Abstract
Fluid balance is a critical prognostic factor for patients with severe acute kidney injury (AKI) requiring continuous renal replacement therapy (CRRT). This study evaluated whether repeated fluid balance monitoring could improve prognosis in this clinical population. This was a multicenter retrospective study that included 784 patients (mean age, 67.8 years; males, 66.4%) with severe AKI requiring CRRT during 2017–2019 who were treated in eight tertiary hospitals in Korea. Sequential changes in total body water were compared between patients who died (event group) and those who survived (control group) using mixed-effects linear regression analyses. The performance of various machine learning methods, including recurrent neural networks, was compared to that of existing prognostic clinical scores. After adjusting for confounding factors, a marginal benefit of fluid balance was identified for the control group compared to that for the event group (p = 0.074). The deep-learning model using a recurrent neural network with an autoencoder and including fluid balance monitoring provided the best differentiation between the groups (area under the curve, 0.793) compared to 0.604 and 0.606 for SOFA and APACHE II scores, respectively. Our prognostic, deep-learning model underlines the importance of fluid balance monitoring for prognosis assessment among patients receiving CRRT.
Similar content being viewed by others
Introduction
For patients with severe acute kidney injury (AKI) admitted to the intensive care unit (ICU), continuous renal replacement therapy (CRRT) is recommended according to their clinical condition. In Korea, the number of patients on CRRT for AKI has increased rapidly, from 4,667 patients in 2005–2007 to 13,414 patients in 2014–20161. Severe AKI requiring CRRT increases the risk of mortality and is also a significant medical concern because of its impact on the incidence of cardiovascular disease (CVD) and chronic kidney disease (CKD)2. The epidemiology and outcomes of AKI across multiple cohorts have been reported by Hostes et al.3: renal replacement therapy (RRT) has been found to be required in 15% of patients with septic AKI and in 5–11% of non-septic patients in the ICU. Moreover, as more than one-third of patients with AKI admitted to the ICU require CRRT, management of AKI is a significant challenge for nephrologists and intensivists4.
The mechanisms underlying the progression from pre-renal AKI to AKI have been studied, and early supportive fluid therapy has been recognized to improve outcomes using various automated methods5,6,7. Notably, a deep-learning approach using information extracted from electronic health records has accurately predicted the development of AKI among in-hospital patients7. To date, however, there is a paucity of accurate prediction models for patients with AKI and a poor prognosis who require CRRT. Machine learning (ML) has been extensively tested in the field of kidney disease, although ML approaches to predict prognosis among patients undergoing CRRT are limited8,9. The technological purpose of deep learning is not to perform a comprehensive statistical analysis of existing data but, rather it is to increase the capacity to forecast the necessary acquisition of future data10. Deep-learning algorithms allow complex nonlinear patterns to be detected in a high-dimensional space, which cannot be easily achieved using standard ML algorithms. To our knowledge, deep learning has not been used to study cases of AKI requiring CRRT. Although the interpretability and explainability of current deep-learning algorithms are limited compared to traditional statistical methods, deep-learning algorithms can predict the realization of dependent variables more accurately on unseen data than traditional methods. Hence, we employed deep-learning models in this study to improve therapeutic considerations instead of building complex and novel artificial intelligence (AI) technologies6,11. Previous prognosis prediction studies have adequately shown that AI technology may be used to predict the risk of AKI using well-known technology6,11. However, identifying instances of significant AKI progression among patients requiring CRRT remains difficult6,11 as the critical issue of fluid balance in these patients has not been accurately addressed12,13. Precision CRRT allows the adjustment of the ultrafiltration rate and body composition monitoring (BCM) based on each patient’s phenotype. Accordingly, clinical outcomes may be improved in certain populations, allowing for individualized therapy based on each patient’s volume status13,14,15,16.
Excessive volume loss may precipitate hypotension and impair renal survival, resulting in reduced RRT-free survival12. Excessive volume overload may exacerbate pulmonary congestion, resulting in reduced ventilator-free survival12. Numerous studies have demonstrated an association between fluid overload and poor clinical outcomes in critically ill patients17,18 and patients with severe AKI19. Elevated cumulative fluid balance during the initial 72 h of ICU admission increases the risk of in-hospital mortality in patients with septic AKI20. Nevertheless, a negative daily fluid balance has been consistently associated with superior clinical outcomes17,21. However, the physical measures of fluid balance utilized in these studies have been inconsistent and based only on body weight measurements. Therefore, in this study, we aimed to determine whether repeated recording of fluid balance, using BCM values, could improve prognostic prediction using various ML methods, including deep-learning techniques, such as recurrent neural networks (RNNs), for patients with AKI requiring CRRT.
Results
Baseline characteristics
After selection, 784 patients were included in the final analysis (Fig. 1). Table 1 presents patient information. The mortality rate was 61.6% (483 patients) overall. End-stage renal disease, defined as the continued need for RRT at 3 months after CRRT initiation, was identified in 77 (9.8%) patients. There was no significant difference in baseline variables, such as age, sex, and age-modified Charlson Comorbidity Index (CCI), between the event and control groups. Sepsis was the most frequently reported cause of AKI in both groups, with a higher prevalence in the event group (57.6%) than in the control group (47.5%; p = 0.06).
Clinical and biochemical data were obtained at the time of CRRT initiation. There were no between-group differences in hemoglobin level, leukocyte count (white blood cell count), electrolyte (Na, K, and Ca) level, and C-reactive protein level. However, serum albumin level and blood venous pH were significantly lower in the event group than in the control group (Table 1). The mean delivered CRRT dose was 34.3 ± 8.6 mL/kg/h in the event group and 33.7 ± 8.7 mL/kg/h in the control group (p = 0.358).
Clinical parameters and volume status assessments during CRRT
Clinical and biochemical volume status assessments, using BCM, are presented in Tables 2 and 3. There were no between-group difference in urine output for 2 h and 6 h after CRRT initiation. However, the clinical severity indexes and Acute Physiology and Chronic Health Evaluation II (APACHE) II, Sequential Organ Failure Assessment (SOFA), and Glasgow Coma Scale (GCS) scores were relatively higher in the event group than in the control group. The mean APACHE II score was higher in the event group than in the control group (29.2 ± 10.3 vs. 26.5 ± 9.0; p < 0.001). Patients who died while receiving CRRT had lower systolic blood pressure, lower mean arterial pressure, and a greater rate of vasopressor use at the time of CRRT initiation. Mechanical ventilation was used to a greater extent in the event group than in the control group (Table 2). There were no between-group differences in volume status, as assessed by body weight and BCM, at baseline (day 0) and no between-group differences in body weight at days 1, 2, and 7 after CRRT initiation. Notably, the total body water/height2 (TBW/H2) level was higher in the event group than in the control group, at day 2 (13.9 ± 2.7 L/m2 vs. 13.4 ± 2.3 L/m2; p = 0.071). Seven days following CRRT initiation, the TBW/H2 level was 13.3 ± 2.5 L/m2 in the event group and 13.2 ± 1.9 L/m2 in the control group (p = 0.669; Table 2).
Modeling process
For the initial implementation of ML, we divided the model approach into five settings (Fig. 2). Model setting 1 involved the application of global characteristics through multiple imputations using 68 variables, whereas model setting 2 included all characteristics, with the exception of the CRRT * startup time from the time of ICU admission, and, thus, did not capture early CRRT initiations; instead, CRRT was entered as a continuous variable. Model setting 3 only used the APACHE II score, and model setting 4 only used the SOFA score. Finally, in model setting 5, an RNN and long short-term memory (LSTM) network were employed to examine all 76 variables collected, including the BCM data.
Between-group comparison of changes in TBW/H2 and body weight
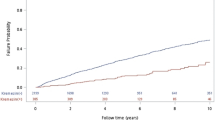
The total TBW/H2 level was higher in the event group than in the control group (Fig. 3). The changes in the TBW/H2 level over time were considerably different between the two groups (p < 0.001). The linear mixed model analysis revealed a time-dependent increase in between-group differences in TBW/H2, although this difference was not significant (p = 0.074) after adjusting for confounding factors of age, sex, age-modified CCI, history of diabetes mellitus, hypertension, SOFA score, urine output for 6 h, serum albumin, and the Modification of Diet in Renal Disease equation, which estimates the glomerular filtration rate at CRRT initiation. Specifically, in the control group, the TBW/H2 level steadily dropped from 13.39 ± 0.16 L/m2 at day 0 to 12.67 ± 0.19 L/m2 at day 7, while in the event group, the TBW/H2 remained steadily high from day 0 (13.47 ± 0.13 L/m2) to day 7 (13.26 ± 0.17 L/m2), as shown in Fig. 3. With regard to change in body weight estimates, the linear mixed-effect model analysis did not reveal a between-group difference (p = 0.534), despite a significant time-dependent effect on body weight in both groups (p < 0.001; Fig. 4). A comparison of the changes in TBW/H2 and body weight between HD-dependent and non-HD-dependent patients on CRRT is shown in Fig. 5. Interestingly, the linear mixed model analysis revealed a substantial difference in body weight change over time between HD-dependent and non-HD-dependent patients (p = 0.060; Fig. 6). In the HD-dependent group, body weight declined quickly over time, from 61.02 ± 1.63 kg at day 0 to 59.98 ± 1.64 kg at day 1, 59.06 ± 1.65 kg at day 2, and 57.77 ± 1.67 kg at day 7. By contrast, the decline in body weight was modest in the non-HD-dependent group, from 63.35 ± 0.50 kg at day 0 to 63.22 ± 0.50 kg at day 1, 62.83 ± 0.51 kg at day 2, and 61.19 ± 0.53 kg at day 7.
Comparison of the change in TBW/H2 estimates between the mortality and alive groups. (A) Univariate and (B) multivariate mixed-effect linear regression analyses are shown. The mixed-effects linear regression analysis between the two groups included the association with volume status at CRRT initiation, adjusted for age, sex, age-modified CCI, DM, hypertension, SOFA score, urine output for 6 h, and serum albumin level, MDRD, and eGFR at CRRT initiation.
Comparison of the change in body weight estimates between the mortality and alive groups. (A) Univariate and (B) multivariate mixed-effect linear regression analyses are shown. The mixed-effects linear regression analysis between the two groups included the association with volume status at CRRT initiation, adjusted for age, sex, age-modified CCI, DM, hypertension, SOFA score, urine output for 6 h, and serum albumin level, MDRD, and eGFR at CRRT initiation.
Comparison of the change in TBW/H2 estimates between the dialysis dependent group and non-dialysis dependent groups. (A) Univariate and (B) multivariate mixed-effect linear regression analyses are shown. The mixed-effects linear regression analysis between the two groups included the association with volume status at CRRT initiation, adjusted for age, sex, age-modified CCI, DM, hypertension, SOFA score, urine output for 6 h, and serum albumin level, MDRD, and eGFR at CRRT initiation.
Comparison of the change in body weight estimates between the dialysis dependent group and non-dialysis dependent groups. (A) Univariate and (B) multivariate mixed-effect linear regression analyses are shown. The mixed-effects linear regression analysis between the two groups included the association with volume status at CRRT initiation, adjusted for age, sex, age-modified CCI, DM, hypertension, SOFA score, urine output for 6 h, and serum albumin level, MDRD, and eGFR at CRRT initiation.
Comparison of the classification model for mortality (event group) using conventional algorithm and severity scores
The findings regarding the model parameters used in this study, including the imputation technique, validation method and ratio, size of the test set, and performance of the test set are shown in Fig. 2 and Table 4. The performance of the ML method for classification was compared with various parameters using the area under the curve (AUC) according to the model setting (Fig. 2; Table 4). We separated model settings 1 and 2 to evaluate whether the prognostic predictive power would be changeable if the early start of the CRRT treatment regimen was included as a variable. The overall attributes with multiple imputations (68 variables), including the initiation time of CRRT, of setting 1 are presented in the Supplemental Table S2. The overall attributes with multiple imputations (67 variables), excluding the initiation time of CRRT, of setting 2 are presented in Table 2. The random forest (AUC, 0.7678) and bagging (AUC, 0.7494) models with cross-validation, which were built from the dataset using the ensemble technique, were found to have enhanced predictive performance compared to the logistic regression analysis (AUC, 0.6770) and APACHE II (AUC, 0.5646) and SOFA (AUC, 0.6505) scoring systems.
Comparison of the classification model for mortality (event group) using the deep-learning model with RNN
We performed an additional analysis to apply a deep-learning algorithm using longitudinal data to evaluate further enhancement of the prediction model. The mortality risk model was validated using deep neural network algorithms and compared it with conventional algorithms. Our proposed deep-learning model comprised LSTM networks and an autoencoder. The former was introduced to deal with time-series data, whereas the latter was used to compensate for missing data. Figure 2 demonstrates the analyzed records for model setting 5, which used 76 independent attributes to guide learning, including repeated fluid balance data and BCM results. Among the various conventional algorithms used, the RNN with LSTM networks model yielded the highest AUC value (0.7938), with inclusion of an autoenconder using 68 variables (setting 1) yielding an AUC value of 0.7912 (Table 5).
Discussion
The main finding of our study is that repeated monitoring of fluid balance among patients with AKI requiring CRRT in the ICU can improve the predictability of prognosis. Our findings, based on a retrospective analysis of a multicenter prospective cohort, underlines the significance of repeated BCM using BIA as a treatment guide for the aforementioned clinical population, with excess body water possibly being an independent risk factor for death, as determined using conventional analysis with a linear mixed-effect model. We further demonstrated that the inclusion of repeated measurements of BCM in a deep-learning approach better predicted mortality than classical ML methods.
Optima fluid status is pivotal, and the concept of “dry weight” is an established performance to guide HD for patients with end-stage kidney disease (ESKD). However, as sustained euvolemia is difficult to achieve in patients without residual kidney function, various efforts have been made to enhance the accuracy of dry weight estimates to guide HD. Randomized controlled studies have provided evidence for the combined use of BIA with other markers to assess weight gain in patients undergoing peritoneal dialysis22 and chronic HD23. Three meta-analyses of RCTs found BIA-guided therapy to be somewhat beneficial in controlling blood pressure and left ventricular hypertrophy among patients on HD, including peritoneal dialysis, but not for improving overall survival24,25,26. Consequently, whether further BIA measures would improve clinical outcomes in patients with severe AKI requiring CRRT has been raised as a clinical issue of interest. Only a few studies conducted in the recent past have shown that BIA is effective in determining the volume status of critically ill patients in the ICU27. The InBody S10 (InBody, Seoul, Korea)28, a body composition analyzer, has touch-type or adhesive electrodes, and is intended for patients who are immobile or amputees necessitating BIA. In this study, BIA was performed repeatedly using InBody, which has been validated to allow for body composition monitoring in the Korean surgical ICU after major surgery29, predicting the severity of patient condition in surgical ICU30. It has also been revealed to be not inferior to CT for the measurement of muscle mass31. At first, in this study, using the linear mixed-effect model approach, we first determined whether the BIA values of the non-survivor (event) and survivor (control) groups were different by repeating BIA measurements. In the univariate analysis, TBW/H2 values revealed a significant effect of time between groups (p = 0.003), with positive fluid balance being higher in the event group than in the control group after adjusting for covariates, although this difference was not significant (p = 0.074; Fig. 2). Interestingly, despite the protocol for fluid balancing therapy used, a significant time-dependent effect on body weight control was observed in both groups, with no between-group difference (p = 0.534; Fig. 3). Our findings are important in this regard as volume status might be difficult to evaluate from body weight alone. Our results show that repeated BCM monitoring and guiding the treatment process for CRRT may provide a more accurate estimate of fluid overload than body weight and, thus, provide improved treatment outcomes. These results are corroborated by our finding that, at 3 months after CRRT initiation, a substantially lower retained body weight was associated with a higher risk of dialysis-dependent status. A previous study using BIA23, in which residual renal function was defined by urine volume, reported a considerable increase in the risk of anuria with a substantial decrease in body weight among patients on HD. In addition, our findings indicate that the use of the TBW level, which is concurrent with body weight, may be preferable to prevent excessive ultrafiltration and a resulting decrease in residual renal function.
Although ML has been widely studied in the context of patients admitted to the ICU, little is known regarding the use of ML methods for patients with CCRT8,9. Considering the heterogenicity and medical severity of patients requiring CCRT12,13, various critical patient prognostic scoring systems have been introduced, even before ML was introduced, with previous attempts to augment the prognostic value of scoring systems, including the SOFA and APACHE scores, using deep learning32. Although these existing scoring systems indicate systemic disease severity among patients in the ICU, they do not include changes in fluid dynamics, which are critical for patients with severe AKI. Monitoring fluid balance status is specifically important for CRRT, which is used for organ support in patients presenting with multi-organ failure, unlike maintenance HD, which is designed as a chronic RRT33,34. Body fluid balance, which reflects the systemic disease state, is a significant clinical variable related to the expansion of extracellular fluid balance and lung congestion; as such, it is an important predictor of death not only for patients with severe AKI19 but also for patients with CKD20. Several studies have tried to overcome the problem of fluid accumulation in pulmonary conditions related to ventilator-free survival and mortality among patients receiving ventilator support using ML35,36. However, in both of the abovementioned studies, only 10%35 and 14%36 of patients with renal failure requiring RRT were included, respectively. By comparison, 62% of patients in our study cohort were on ventilator support, with our findings indicating that fluid balance may be an essential prognostic variable in this clinical population. This is the reason we chose setting 5 in our deep-learning model, which included BCM data, with this setting improving the predictive performance of the model (AUC 0.7938).
Euvolemia is a critical goal of CRRT in both patients on maintenance dialysis and patients with AKI. Adequate volume status is difficult to achieve due to the lack of established criteria for defining excess volume and a clear definition of “euvolemia” In patients with AKI requiring CRRT, the prescription CRRT dose, including the ultrafiltration rate, is typically influenced by each clinician’s considerations. This is troublesome because, if ultrafiltration is insufficient, persistent fluid overload occurs, jeopardizing organ function. However, excessive ultrafiltration for decongestion might result in iatrogenic hemodynamic instability and end-organ ischemia, especially in patients with AKI requiring CRRT. Bioimpedance integration into patient care may improve the objectivity of volume measurements and, for individuals receiving CRRT, the data presented in this study may serve as a practical guide for the safe and successful administration of ultrafiltration. As a limitation, our study acknowledges the potential interference of multiple devices on BIA measurements in critically ill ICU patients. Accurate measurements may require disconnecting or temporarily turning off certain devices, and the use of BIA should be implemented with consideration of the patient's clinical condition.
In conclusion, this retrospective analysis was conducted using a multicenter prospective cohort in which fluid status techniques were assessed in critically ill patients with acute kidney damage who had begun CRRT. It should not be forgotten that the ultimate purpose of medical AI for patients with CRRT is to assist clinicians in providing more effective CRRT therapy by providing them with an accurate assessment of a patient’s fluid status. Moving forward, it would be valuable to systematically validate the predictive potential and clinical usefulness of the AI models presented herein.
Methods
Study design, ethics statement, and participants
This multicenter cohort study presents an ML approach for the development cohort of the VolumE maNagement Under BCI in critically ill patientS on Continuous Renal Replacement Therapy (VENUS) trial. The detailed protocol of the VENUS trial has been previously described37. Briefly, the VENUS trial is a prospective, multicenter, randomized controlled trial (RCT) study on fluid management using BCM. The trial includes eight tertiary hospitals in Korea: Seoul National University Boramae Medical Center, Seoul National University Bundang Hospital, Seoul National University Hospital, Ewha Womans University Mokdong Hospital, Pusan National University Hospital, Kyungpook national university hospital, Hallym University Dongtan Sacred Heart Hospital, and Korea University Guro Hospital.
The present study is a retrospective analysis of the data from the trial for the purposes of using ML to evaluate the clinical value of the repeated monitoring of volume status in patients with AKI requiring CRRT on predicted prognostic outcomes. The study was approved independently from the VENUS trial by the institutional review boards of the aforementioned institutes for retrospective analysis. The following are the respective approval numbers of each hospital: Seoul National University Hospital Institutional Review Board (IRB No. B-1801/445–106), SMG-SNU Boramae Medical Center Institutional Review Board (IRB No. 20180108/10-2018-5/012), Seoul National University Hospital Institutional Review Board (IRB No. 1801-036-913), Ewha Womans University Medical Center Institutional Review Board (IRB No. EUMC 2018–01–071), Pusan National University Hospital Institutional Review Board (IRB No. H-1804-029-066), Institutional Review Board (IRB) of the Kyungpook National University Hospital (IRB No. KNUH 2020–01-034), Hallym University Dongtan Sacred Heart Hospital Institutional Review Board (IRB No. HDT 2020-01-011, and Korea University Guro Hospital Institutional Review Board (IRB No. 2020GR0197). The requirement for informed consent was waived by the IRB of (Seoul National University Hospital IRB, SMG-SNU Boramae Medical Center IRB, Seoul National University Hospital IRB, Ewha Womans University Medical Center IRB, Pusan National University Hospital IRB, Kyungpook National University Hospital IRB, Hallym University Dongtan Sacred Heart Hospital IRB, and Korea University Guro Hospital IRB owing to the retrospective design of the study. The study was performed in accordance with the principles of the Declaration of Helsinki, and clinical data from patients were obtained after receiving approval from the Institutional Review Board (IRB) at each center.
Patients with AKI requiring CRRT who were admitted to the ICU of the participating hospitals from November 2017 to November 2019 were included (Fig. 1). Patients were excluded if they were ≤ 18 years of age or had no mortality data.
Clinical and laboratory evaluations
Demographic, clinical, and biochemical data were obtained immediately before CRRT initiation. Clinical data, including hemodynamic monitoring and laboratory data, were obtained on the same day before CRRT initiation. To evaluate adverse events, data on the number of hypotension episodes; usage, type, and dosage of vasopressors; and use and dose of diuretics were also collected. Moreover, data on complete blood cell counts, electrolytes, BUN, serum creatinine, albumin, aspartate aminotransferase, alanine aminotransferase, calcium, phosphorus, C-reactive protein, prothrombin time, and lactate levels were collected in the ICU. The variables contributing to AKI were originally classified and validated by the researchers using electronic medical records. The choice to provide vasopressors or mechanical ventilation was made by the attending doctors or intensivists based on the participants’ hemodynamic condition. The Acute Physiology and Chronic Health Evaluation (APACHE) II score38, SOFA score39, and the age-modified CCI were used as indicators of disease severity and comorbidities40.
The InBody S10 (InBody, Seoul, Korea)28, an impedance-based body fat analyzer, was used to measure fluid status at 0, 1, 2, and 7 days after CRRT initiation. TBW, intracellular water, extracellular water (ECW), segmental water, ECW/TBW values, fat-free mass, soft lean mass, segmental lean mass, body fat mass, and percent body fat were measured41. A bioimpedance analysis (BIA) showed differences in electrical conductivity according to the amount of water/electrolytes in each tissue. As BW passes an electrical current, the volume of BW can be obtained by measuring the resistance value obtained41. TBW/H2 and body weight were used as indicators of excess fluid balance.
CRRT protocol
CRRT was performed using the PRISMA FLEX system (Gambro AB, Stockholm, Sweden) and AV1000 (Fresenius Medical Care, Bad Homburg, Germany). The prescribed CRRT dose varied from 35 to 65 mL/h/kg according to each patient’s clinical needs and attending physicians or intensivists’ decision. After initiating CRRT, the attending physicians and experienced nurses evaluated participants’ body weight, urine output, laboratory results, actual administered dosages, and hemodynamic status. These data were reviewed with the nephrologists to ensure that CRRT was adequate. The dialysate and replacement fluid ratio was maintained at 1:1. CRRT was administered using an internal jugular vein or femoral vein dual-lumen catheter. Unfractionated heparin or nafamostat mesilate was used as the anticoagulant. The CRRT maintenance period was based on each patient’s clinical status, with weaning from CRRT performed for any of the following reasons: systolic blood pressure > 120 mmHg and heart rate < 90/min, maintained without the use of vasoactive drugs; urine output maintained at ≥ 1000 mL, with or without the use of diuretics; renal recovery confirmed by the attending physician; and conventional hemodialysis (HD) deemed possible, even if renal function did not recover.
Clinical outcomes
The primary outcome was in-hospital mortality. Secondary outcomes included the comparison of fluid balance by TBW/H2 and body weight between the event (death) and control (alive) groups. Events were defined as in-hospital mortality and RRT dependence among survivors at 90 days.
Analysis using ML methods
We first introduced the traditional ML methods that we used as baseline methods for the experiments, along with an imputation method. Subsequently, a more advanced method, based on modern deep-learning architectures, was introduced. For extended methods related to modern deep learning, please refer to the included online supplemental data (see Supplementary Information).
Statistical analysis
All statistical analyses were performed using the R statistical language (version 3.0.2, The Comprehensive R Archive Network: http://cran.r-project.org). The Multivariate Imputation by Chained Equations (MICE) package was used to compute missing values for continuous and categorical data. Python version 3.6.5 and TensorFlow 1.14.0 were used to implement the deep-learning models. Data were evaluated using the IBM SPSS software (version 22.0; IBM Corp., Armonk, NY, USA). Continuous variables of baseline characteristics and biochemical data are presented as mean ± standard deviation. Student’s t-test was used to test between-group differences in continuous variables, whereas the chi-squared test was used for categorical variables.
Data availability
All data associated with this study are available from the corresponding author upon reasonable request.
References
Park, S. et al. Epidemiology of continuous renal replacement therapy in Korea: Results from the national health insurance service claims database from 2005 to 2016. Kidney Res. Clin. Pract. 37, 119–129 (2018).
Chawla, L. S. et al. Acute kidney disease and renal recovery: Consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat. Rev. Nephrol. 13, 241–257 (2017).
Hoste, E. A. J. et al. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 14, 607–625 (2018).
Hoste, E. A. & Schurgers, M. Epidemiology of acute kidney injury: How big is the problem?. Crit. Care Med. 36, S146-151 (2008).
Park, S. et al. Impact of electronic acute kidney injury (AKI) alerts with automated nephrologist consultation on detection and severity of AKI: A quality improvement study. Am. J. Kidney Dis. 71, 9–19 (2018).
Yuan, Q. et al. Role of artificial intelligence in kidney disease. Int. J. Med. Sci. 17, 970–984 (2020).
Tomasev, N. et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature 572, 116–119 (2019).
Kang, M. W. et al. Machine learning algorithm to predict mortality in patients undergoing continuous renal replacement therapy. Crit. Care 24, 42 (2020).
Kim, Y. et al. Development of a new mortality scoring system for acute kidney injury with continuous renal replacement therapy. Nephrology (Carlton) 24, 1233–1240 (2019).
Bzdok, D., Altman, N. & Krzywinski, M. Statistics versus machine learning. Nat. Methods 15, 233–234 (2018).
Burlacu, A. et al. Using artificial intelligence resources in dialysis and kidney transplant patients: A literature review. Biomed. Res. Int. 2020, 9867872 (2020).
Jamme, M., Legrand, M. & Geri, G. Outcome of acute kidney injury: how to make a difference?. Ann. Intensive Care 11, 60 (2021).
Samoni, S., Husain-Syed, F., Villa, G. & Ronco, C. Continuous renal replacement therapy in the critically Ill patient: From garage technology to artificial intelligence. J. Clin. Med. 11, 172 (2021).
Clark, W. R. et al. Data analytics for continuous renal replacement therapy: Historical limitations and recent technology advances. Int. J. Artif. Organs 39, 399–406 (2016).
Siew, E. D. et al. Optimizing administrative datasets to examine acute kidney injury in the era of big data: Workgroup statement from the 15(th) ADQI consensus conference. Can. J. Kidney Health Dis. 3, 12 (2016).
Villa, G. et al. oXirisNet registry: A prospective, national registry on the oXiris membrane. Blood Purif 47(Suppl 3), 1–8 (2019).
Malbrain, M. L. et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anaesthesiol. Intensive Ther. 46, 361–380 (2014).
Messmer, A. S. et al. Fluid overload and mortality in adult critical care patients-A systematic review and meta-analysis of observational studies. Crit. Care Med. 48, 1862–1870 (2020).
Bouchard, J. et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 76, 422–427 (2009).
Neyra, J. A. et al. Cumulative fluid balance and mortality in septic patients with or without acute kidney injury and chronic kidney disease. Crit. Care Med. 44, 1891–1900 (2016).
Zarbock, A. et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically Ill patients with acute kidney injury: The ELAIN randomized clinical trial. JAMA 315, 2190–2199 (2016).
Oh, K. H. et al. Does routine bioimpedance-guided fluid management provide additional benefit to non-anuric peritoneal dialysis patients? Results from COMPASS clinical trial. Perit. Dial. Int. 38, 131–138 (2018).
Hur, E. et al. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: A randomized controlled trial. Am. J. Kidney Dis. 61, 957–965 (2013).
Beaubien-Souligny, W. et al. Meta-analysis of randomized controlled trials using tool-assisted target weight adjustments in chronic dialysis patients. Kidney Int. Rep. 4, 1426–1434 (2019).
Tabinor, M. & Davies, S. J. The use of bioimpedance spectroscopy to guide fluid management in patients receiving dialysis. Curr. Opin. Nephrol. Hypertens. 27, 406–412 (2018).
Covic, A. et al. Value of bioimpedance analysis estimated “dry weight” in maintenance dialysis patients: A systematic review and meta-analysis. Int. Urol. Nephrol. 49, 2231–2245 (2017).
Basso, F. et al. Fluid management in the intensive care unit: Bioelectrical impedance vector analysis as a tool to assess hydration status and optimal fluid balance in critically ill patients. Blood Purif. 36, 192–199 (2013).
D’Hondt, J., Waterplas, J., Chapelle, L., Clarys, P. & D’Hondt, E. A comparative and sex-specific study of bio-electrical impedance analysis and dual energy X-ray absorptiometry for estimating whole-body and segmental body composition in healthy young adults. Appl. Sci. 12, 7686 (2022).
Park, S. R. et al. Sequential changes in body composition using bioelectrical analysis during the metabolic response in critically Ill surgical patients. Surg. Metab. Nutr. 8, 13–16 (2017).
Lee, Y. H., Lee, J. D., Kang, D. R., Hong, J. & Lee, J. M. Bioelectrical impedance analysis values as markers to predict severity in critically ill patients. J. Crit. Care 40, 103–107 (2017).
Kim, D. et al. Comparative assessment of skeletal muscle mass using computerized tomography and bioelectrical impedance analysis in critically ill patients. Clin. Nutr. 38, 2747–2755 (2019).
Shickel, B. et al. DeepSOFA: A continuous acuity score for critically Ill patients using clinically interpretable deep learning. Sci. Rep. 9, 1879 (2019).
Claure-Del Granado, R. & Mehta, R. L. Fluid overload in the ICU: evaluation and management. BMC Nephrol. 17, 109 (2016).
Murugan, R. et al. Precision fluid management in continuous renal replacement therapy. Blood Purif. 42, 266–278 (2016).
Lin, M. Y. et al. Explainable machine learning to predict successful weaning among patients requiring prolonged mechanical ventilation: A retrospective cohort study in central Taiwan. Front. Med. 8, 663739 (2021).
Kim, J. H., Kwon, Y. S. & Baek, M. S. Machine learning models to predict 30-day mortality in mechanically ventilated patients. J. Clin. Med. 10, 2172 (2021).
Oh, H. J. et al. VolumE maNagement under body composition monitoring in critically ill patientS on CRRT: Study protocol for a randomized controlled trial (VENUS trial). Trials 19, 681 (2018).
Knaus, W. A., Draper, E. A., Wagner, D. P. & Zimmerman, J. E. APACHE II: A severity of disease classification system. Crit. Care Med. 13, 818–829 (1985).
Minne, L., Abu-Hanna, A. & de Jonge, E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit. Care 12, R161 (2008).
Charlson, M. E., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40, 373–383 (1987).
Moissl, U. M. et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol. Meas. 27, 921–933 (2006).
Funding
This study was funded by Grant 03-2020-2130 from the Seoul National University Hospital Research Fund and by grant HI17C1827 from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), Ministry of Health and Welfare, Republic of Korea.
Author information
Authors and Affiliations
Consortia
Contributions
Each author contributed to the intellectual content during drafting and revision of the manuscript and accepts accountability for the overall work. K.D.Y., J.P.L., and S.K. developed the main conceptual ideas and proof of concept for the project. J.N. and W.B. developed the technical details and performed the numerical calculations for machine learning. K.D.Y., J.P.L., J.N., and W.B. wrote the main manuscript text. J.N.A., H.J.O., H.R., E.Y.S., S.H.B., S.Y.A., J.-H.C., D.K.K., D.-R.R., and C.S.L. contributed to the research idea, study design, data acquisition, and interpretation. All authors reviewed and accepted the manuscript submitted. The final submitted version of the work was critically evaluated and approved by all authors. Prior to submitting the work for publication, all data and statistical analysis have been reviewed for correctness, and any issues have been thoroughly addressed.
The Korean Association for the Study of Renal Anemia and Artificial Intelligence (KARAI)—available at https://karai.or.kr/content/staff [accessed on 19 October 2021].
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoo, K.D., Noh, J., Bae, W. et al. Predicting outcomes of continuous renal replacement therapy using body composition monitoring: a deep-learning approach. Sci Rep 13, 4605 (2023). https://doi.org/10.1038/s41598-023-30074-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30074-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.