of ethylamine and 2 in a solvent such as water or DMF for 1 h at room temperature, followed by the addition of nitroepoxide (1 equivalent) and further stirring for 6 h at room temperature afforded a trace amount of corresponding product 4a (Table 1, entries 1 and 3). By decreasing the reaction temperature to 10–15 °C for the second step, the yield was improved to 25% and 18% in water and DMF, respectively (Table 1, entries 2 and 4). By performing the reaction in CH2Cl2 at the same temperature, the yield was improved to 55% (Table 1, entry 5). Using ethanol as reaction solvent increased the yield to 70% (Table 1, entry 6). In addition, using THF as solvent improved the yield to 80% at 10–15 °C for the total time of 7 h (Table 1, entry 7). Interestingly, by using 1.2 equivalent of amine, the yield was improved to 89% (Table 1, entry 8). Prolonging the reaction time to 10 h and overnight didn’t improve the reaction yield (Table 1, entries 9 and 10). It is notable that premixing of ethylamine and phenyl isothiocyanate for 1 h at room temperature before the addition of nitroepoxide is crucial to obtain high yield. Finally, reaction of 1.2 equivalent of an amine with an equivalent of isothiocyanate in THF for 1 h at room temperature, followed by the addition of a nitroepoxide (1 equiv.) and further stirring for 6 h at 10–15 °C was considered as optimal reaction conditions for further derivatization.
With optimized reaction conditions in hand, the generality and scope of this protocol was examined using various amines, isothiocyanates, and nitroepoxides and the results are summarized in Table 2. Diversities of primary aliphatic amines including ethylamine, propylamine, buthylamine, isobuthylamine, benzylamine were applied successfully in this protocol. All aliphatic primary amines afforded high to excellent yields in this protocol. In contrast to aliphatic amines, aromatic amines were not compatible with this protocol. Slightly higher yields were obtained with isothiocyanates with an electron-withdrawing group on the phenyl ring compared to simple phenyl isothiocyanate (for example 4r vs. 4 g). In addition, the nature and position of the substituents on the phenyl ring of nitroepoxides did not have significant impact on the reaction yield. High to excellent yields were obtained with both electron-donating and -withdrawing substituents on the phenyl ring. The structures of products were confirmed using IR, 1H and 13C NMR, and HRMS analyses. Characteristic signal in 1H NMR spectra is related to the methyl group which appeared around 2.2 ppm in all products. In addition, a signal at 158 ppm in 13C NMR was assigned for the carbon of the imine moiety in the products. In principle, two regioisomers may be generated in the reaction of in situ prepared thiourea with nitroepoxide, which is difficult to differentiate by 1H and 13C NMR analyses. For this purpose, to confirm the proposed structure for the products, the structure of 4p was elucidated using single-crystal X-ray analysis. ORTEP representations of 4p is shown in Fig. 2 (CCDC no. 217830; for details of the crystal structure data and refinement of 4p see the Supporting Information). X-ray analysis revealed that the configuration around the imine bond is Z, in which the substituent on the nitrogen of imine is in the same side with sulfur atom in the ring.
ORTEP representation of the structure 4p. The figure was drawn by DIAMOND (https://www.crystalimpact.com/diamond/)53.
To propose a reasonable mechanism, two control experiments were carried out. Reaction of phenylisothiocyanate 2 with isobutyl amine was performed in THF for 1 h and the corresponding thiourea 5 was separated in 95% yield and characterized by 1H NMR analysis (See supporting information for 1H NMR spectra) (Scheme 2a). In continue, the reaction of freshly prepared thiourea 5 with nitroepoxide was carried out and the corresponding 2-iminothiazole 4e was obtained in 90% isolated yield (Scheme 2b). According to these findings and literature reports27,28,40,41, a proposed mechanism for this reaction is depicted in Scheme 2c. Initially, reaction of amine with phenyl isothiocyanate furnish the corresponding N,N’-disubstituted thiourea A, which undergoes nucleophilic addition to nitroepoxide by sulfur atom to produce intermediate B. Elimination of nitrous acid from B affords the intermediate C. Then, intramolecular cyclization via the addition of aliphatic nitrogen to the carbonyl group in intermediate C provides the intermediate D, which undergoes dehydration reaction to afford the final structure 4. The nitrogen of aliphatic amine is superior to the nitrogen of aromatic amine for cyclization reaction due to the higher electron density and nucleophilic character.
Conclusion
In conclusion, we have developed an efficient catalyst-free one-pot three-component procedure for the synthesis of 2-imino-1,3-thiazoles in high to excellent yields. The reaction proceeds via the ring-opening of nitroepoxides with in situ prepared thiourea derivatives. By this protocol, it is possible to prepare diversities of 2-imino-1,3-thiazolines with various substitution pattern. The main advantages of this protocol include catalyst-free conditions, simple reaction conditions, high to excellent yields and convenient operations without extra purification.
Data availability
All data generated or analyzed during this study are included in this published article [and its Supplementary Information file].
References
de Santana, T. I. et al. Synthesis, anticancer activity and mechanism of action of new thiazole derivatives. Eur. J. Med. Chem. 144, 874–886. https://doi.org/10.1016/j.ejmech.2017.12.040 (2018).
Ali, S. H. & Sayed, A. R. Review of the synthesis and biological activity of thiazoles. Synth. Commun. 50, 670–700. https://doi.org/10.1080/00397911.2020.1854787 (2020).
Metzger, J. V. In Comprehensive Heterocyclic Chemistry (Ed. Katritzky, A. R. & Rees, C. W.) 235 (1984) and references cited therein.
Ayati, A., Emami, S., Asadipour, A., Shafiee, A. & Foroumadi, A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 97, 699–718. https://doi.org/10.1016/j.ejmech.2015.04.015 (2015).
Rouf, A. & Tanyeli, C. Bioactive thiazole and benzothiazole derivatives. Eur. J. Med. Chem. 97, 911–927. https://doi.org/10.1016/j.ejmech.2014.10.058 (2015).
Morigi, R., Locatelli, A., Leoni, A. & Rambaldi, M. Recent patents on thiazole derivatives endowed with antitumor activity. Rec. Pat. Anti-cancer drug discov. 10, 280–297 (2015).
Vittoria, D. M., Mazzoni, O., Piscopo, E., Caligmano, A. & Bolognese, A. Synthesis and antihistaminic activity of some thiazolidin-4-ones. J. Med. Chem. 35, 2910–2912. https://doi.org/10.1021/jm00093a025 (1992).
Omar, A. M. & Eshba, N. H. Synthesis and biological evaluation of new 2,3-dihydrothiazole derivatives for antimicrobial, antihypertensive, and anticonvulsant activities. J. Pharm. Sci. 73, 1166–1168. https://doi.org/10.1002/jps.2600730837 (1984).
Chaudhary, M., Parmar, S. S., Chaudhari, S. K. & Ramasastry, B. V. CNS depressant activity of pyrimidylthiazolidones and their selective inhibition of NAD-dependent pyruvate oxidation. J. Pharm. Sci. 65, 443–446. https://doi.org/10.1002/jps.2600650336 (1976).
Hassan, H. Y., El-Koussi, N. A. & Farghaly, Z. S. Synthesis and antimicrobial activity of pyridines bearing thiazoline and thiazolidinone moieties. Chem. Pharm. Bull. 46, 863–866. https://doi.org/10.1248/cpb.46.863 (1998).
Turan-Zitouni, G., Sivaci, D. M., Kaplancikli, Z. A. & Ozdemir, A. Synthesis and antimicrobial activity of some pyridinyliminothiazoline derivatives. Farmaco 57, 569–572. https://doi.org/10.1016/S0014-827X(02)01250-8 (2002).
Bonde, C. G. & Gaikwad, N. J. Synthesis and preliminary evaluation of some pyrazine containing thiazolines and thiazolidinones as antimicrobial agents. Bioorg. Med. Chem. 12, 2151–2161. https://doi.org/10.1016/j.bmc.2004.02.024 (2004).
Fritch, P. C. et al. Novel KCNQ2/Q3 agonists as potential therapeutics for epilepsy and neuropathic pain. J. Med. Chem. 53, 887–896. https://doi.org/10.1021/jm901497b (2010).
Bodtke, A., Pfeiffer, W. D., Ahrens, N. & Langer, P. Peroxidase catalyzed formation of azine pigments-a convenient and sensitive method for the identification of human cells with positive myeloperoxidase reactivity. Bioorg. Med. Chem. Lett. 14, 1509–1511. https://doi.org/10.1016/j.bmcl.2004.01.001 (2004).
Manaka, A. et al. 2-Acylimino-3H-thiazoline derivatives: A novel template for platelet GPIIb/IIIa receptor antagonists. Bioorg. Med. Chem. Lett. 11, 1031–1035. https://doi.org/10.1016/S0960-894X(01)00123-8 (2001).
Chavan, A. A. & Pai, N. R. Synthesis and antimicrobial screening of 5-arylidene-2-imino-4-thiazolidinones. ARKIVOC 16, 148–155. https://doi.org/10.3998/ark.5550190.0008.g16 (2007).
Kim, D. S. et al. A new 2-imino-1,3-thiazoline derivative, KHG22394, inhibits melanin synthesis in mouse b16 melanoma cells. Biol. Pharm. Bull. 30, 180–183. https://doi.org/10.1248/bpb.30.180 (2007).
Zhu, J., Singh, M., Selivanova, G. & Peuget, S. Pifithrin-α alters p53 post-translational modifications pattern and differentially inhibits p53 target genes. Sci. Rep. 10, 1049. https://doi.org/10.1038/s41598-020-58051-1 (2020).
Bassi, L. et al. Pifithrin-α, an inhibitor of p53, enhances the genetic instability induced by etoposide (VP16) in human lymphoblastoid cells treated in vitro. Mutat. Res. 499, 163–176. https://doi.org/10.1016/S0027-5107(01)00273-1 (2002).
Nagasaki, F., Yamada, T., Takahashi, E., Kitagawa, Y., & Hatano, R. Jpn. Kokai Tokkyo Koho JP 63250371, 18/10/1988; Chem. Abstr. 110, 192810 (1989).
Hoelzel, H. et al. Ger. DD 258168, 13/07/1988; Chem. Abstr. 111, 2681g (1989).
Ibrahim, M. M., Grau, D., Hampel, F. & Tsogoeva, S. B. α-nitro epoxides in organic synthesis: Development of a one-pot organocatalytic strategy for the synthesis of quinoxalines. Eur. J. Org. Chem. https://doi.org/10.1002/ejoc.201301591 (2014).
Ziyaei Halimehjani, A. & Lotfi Nosood, Y. Synthesis of N, S-heterocycles and dithiocarbamates by the reaction of dithiocarbamic acids and S-alkyl dithiocarbamates with nitroepoxides. Org. Lett. 19, 6748–6751. https://doi.org/10.1021/acs.orglett.7b03501 (2017).
Newman, H. & Angier, R. The preparation and chemistry of α-nitroepoxides. Tetrahedron 26, 825–836. https://doi.org/10.1016/S0040-4020(01)97880-9 (1970).
Badali, E., Rahimzadeh, H., Sharifi, A., Habibi, A. & Ziyaei Halimehjani, A. Nitroepoxide ring opening with thionucleophiles in water: Synthesis of α-xanthyl ketones, β-keto sulfones and β-keto sulfonic acids. Org. Biomol. Chem. 18, 4983–4987. https://doi.org/10.1039/D0OB00941E (2020).
Capel, E., Vidal-Albalat, A., Rodríguez, S. & Gonzalez, F. Preparation of morpholines and benzoxazines starting from nitroepoxides. Synthesis 48, 2572–2580. https://doi.org/10.1055/s-0035-1561466 (2016).
Guo, X. et al. One-pot three-component strategy for functionalized 2-aminoimidazoles via ring opening of α-nitro epoxides. Org. Lett. 17, 1157–1159. https://doi.org/10.1021/acs.orglett.5b00289 (2015).
Vidal-Albalat, A., Rodriguez, S. & Gonzalez, F. V. Nitroepoxides as versatile precursors to 1, 4-diamino heterocycles. Org. lett. 16, 1752–1755. https://doi.org/10.1021/ol500444z (2014).
Zhao, D., Guo, S., Guo, X., Zhang, G. & Yu, Y. Facile, efficient synthesis of polysubstituted thiazoles via α-nitroepoxides and thioureas. Tetrahedron 72, 5285–5289. https://doi.org/10.1016/j.tet.2016.05.010 (2016).
Liu, X., Nie, Z., Shao, J., Chen, W. & Yu, Y. A multi-component synthesis of N-substituted 2-amino-3-cyano pyrroles via ring-opening of nitroepoxides. New J. Chem. 42, 2368–2371. https://doi.org/10.1039/C7NJ04584K (2018).
Ranjbari, M. A. & Tavakol, H. Catalyst-free synthesis of benzofuran derivatives from cascade reactions between nitroepoxides and salicylaldehydes. J. Org. Chem. 86, 4756–4762. https://doi.org/10.1021/acs.joc.1c00143 (2021).
Dömling, A., Wang, W. & Wang, K. Chemistry & biology of multicomponent reactions. Chem. Rev. 112, 3083–3135. https://doi.org/10.1021/cr100233r (2012).
Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 106, 17–89. https://doi.org/10.1021/cr0505728 (2006).
Echemendía, R. et al. Highly stereoselective synthesis of natural-product-like hybrids by an organocatalytic/multicomponent reaction sequence. Angew. Chem. Int. Ed. 54, 7621–7625. https://doi.org/10.1002/anie.201412074 (2015).
Kobayashi, S. New methodologies for the synthesis of compound libraries. Chem. Soc. Rev. 28, 1–15. https://doi.org/10.1039/A707429H (1999).
Wu, X., Lin, H., Dai, F., Hu, R. & Tang, B. Z. Functional polyselenoureas for selective gold recovery prepared from catalyst-free multicomponent polymerizations of elemental selenium. CCS Chem. 2, 191–202. https://doi.org/10.31635/ccschem.020.201900080 (2020).
Zhang, M. & Zeng, X. Metal-free radical thiocyanatosulfonation of terminal alkynes in aqueous medium. Org. Lett. 23(9), 3326–3330. https://doi.org/10.1021/acs.orglett.1c00820 (2021).
Ma, C., Xie, J., Zeng, X., Wei, Z. & Wei, Y. Radical-mediated carboselenation of terminal alkynes under mild conditions. Org. Chem. Front. 9, 4441–4446. https://doi.org/10.1039/D2QO01024K (2022).
Yavari, I., Malekafzali, A. & Seyfi, S. A synthesis of functionalized 2-imino-1,3-thiazoles from tetramethylguanidine, isothiocyanates, and 2-chloro-1,3-dicarbonyl compounds. J. Iran. Chem. Soc. 11, 285–288. https://doi.org/10.1007/s13738-013-0299-0 (2014).
Kumar, G. S., Ragini, S. P. & Meshram, H. Catalyst free, regioselective one-pot three-component synthesis of thiazol-2-imine derivatives in ionic liquid. Tetrahedron Lett. 54, 5974–5978. https://doi.org/10.1016/j.tetlet.2013.08.056 (2003).
Raja, R. et al. Sequential change in one-pot, three-component protocol: A stepping stone in heterocyclic synthesis. Synth. Commun. 46, 942–948. https://doi.org/10.1080/00397911.2016.1178775 (2016).
Chen, C. Y., Barve, I. J. & Sun, C. M. One-pot three-component synthesis of 2-imino-1,3-thiazolines on soluble ionic liquid support. ACS Comb. Sci. 18, 638–643. https://doi.org/10.1021/acscombsci.6b00106 (2016).
Guo, S. et al. One-pot three-component protocol for the synthesis of substituted 2-aminothiazoles. Synth. Commun. 47, 1758–1764. https://doi.org/10.1080/00397911.2017.1350275 (2017).
Haouas, B., Sbei, N., Ayari, H., Benkhouda, M. L. & Batanero, B. Efficient synthetic procedure to new 2-imino-1,3-thiazolines and thiazolidin-4-ones promoted by acetonitrile electrogenerated base. New J. Chem. 42, 11776–11781. https://doi.org/10.1039/C8NJ01992D (2018).
Hantzsh, A. & Weber, J. H. Ueber verbindungen des thiazols (pyridins der thiophenreihe). Ber. Dtsch. Chem. Ges. 20, 3118–3132. https://doi.org/10.1002/cber.188702002200 (1887).
D’hooghe, M., Waterinckx, A. & Kimpe, N. D. A novel entry toward 2-imino-1,3-thiazolidines and 2-imino-1,3-thiazolines by ring transformation of 2-(thiocyanomethyl)aziridines. J. Org. Chem. 70, 227–232. https://doi.org/10.1021/jo048486f (2005).
Kumar, G. S., Kumar, A. S. & Meshram, H. M. A base-free multicomponent domino approach: one-pot synthesis of 2-iminothiazolines via oxy-iodination of arylacetylenes. Synlett 27, 399–403. https://doi.org/10.1055/s-0035-1560502 (2016).
Nosood, Y. L., Ziyaei Halimehjani, A. & Gonzalez, F. V. Regioselective opening of nitroepoxides with unsymmetrical diamines. J. Org. Chem. 83, 1252–1258. https://doi.org/10.1021/acs.joc.7b02795 (2018).
Ziyaei Halimehjani, A., Khalesi, M. & Breit, B. Amino acid-based dithiocarbamates as efficient intermediates for diversity-oriented synthesis of thiazoles. Eur. J. Org. Chem. https://doi.org/10.1002/ejoc.202001050 (2020).
Khalili Foumeshi, M., Ziyaei Halimehjani, A., Alaei, A., Klepetářová, B. & Beier, P. Tandem alkylation/michael addition reaction of dithiocarbamic acids with alkyl γ-bromocrotonates: Access to functionalized 1,3-thiazolidine-2-thiones. Synthesis 53, 2219–2228. https://doi.org/10.1055/a-1372-1619 (2021).
Ziyaei Halimehjani, A., Saeb, M. & Khalesi, M. Multicomponent synthesis of fully substituted thiazoles using glycine-based dithiocarbamates, acetic anhydride and nitroalkenes. Org. Biomol. Chem. 20, 3763–3766. https://doi.org/10.1039/D2OB00448H (2022).
Khoshdoun, M., Taheri, S., Daneshgar, P., Jamshidi, H. R. & Ziyaei Halimehjani, A. Synthesis of 5-amino-1,3,4-thiadiazoles containing dithiocarbamate groups. J. Heterocycl. Chem. 57, 1063–1070. https://doi.org/10.1002/jhet.3841 (2020).
Pennington, W. T. DIAMOND–Visual crystal structure information system. J. Appl. Crystallogr. 32, 1028–1029. https://doi.org/10.1107/S0021889899011486 (1999).
Acknowledgements
We are grateful to the research council of Kharazmi University and Sharif University of Technology for supporting this work. In addition, we thank the Alexander von Humboldt foundation for supporting this work.
Author information
Authors and Affiliations
Contributions
E.B. Writing and Running the experiments; A.Z.H. supervision, conceptualization, writing-review & editing; A.H. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Badali, E., Ziyaei Halimehjani, A. & Habibi, A. Catalyst free one pot three components synthesis of 2-iminothiazoles from nitroepoxides and thiourea. Sci Rep 13, 3079 (2023). https://doi.org/10.1038/s41598-023-30243-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30243-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.