Abstract
To date, the impossibility of treating resistant forms of bacteria and fungi (AMR) with traditional drugs is a cause for global alarm. We have made the green synthesis of Argirium silver ultra nanoclusters (Argirium-SUNCs) very effective against resistant bacteria (< 1 ppm) and mature biofilm (0.6 ppm). In vitro and preclinical tests indicate that SUNCs are approximately 10 times less toxic in human cells than bacteria. Unique chemical-physical characteristics such as particle size < 2 nm, a core composed of Ag0, and a shell of Ag +, Ag2+ , Ag3+ never observed before in stable form in ultra pure water, explain their remarkable redox properties Otto Cars (Lancet Glob. Health 9:6, 2021). Here we show that Argirium-SUNCs have strong antimicrobial properties also against resistant Aspergillus niger GM31 mycelia and spore inactivation (0.6 ppm). The membrane depolarization is a primary target leading to cell death as already observed in bacteria. Being effective against both bacteria and fungi Argirium-SUNCs represent a completely different tool for the treatment of infectious diseases.
Similar content being viewed by others
Introduction
In the last years there has been a fast spread of multi- and pan-drug resistant bacteria (also known as “superbugs”) which are responsible of infections not treatable with existing antibiotics. Moreover, the rise of multi-drug resistance in fungi is also a cause for alarm1,2. Growing antimicrobial resistance (AMR) could spiral out of control of the healthcare system, according to estimates for the year 2050, deaths attributable to AMR could reach 10 million1,3. In addition to this, it should be considered the economic consequences due to continuous increase in health care costs. The development of new antimicrobial agents is necessary in order to overcome antimicrobial resistance. Silver nanoparticles (AgNPs) have attracted the attention of many researchers for their antibacterial and anti-fungal properties, varying between 10 and 100 ppm4 .The plethora of AgNPs synthesis protocols can be grouped in physical, chemical, and biological green methods5. Important problems have to be overcome to make the synthesis methods reproducible. The presence of reducing agents, stabilizers, and contaminants can interfere in terms of efficacy and toxicity by altering the expected results from nanoparticle formulations5,6. Structural characterizations are often incomplete so that, in many cases, the comparison of results is not possible4,7. To overcome these issues, we have recently modified an old synthesis8 for generate a novel silver nanoparticles, using a reproducible electrochemical method (Patent EP-18181873). Our nanoparticles exhibited antibacterial properties even against resistant strains at a very low concentration (< 1 ppm), a value much lower than that reported for other silver formulations4and are also very effective at deconstructing mature biofilm (0.625 ppm)9,10,11. This is because they are a novel nano material characterized by unique chemo-physical properties. Their size (< 2 nm) is the smallest of all nanoparticles so far studied, which is also why we named them Argirium Silver Ultra Nano Clusters (Argirium-SUNCs)12. All studies indicate that small nanoparticles induce greater cytotoxicity13 than larger ones because of the higher surface area/volume ratio that facilitates the binding around the cell membrane with its consequent damage13. Structural studies12exhibited other unique SUNCs features. The presence of metallic Ag0 in the core of the nanoparticle while in the external shells, due to the electron-attracting action of the water oxygen atoms, are present Ag+, Ag2+ and Ag3+ silver oxides never observed in a stable form before.
The consequent high anionic salvation surrounding of SUNCs (Zpuls value > − 50 mV) explains their stability for several months in ultra-pure water solution without large aggregates while the presence of silver oxides on the clusters surface explains their enhanced redox properties towards biological targets. Different mechanisms have been proposed to explain the AgNPs antimicrobial action such as oxidative stress, reduced ATP synthesis, reduced GSH concentration and enzymes inhibition13,14,15.
However, from the results reported in the literature13,15, the events leading to cell death are not always clearly distinguished from side effects/epiphenomena. We have recently identified the membrane depolarization and increased intracellular calcium level as primary events leading to bacterial death11. Finally, in vitro and in vivo toxicity studies reported that silver nanoparticles are no toxic against lung epithelial cells up to 100 µg/ml16. Also our Argirium-SUNCs resulted about 10 times less toxic in human cells than in bacteria and, in addition, we tested them with Galleria mellonella as a pre-clinical toxicity test10. Survival curves indicated that Argirium-SUNCs were no-toxic toward larvae up to the highest concentration possible used 6.8 µg/ml.
Previous studies indicated differences in anti-fungal properties of different Ag-NPs used5,17. In addition, in most of these studies very rarely the same nanoparticles have been tested on both bacteria and fungi. This prompted us to investigated, in addition to being antibacterial, the anti-fungal properties of Argirium-SUNCs.
The aim of this study was to investigate the effects of Argirium-SUNCs on Aspergillus niger , an ubiquitous fungus, which causes various diseases in different living organisms, producing mycotoxins. In humans, it causes invasive aspergillosis, particularly in immunosuppressed patients, undergoing transplants and blood cancers18. Several synthetic fungicides are used to combat Aspergillus niger, contamination in food and various environments. Aspergillus niger, has developed resistance to antifungal agents and is considered as “model organism” to investigate anti-fungal performance of different molecules19.
The results indicate that they are very effective against resistant Aspergillus niger GM31 (0.625 ppm) and the anti-fungal mechanism was similar of SUNCs to what we have previously described in bacteria9,10 even if with differences related eukaryotic nature of A. niger GM31.
This indicates that our Ag formulation could represent a new class of molecules that overcome antimicrobial resistance. Unlike traditional antibiotics, Argirium-SUNCs being effective against bacteria and fungi represent an entirely different pharmacological strategy for dealing with infectious diseases20.
Effect of SUNCs on Aspergillus niger GM31
Argirium-SUNCs showed strong antimicrobial properties against mycelia and spore inactivation with a MIC value of 1.25 ppm (Fig. 1A,B). In particular 0.625 ppm of Argirium-SUNCs treatments significantly decreased the mycelia production (P < 0.05) of about 80% (Fig. 1A,B). With the enhancement of Argirium-SUNCs concentration the mycelia growth was further reduced reaching 100% already at 1.25 ppm after 7 days of incubation at 28 °C. To observe if the anti-fungal activity was fungicidal or fungistatic the treated mycelia with 1.25, 2.5, 4, 8 and 10 ppm were washed with PBS buffer and further inoculated in a new liquid media (50 mL of malt extract broth) for 7 days more at the same temperature. After this incubation period, we did not register any mycelia growth suggesting a fungicidal effect of the Argirium-SUNCs already at 1.25 ppm. As regard spore germination, also in this case we observed a strong reduction of germination of about 87% of the spores at 0.625 ppm in relation to the 98% of germinated spores of the control group (CTR) (Fig. 1C). Since the first morphological change in spore germination of many fungal species is the isotropic growth, in which the spore starts to swell and consequently increases its volume due to the water uptake, which is accompanied by numerous metabolic activities including respiration, RNA and protein synthesis21,22 , as well as trehalose breakdown21, we measured the dimension of the spores during this first phase (Fig. 1C). As evidence spores treated with Argirium-SUNCs delay this phase achieving only 3.66 ± 0.2 um (95% of spore) within 4 h at 28 °C (Fig. 1B and C), in contrast, with the CTR spores, which measured 6.28 ± 0.7 um (98% of spore) during the time. Figure 2 shows the cells viability. At is well-known dyes of SYTO 9 (green) and propidium iodide (PI) (red) have diverse permeability to healthful cells.
(A) Reduction of mycelia growth. 0,2 g of A. niger GM31 mycelia was growth at different concentrations of SUNCs in MEB at 28 °C for 7 days.Untreated mycelia was used as a control. The mycelial growth inhibition, measured as the percentage of dried mycelia biomass, was determined according to Eq. (1) (materials and methods). (B) The effect of Arg-SUNCs on the conidia germination. Conidia suspension (9.8 × 106 CFU mL−1) were incubated at different concentrations of SUNCs at 28 °C at different time. (C) Dimension of the spores during this first phase at different concentrations of SUNCs within 4 h at 28 °C. The analysis of variance (ANOVA) was carried out to every variable studied (P < 0.05).
Effect of sublethal concentration (0.625 ppm) of Argirium-SUNCs on A. niger GM31 cell viability. Live and death as (green) intact fungal structure; (red) damaged fungal structure were visualized by fluorescence microscopy after staining with CFDA (carboxyfluorescein diacetate) and PI (propidium iodide). Scale bar 20 μm.
In fact, while live cells are stained with SYTO 9, dead cells with damage in cellular membrane stained with PI. As shown in Fig. 2, in CTR samples almost all cells exhibited a green fluorescence indicating that cell hyphae are alive. With the increase of Argirium-SUNCs concentration and exposure time, a significant reduction in green fluorescence was evidenced and the red fluorescence was markedly intensified. In particular we observed after 1 h of exposure a significant number of the spores showed a membrane disruption since PI stain the DNA of a high percentage of cells. The maximum cells killing were achieved already at 24 h after treatments (Fig. 2).
The cell membrane depolarization, the pivot
As observed above Argirium-SUNCs showed a rapid anti-fungal action, thus we expected that cellular membrane was a principal target of the anti-fungal action of the nano clusters. As it is well known cells accumulate energy produced during respiration by forming both a charge gradient (membrane potential, ΔΨ) and a chemical gradient across a membrane system23. The ΔΨ potential is a component of a large range of essential biological functions24 such as ATP synthesis, flagella rotation and ammonium accumulation. We therefore studied the change in membrane potential using a fluorescent dye DiBAC411 that enters into the cells when they have a depolarised membrane, binding intracellular or membrane proteins and showing green fluorescence.
As observed in Fig. 3, there is no visible fluorescence in CTR cells, indicating that they are not depolarized. In contrast, intense green fluorescence was observed in cells treated with Argirium-SUNCs, indicating a loss of membrane potential. We have previously observed that Argirium-SUNCs favour azobenzenes isomerization in aqueous solution25. In addition disulphides such as cystine and GSSG rapidly interact with Argirium-SUNCs, strongly altering their properties as indicated by the disappearance of the plasmonic spectrum (unpublished results). As shown in Fig. 3, the simultaneous addition of cystine and Argirium-SUNCs to treated cells (Fig. 3) inhibits fluorescence dyeing, confirming the decisive role of Argirium-SUNCs in the membrane depolarization process. Interestingly, the addition of Ag+ in the form of AgNO3 , in contrast to Argirium-SUNCs, does not alter the membrane potential (Fig. 3). This means that the oxidative power of Ag+, which is lower than that of Ag2+ and Ag3+ present in our nanoclusters, is not sufficient to depolarize the membrane. This rare oxidative states of silver were observed for the first time by works reported in26,27. However Ag2+ and Ag3+ species produced by anodic oxidation synthesis and stoked at − 20 °C were found to be too unstable in order to test their possible effects in any field26. In an other work Ag+/Ag2+/Ag3+ nanoparticle composites was obtained by green synthesis. This nanoparticle composition displayed an antibacterial activity in the range 0.7 and 12.5 ppm. However this nano formulation obtained from the pomegranate extract stabilized by contaminant biomolecules resulted complex and authors reported that the mechanism of dispersion and stabilization by biological macromolecules needs further study28.
Effect of Argirium-SUNCs on cellular membrane depolarization. Hyphae of A. niger GM31 were stained with 20 μg/ml of DiBAC4 (Bis-(1,3-Dibutylbarbituric Acid Trimethine Oxonol). (A) Untreated hyphae; (B) hyphae treated with 0.625 mg L−1 Argirium-SUNCs; (C) Hyphae treated with Argirium-SUNCs + Cystine and (D) Hyphae treated with AgNO3. Scale bar of 20 μm.
On the contrary in ultra pure water solution our ultra nanoclusters are characterized by stable Ag2 + and Ag3 + cationic species coordinated in Ag3O412 form as indicated by XRD data already deposited at the International Centre for Diffraction Data (ICDD Reference #56,443) upon request. Thus, the present results indicate for the first time that Argirium-SUNCs have as primary target the membrane depolarization whose loss of function leads to cell death in bacteria and fungi, both of which are characterized by a negative external membrane potential.
To the best of our knowledge, membrane depolarization is not reported as a primary effect in the case of other silver nanoparticles whose the antibacterial activity was dominated by Ag+ ions and by the absence of Ag2+ and Ag3 + cationic forms5,13. As consequence of the depolarization the membrane permeability of the fungal cells is likely compromised due to the Argirium-SUNCs interactions, thus we observed the morphology of the A. niger GM31 hyphae. As reported in Fig. 4D the CTR hyphae displayed a normal morphology with turgid cells, which coincided with the results of cell viability.
Release of intracellular proteins from A. niger GM31 before and after treatments with Argirium-SUNCs: (A) Protein concentration (μg/g dry biomass) in the supernatant medium after 18 h of incubation with the Argirium-SUNCs at 28 °C; (B) morphological change as indicate by arrow (magnification picture) after 18 h of 0.625 mg Argirium-SUNCs exposure at 28 °C treatments.
On the contrary, treated mycelia showed flattened hyphae, due to the leakage of the intracellular compounds as observed by the significant (P < 0.05) increase of the extracellular protein content which major values were reached at 1.25 ppm of Argirium-SUNCs (Fig. 4A).
The depolarization could also alter the membrane channel properties and affect increased intracellular calcium we have already observed in bacteria29. It has been proposed that the calcium level represents an important intracellular messenger that initiates and regulates the apoptotic process29,30.
Methods and protocols
Oxidative stress (ROS) and integrity of membrane structural component
To determine if SUNCs treatment contributes to the generation oxygen radicals (ROS) of A. niger GM31 mycelia, the intracellular ROS were further studied. In this case we used the dye DCFH-DA which can penetrate into the hyphae and after being hydrolysed by intracellular esterase to produce DCFH, which in presence of ROS is oxidised to DCF and emits green fluorescence.
As illustrated in Fig. 5A, a weak fluorescence intensity was detected in CTR hyphae, indicating the normal ROS production during the aerobic metabolism of A. niger GM31. The fluorescence increased drastically after 1 h of Argirium-SUNCs exposure and almost near 75% of the hyphae showed an evident ROS accumulation (Figure. S1, b and c).
Mitochondrial staining of A. niger GM31 with MitoTracker Green before and after treatment with Argirium-SUNCs. From left to right (nucleus-mitochondrial-overlaped): fluorescence intensity of Hoechst (nucleus), MitoTracker Green (mitochondrial viability): (A) untreated hyphae and (B) treated hyphae with 0.625 mg L−1 of Argirium-SUNCs. Scale bar: 20 μm.
The changing pattern of the ROS-positive cells is consistent with the toxicity of metal ions like Ag°/Ag+ suggesting that a high concentration of ROS is a cofactor in the cellular stress of mycelia. In most of the works the Ag+ oxidizing effect is considered the main cause of cellular toxicity31. In the present and previous works we demonstrate that a greater antimicrobial effect is obtained by membrane depolarization caused by Argirium-SUNCs which are, at the same time, less toxic than AgNO3 against human cells12. These data, all together, confirm that the AgNO3 formulation and Argirium-SUNCs exhibit two different antimicrobial mechanisms and that our ultra-nanoclusters, although more effective against fungi and bacteria, are less toxic than AgNO3 against human cells12. We investigated if the depolarization induced by Argirium-SUNCs has any effects on energy metabolism, and for this purpose the mitochondrial membrane integrity was measured. It is well known that the production of energy is one of the most principal roles of the mitochondria, it also control key physiological activities, such as lipid synthesis and trafficking, aging, reactive oxygen species production, apoptosis, and cellular signaling32. It has been well documented that when the mitochondrial membrane is damaged, ΔΨm decreases33. The confocal images of CTR of the A. niger GM31 hyphae cells (Fig. 5A) showed an intense green fluorescence after the staining with MitoTracker dye suggesting a mitochondrial potential activity. These ΔΨm decreased upon to Argirium-SUNCs treatment (Fig. 5B), affecting the energy metabolism, inhibits the normal growth of A. niger GM31. It is well known that ΔΨm is an indicator of the integrity of the mitochondrial membrane. In a previous work it has been demonstrated that mitochondrial dysfunction participates in the induction of apoptosis as a response to some cellular damage/stress and it has been underlined how it plays a central role34. This pathway induces depolarization of the transmembrane potential ΔΨm, release of apoptogenic factors and loss of oxidative phosphorylation.
As the fungal cell wall is a dynamic and developmentally plastic construction, capable of compensating for the loss of β-1,3-glucan by increased chitin deposition, we investigated the accumulation of chitin and glucan after Argirium-SUNCs treatment through the calcofluor white probe (Figure S2). Chitin is one of the major structural components of the fungal cell wall, which plays an important role in pathogen resistance and environmental stress3. The microscopic examination of the chitin and glucan-stained hyphae treated with Argirium-SUNCs revealed an irregular deposition of chitin spots within the hyphae or spore cell wall (Figure S2 (b,c)). This could mean that irregular cell wall synthesis occurs as a adaptive response to Argirium-SUNCs treatments.
Toxicity of SUNCs on mammalian cells
We have shown that treatment of the sheep fibroblasts (SAF) with SUNCs (0.625 ppm) does not affect the somatic cell's vitality. Counting the number of the live cells using Trypan blue staining there was no observed statistical difference between the treated and CTR. In addition, the absence of cytotoxic effect was confirmed using live/dead assay with Calcein-AM/PI (Fig xx).
We assessed the functionality of mitochondria using the Mito-Tracker Green dye. There were no observed important reductions in dye accumulation between the treated Argirium-SUNCs and CTR group (Fig. 6D,E). Treated SAF displayed only a lower proliferation rate compared to the CTR group, as shown by the significant difference in the percentage of BrdU-positive cells between SAF exposed to Argirium-SUNCs for 24 h compared to the CTR group (9% vs. 35%, respectively; p < 0.001) (Fig. 6A–C).
Effect of Argirium-SUNCs on fibroblasts. (A, B) The BrdU assay revealed that Argirium-SUNCs reduced cell proliferation (B) in SAF compared to control (B). The difference in the proliferating cells (%) is reported in histogram (C). The images show BrdU positive cells (green), indicative of replicating cells. All nuclei were counterstained with Hoechst 33342 (blue). Merge means Hoechst + BrdU (A, B) (n = 3 images) (*p < 0.0005; C) . Scale bar = 100 μm; magnification is 20X (D, E) Sheep fibroblasts, that were previously subjected to Arg-SUNCs treatment for 24 h (D) and control non-treated (E), were stained with Mitotracker green dye. Pictures show the amount and localisation of the active mitochondria. No observed important reductions in dye accumulation between the treated Argirium-SUNCs and CTR group indicating that SUNCs does not affect mitochondrial functionality; nuclei were stained with Hoechst 33342; Experiments were conducted in triplicate. Scale bars: 10 μm.
These results indicate that SAFs are more resistant than A. niger GM31 to SUNCs.This is in agreement with our previous toxicity studies in which the MTT values of mammalian cells (HEK-293, HaCaT, HMEC) were always higher9,10,12 than the MIC values observed in bacteria. A preclinical toxicity model also indicated that Argirium-SUNCs was not toxic to Galleria mellonella larvae up to the highest concentration of 6.8 ppm that could be used. The presence of metal ion scavenger systems such as metallothionein and/or the positive external potential of the mammalian membrane could be some of the characteristic properties of human cells that explain their greater resistance to Argirium-SUNCs than bacteria and fungi.
Synthesis and characterization of Arg-SUNCs
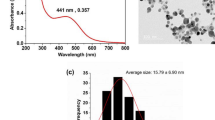
ARGIRIUM-SUNCs (Arg-SUNCs) were synthesized with a reproducible method at 20–40 ppm water solution concentration as measured by Ionic selective Electrode (ISE) and TEM (Fig. 7) in ultra-pure water without stabilizing agents or other chemical components as previously reported12. The method is protected by European Patent (EP-18181873.3) and trade mark (Argirium SUNCs ™).
The main chemical–physical properties (Table 1) of Arg-SUNCs as determined by TEM , XR-SEM, X-Ray Diffraction (XRD) , X-ray photoelectron spectroscopy (XPS) , MALDI TOF, Dynamic light scattering (DLS), UV–Vis, ISE are : a very small size 1.79 nm ± 1.004 (ultra nano clusters), plasmonic resonance spectrum (λ max at 410 nm) and non-spherical shape11.
The X-ray diffraction and XPS analysis revealed the presence of Ag3O4 and AgO crystalline phase corresponding to Ag +, Ag2+, Ag3+ silver oxides on the surface of the Arg-SUNCs and Ag° in the core. As a consequence SUNCs are characterized by negative solvation shell (Zeta Potential values in the range − 40/ − 70 mV) (Figure S3). The high absolute zeta potential value suggests that nanoparticles surrounded by anionic salvation tend to repulse each other, avoiding any aggregation process and explains the long stability (> 1 year). The Oxidation–reduction potential (ORP) is resulted + 400 mV in comparison to − 90 mV obtained from the Dithiothreitol (DTT) Reducing Agent standard solution (Thermo Scientific™).
This surprisingly high ORP value indicates an high oxidative capacity above the Ag ° torque (+ 0.80 Volt)//H2O2 (+ 1.78 Volt) as deduced from the redox values of standard potentials35and compatible with an unusual superficial presence of Ag2+, Ag3+ was never observed before in stable form in ultra-pure water.
Result and discussion
Antifungal activity
Strain and inoculum
In this study we used the strain A. niger GM31 provided by the Faculty of Bioscience and Technology for Food, Agriculture and Environment, University of Teramo. The strain was isolated previously from food sources and identify by molecular methods by using the internal transcribed spacer (ITS) region. Primers used were ITS1/ITS4 as described by Molina Henandez et al.11.
The strain from stock culture tubes was reactivated and cultivated in malt extract agar (MEA-OXOID) medium plates and incubated for 5 days at 28 °C, in the dark. Conidia formed were collected in 1 mL of sterile physiological solution and washed twice. Successively the concentration of the spores was adjusted to 105/ml and 100μL of conidia suspension were inoculated in 250 mL of MEA and incubated for 5 days, at 28 °C in a rotary shaker (110 g) incubator (Centomat BS-T, B.Braun, Milan, Italy). Successively fungal mycelium was collected by centrifugation (15 min, 4 °C, 100 g), and used for further experiments.
Determination of the antifungal activity of NANO
Following this equation:
where, the dried mass t indicates the dried mycelia biomass after a determined incubation time (t), while the dried mass control indicates the dried mycelial biomass of the untreated sample (at the same time of incubation).
The minimal inhibitory concentrations (MIC) of SUNCs against the A. niger GM31 mycelia was determined in flasks of 100 ml containing 40 ml of malt extract broth (MEB) in which 0.625, 1.25, 2.5, 4, 8 and 10 mg L−1 of Argirium-SUNCs previously filtered (0.22 μm pore filter -Whatman International, Maidstone, UK) were added, successively 0.20 g of mycelium were inoculated and incubated at 28 °C in a rotary shaker (110 g). Untreated mycelia were used as a control. Three different replicates for each concentration were performed and the experiment was repeated two times. The mycelial growth inhibition, measured as the percentage of dried mycelia biomass, was determined according to Scroccarello et al.19.
The effect of Argirium-SUNCs on the conidia germination was evaluated following the methodology reported by Peralta-Ruiz et al.36. Briefly, twenty microliters of conidia suspension (9.8 × 106 CFU mL −1) were deposited onto slides previously coated with a thin layer of MEA medium, and immediately added with the different concentration of Arg-SUNCs. The slides were incubated at 28 °C and observed with a phase-contrast optical microscope after 4, 6, 8, 10 and 24 h of treatment. Conidia were considered germinated when the germinative tube resulted longer than the same conidia.
Impact of Arg-SUNCs on fungal membrane depolarization
Cytoplasmic membrane depolarization of Aspergillus nigerMG13 was measured using a membrane potential sensitive probe DiBAC4 (Bis-(1,3-Dibutylbarbituric Acid Trimethine Oxonol, Invitrogen). Dye uptake and resultant self-quenching are modulated by the membrane potential; thus the samples were prepared by putting 100 μL of conida solution standardized in 1 ml MEB growing medium on a sterile coverslip (22 mm × 22 mm), incubated at 28 °C for 18 h. Then, one aliquoted equivalent of sub MIC concentration (0.625 mg L−1 Arg-SUNCs) were added onto coverslips and incubated for 6 h, the control was prepared by adding 100 μL of Milli-Q water. Subsequently, coverslips were washed with PBS and the adherent hyphae were treated with an equivalent volume of 20 μg/ml of DiBAC4 and incubated for 30 min in the dark at room temperature and finally washed twice with PBS. Stained Aspergillus niger GM31 hyphae were examined and the decrease in potential was monitored by the increase in fluorescence under a fluorescence microscope at 493 nm excitation and 516 nm emission wavelengths. Measurements were repeated at least twice.
Intracellular ROS accumulation
To evaluate the intracellular ROS accumulation after Argirium-SUNCs treatment, we used also sublethal concentration (0.625 mg L−1 Arg-SUNCs) of the nano material. The ROS detection was assessed using 2´,7´- dichloro dichlorofluorescein diacetate (H2DCFDA; Molecular Probes®) described by Lui et al.37 with some modification. Condia were cultured in MEB medium and treated as described above. After treatments with Argirium-SUNCs, the adherent hyphae were washed with phosphate buffer saline (PBS- pH 7). Then 10 µM H2DCFDA (dissolved in dimethyl sulfoxide) was added and incubated for 1 h and washed twice with PBS. For fluorescence detection was measured at excitation and emission wavelengths of 485 nm and 535 nm.
Chitin accumulation
For detection of Chitin accumulation, germinated conidia were stained with calcofluor white and Blue Evans (Sigma), according to the methodology reported by Geißel et al. 2018 with some modification. After 18 h of incubation hyphae were treated with 0.625 mg L-1 of Arg-SUNCs for 18 h, immediately remove supernatant, and resuspended in MEB for 14 h of incubation. Samples were stained with 1 ml (10 mg ml−1) calcofluor white dissolved in Evans Blue for approximately 1 min. For fluorescence detection was measured at excitation and emission wavelengths of 423–443 nm.
Changes in mitochondrial activity
To detect if Argirium-SUNCs induce a change of mitochondrial and nuclei, hyphae were stained with the fluorescent dye MitoTracker (MT) Green FM (Invitrogen) and Hoechst 33,342 (Sigma) according to the manufacturer’s protocol. Also, in this case we used 0.625 mg L−1 Arg-SUNCs. The procedure was modified for Aspergillus niger staining. Spores were cultured in MEB medium and treated as described above. After of incubation period, hyphae were stained with MT green at 37 °C /45 min, the medium was removed and immediately counterstained with Hoechst 33342 and incubated at 25 °C/for 10 min. Finally, fluorescence was measured.
Release intracellular proteins
The release of intracellular protein after treatment with Argirium-SUNCs was estimated using the method of Bradford. 10 ul of supernatant (MEB) was used for measuring the protein contents and analyzed using UV spectrophotometer (Jenway™ Spectrophotometry UV–Visible Jenway™ 6305, Dunmow, EU). For quantitative analysis, bovine serum albumin (BSA) was used as a standard protein. Data was statistical analyzed for five repetitions using ANOVA (p ≤ 0.05; Tukey HSD post-hoc test).
Cell toxicity
Cell culture and treatments
Sheep adult fibroblasts (SAF) used for the experiments have been derived from one month-old sheep, previously collected from a local slaughterhouse as refuse animal material. Primary cultures of SAF were obtained from small pieces of ears by mechanical (by blades) and enzymatic (by trypsin digestion at 38.5 °C) disaggregation. Cells were expanded in Minimum Essential Medium (MEM) enriched with 10% Fetal Bovine Serum (FBS), 2 mM l-Glutamine, 26 mM NaHCO3 and 50 μg/ml Gentamicin. After three passages, cells were assigned to the following groups: (i) treated group (NP), cultured in the above medium supplemented with 0.625 ppm of Arg-SUNCs for 24 h; (ii) control group (CTR), cultured in medium without NP. The working dose of the Argirium-SUNCs tested on SAF in this study (0.625 ppm) was chosen on the basis of preliminary experiments carried out to assess the NP concentration that produced significant effects on fungal cells. Cell vitality was evaluated using Trypan blue staining.
In addition to evaluate the cytotoxic effect in SAF cultured for 24 h in presence of NP 0.625 ppm, a LIVE/DEAD assay (Calcein AM/Propidium Iodide, PI, Invitrogen) was used too (Fig. 8). This cell viability assay allows the simultaneous fluorescence staining of viable cells (1 uM Calcein-AM for 20 min, green fluorescence) and dead cells (5 μM Propidium Iodide/PI for 5 min, red fluorescence). Calcein-AM fluoresces green, relying on esterase activity present only in metabolically active viable cells. PI is a polar nucleic acid dye that is excluded by the membrane of live cells, but enters the damaged membrane of dead cells and emits nuclear red fluorescence.
LIVE/DEAD assay in SAF cultured for 24 h with or without NP 0,625 ppm. A cell viability assay was used with SAF cultures for simultaneous fluorescence staining of viable cells (Calcein-AM, green fluorescence) and dead cells (Propidium Iodide PI, red fluorescence). The total cell number was evaluated using the viable nucleic acid staining Hoechst 33,342 (blue fluorescence). Confocal microscopy was used for image acquisition. As shown in images, both in CTRL and in NP 0,625 ppm groups, cells emit a bright green fluorescence. On the contrary, in POSITIVE CTRL, cells did not show any green fluorescence while nuclei emit red fluorescence. CTRL: SAF fibroblast cultured for 24 h without NP; NP: SAF cultured for 24 h with NP at the concentration of 0,625 ppm; POSITIVE CTRL: a sample of SAF cultured for 24 h in presence of very high/cytotoxic concentration (12,5 ppm) of NP was used as a positive control.
The total cell number was evaluated using the viable nucleic acid staining Hoechst 33342 (1 ug/ml for 5 min, blue fluorescence). SAF cultured for 24 h in presence of very high/cytotoxic concentration (12.5 ppm) of NP were used as a positive control. Images were acquired by confocal microscopy at 20X magnification, using appropriate filter sets for the three dyes.
Cell proliferation assay
Cell proliferation was evaluated by an immunocytochemistry assay for 5-bromo-2′-deoxyuridine (BrdU), a thymidine analog incorporated during S-phase in replicating cells if previously added to culture medium. One day before the immunocytochemistry, cells were plated in multiwell slides (Millicell EZ Slide, Millipore), in number of 10,000/well. Briefly, 24 h post treatment with Argirium-SUNCs, SAF were incubated with 100 μM BrdU for 4–6 h, fixed in cold methanol for 20 min and permeabilized with 0.1% Triton X-100, 15 min at room temperature (RT). Next, cells were treated with 4 N HCl 30 min, RT, and incubated with primary antibody (Ab I) (mouse anti-BrdU, monoclonal antibody, B2531, Sigma) 1:100 in blocking solution (BS) (0.1% Bovine Serum Albumin (BSA) in PBS) at 4 °C, overnight. Then, cells were incubated with secondary antibody (Ab II) (rabbit anti-mouse IgG-FITC polyclonal antibody, F9137, Sigma) 1:500 in BS for 2 h RT, and counterstained with 0.5 μg/ml Hoechst for 5 min. Between all steps, cells were washed twice in PBS, 5 min, RT. Finally, slides were mounted with Fluoromount and observed under an epifluorescent microscope Nikon Eclipse E600, at 20 × magnification. All nuclei were counted, while only the BrdU-positive nuclei have been considered as replicating cells. Cell proliferation rate was obtained by the ratio between the number of BrdU-positive cells (green) and the total number of cells (blue). For statistical valence, we considered at least 200 nuclei for each group.
Materials and methods
MitoTracker assay
To assess mitochondrial functionality, SAF cells after incubation with NP were further incubated at 38.5 °C for 30 min in medium supplemented with 0.5 mM MitoTracker green (Invitrogen, Milan, Italy), a cell permeant fluorescent probe that accumulates in active mitochondria. Then, cells were counterstained with 0.5 μg/ml Hoechst for 5 min, washed in PBS and mounted on glass slides. The intensity of the fluorescent signal indicating mitochondrial activity was measured using the confocal microscope Nikon Eclipse E600.
Statistical analysis
The analysis of variance (ANOVA) was carried out to every variable studied and Tukey post-hoc test (HSD) was performed to analyze the statistical difference among means. Principal Components Analysis (PCA) based on Spearman correlation matrix, was obtained using all the variables to reduce dimensionality and better represent the whole dataset. Data were analyzed using both STATISTICA software (StatSoft, Hamburg, Germany) and XLSTAT 2021 software (Addinsoft, Paris, France).
Inactivation of mycelia and spore germination
3.4 Argirium-SUNCs showed strong antimicrobial properties against mycelia and spore Inactivation MICS values of 1.25 ppm (Fig. 1A). In particular 0.625 ppm of Argirium-SUNCs treatments significantly decreased the mycelia production (P < 0.05) of about 80%. With the enhancement of Arg-SUNCs concentration the mycelia growth was further reduced reaching 100% already at 1.25 ppm after 7 days of incubation at 28 °C. To observe if the anti-fungal activity was fungicidal of fungistatic the treated mycelia with 1.25, 2.5, 4, 8 and 10 ppm were washed with PBS buffer and further inoculated in a new liquid media for 7 days more at the same temperature. After these incubation period, we did not register any mycelia growth suggesting a fungicidal effect of the Arg-SUNCs already at 1.25 ppm.
Conclusions
The presence of stable Ag2+ and Ag3+ cationic forms in ultra pure water solution is a novelty that distinguishes Argirium-SUNCs from any other nanomaterial. The higher redox capacities of our ultra-nano clusters compared to Ag+ explain their antibacterial and anti-fungal efficacy at a very low concentration, as well as their unique antimicrobial mechanism. The main biological target of Argirium-SUNCs is the cell membrane whose depolarization and subsequent loss of function leads to bacterial and fungal death. Not surprisingly, the same mechanism is not reported in other silver formulations usually characterized by Ag0 and Ag+ elements which we have found to be incapable of depolarizing the cell membrane. In vitro and preclinical toxicity studies indicate that Argirium-SUNCs are about 10 times less toxic in human cells than in bacteria and fungi.
The specific antibacterial mechanism and experimental evidence indicate that Argirium-SUNCs overcome the phenomenon of the antibiotic resistance. Unlike traditional drugs, being effective against both bacteria and fungi, they represent a new and completely different pharmacological strategy against infectious diseases.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author. The patent referenced in the manuscript is held only by Luca Scotti without any potential conflict of interest.
References
Otto Cars, S. J. C., Mpundu, M., Peralta, A. Q., Zorzet, A. & So, A. D. Resetting the agenda for antibiotic resistance through a health systems perspective. Lancet Glob. Health 9, 6. https://doi.org/10.1016/S2214-109X(21)00163-7 (2021).
A.A. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022. report WHO, 71, doi:https://doi.org/10.1073/pnas.1717295115 (2022).
Moebius, N., Uzum, Z., Dijksterhuis, J., Lackner, G. & Hertweck, C. Active invasion of bacteria into living fungal cells. Elife 3, e03007. https://doi.org/10.7554/eLife.03007 (2014).
Duval, R. E., Gouyau, J. & Lamouroux, E. Limitations of recent studies dealing with the antibacterial properties of silver nanoparticles: Fact and opinion. Nanomaterials (Basel) https://doi.org/10.3390/nano9121775 (2019).
Siddiqi, K. S., Husen, A. & Rao, R. A. K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 16, 14. https://doi.org/10.1186/s12951-018-0334-5 (2018).
Mousavi, S. M. et al. Green synthesis of silver nanoparticles toward bio and medical applications: Review study. Artif. Cells Nanomed. Biotechnol. 46, S855–S872. https://doi.org/10.1080/21691401.2018.1517769 (2018).
Salem, S. S. & Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 199, 344–370. https://doi.org/10.1007/s12011-020-02138-3 (2021).
Scotti, L., Angelini, G., Gasbarri, C. & Bucciarelli, T. Uncoated negatively charged silver nanoparticles: Speeding up the electrochemical synthesis. Mater. Res. Exp. https://doi.org/10.1088/2053-1591/aa8c39 (2017).
Grande, R. et al. Antimicrobial and antibiofilm activities of new synthesized silver ultra-nanoclusters (SUNCs) against helicobacter pylori. Front. Microbiol. 11, 1705. https://doi.org/10.3389/fmicb.2020.01705 (2020).
Pompilio, A. et al. Electrochemically synthesized silver nanoparticles are active against planktonic and biofilm cells of pseudomonas aeruginosa and other cystic fibrosis-associated bacterial pathogens. Front. Microbiol. 9, 1349. https://doi.org/10.3389/fmicb.2018.01349 (2018).
Molina-Hernandez, J. B. et al. The membrane depolarization and increase intracellular calcium level produced by silver nanoclusters are responsible for bacterial death. Sci. Rep. 11, 21557. https://doi.org/10.1038/s41598-021-00545-7 (2021).
Gasbarri, C. et al. Structure and properties of electrochemically synthesized silver nanoparticles in aqueous solution by high-resolution techniques. Molecules https://doi.org/10.3390/molecules26175155 (2021).
Khalandi, B. et al. A review on potential role of silver nanoparticles and possible mechanisms of their actions on bacteria. Drug Res. (Stuttg) 67, 70–76. https://doi.org/10.1055/s-0042-113383 (2017).
Adeyemi, O. S., Shittu, E. O., Akpor, O. B., Rotimi, D. & Batiha, G. E. Silver nanoparticles restrict microbial growth by promoting oxidative stress and DNA damage. EXCLI J. 19, 492–500. https://doi.org/10.17179/excli2020-1244 (2020).
Yuan, Y. G., Peng, Q. L. & Gurunathan, S. Effects of silver nanoparticles on multiple drug-resistant strains of staphylococcus aureus and pseudomonas aeruginosa from mastitis-infected goats: An alternative approach for antimicrobial therapy. Int. J. Mol. Sci. https://doi.org/10.3390/ijms18030569 (2017).
Pokhrel, L. R., Jacobs, Z. L., Dikin, D. & Akula, S. M. Five nanometer size highly positive silver nanoparticles are bactericidal targeting cell wall and adherent fimbriae expression. Sci. Rep. 12, 6729. https://doi.org/10.1038/s41598-022-10778-9 (2022).
Salem, S. S., Ali, O. M., Reyad, A. M., Abd-Elsalam, K. A. & Hashem, A. H. Pseudomonas indica-mediated silver nanoparticles: Antifungal and antioxidant biogenic tool for suppressing mucormycosis fungi. J. Fungi (Basel) https://doi.org/10.3390/jof8020126 (2022).
Cornet, M. et al. Epidemiology of invasive aspergillosis in France: A six-year multicentric survey in the Greater Paris area. J. Hosp. Infect. 51, 288–296. https://doi.org/10.1053/jhin.2002.1258 (2002).
Scroccarello, A. et al. Effect of phenolic compounds-capped AgNPs on growth inhibition of Aspergillus niger. Colloids Surf. B Biointerfaces 199, 111533. https://doi.org/10.1016/j.colsurfb.2020.111533 (2021).
Stater, E. P., Sonay, A. Y., Hart, C. & Grimm, J. The ancillary effects of nanoparticles and their implications for nanomedicine. Nat. Nanotechnol. 16, 1180–1194. https://doi.org/10.1038/s41565-021-01017-9 (2021).
Ojha, M. & Barja, F. Spatial and cellular localization of calcium-dependent protease (CDP II) in Allomyces arbuscula. J. Cell Sci. 116, 1095–1105. https://doi.org/10.1242/jcs.00307 (2003).
Osherov, N. & May, G. S. The molecular mechanisms of conidial germination. FEMS Microbiol. Lett. 199, 153–160. https://doi.org/10.1111/j.1574-6968.2001.tb10667.x (2001).
Craig, C., Wood, R. J. R. & Kennedy, I. R. Membrane potential, proton and sodium motive forces in Azospirillum brasilense Sp7-S. FEMS Microbiol. Lett. 164, 7. https://doi.org/10.1111/j.1574-6968.1998.tb13101.x (1998).
Abdul Kadir, L., Stacey, M. & Barrett-Jolley, R. Emerging roles of the membrane potential: Action beyond the action potential. Front. Physiol. 9, 1661. https://doi.org/10.3389/fphys.2018.01661 (2018).
Guido Angelini, L., Aceto, A. & Gasbarri, C. Silver nanoparticles as interactive media for the azobenzenes isomerization in aqueous solution: From linear to stretched kinetics. J. Mol. Liq. 284, 7. https://doi.org/10.1016/j.molliq.2019.04.048 (2019).
Burkhard Standke, M. J. Ag3O4, the first silver(II, III) oxide. Angewandte 25, 2. https://doi.org/10.1002/anie.198600771 (1986).
Burkhard Standke, M. J. Ag, 03, a novel binary silver oxide. Angen. Chem. Int. 2, 2. https://doi.org/10.1002/anie.198501181 (1985).
Yang Hui, R.-Y., Tao, W. & Chuang, W. Preparation and antibacterial activities of Ag/Ag+/Ag3+ nanoparticle composites made by pomegranate (Punica granatum) rind extract. Results Phys. 6, 299–304. https://doi.org/10.1016/j.rinp.2016.05.012 (2016).
Rosch, J. W., Sublett, J., Gao, G., Wang, Y. D. & Tuomanen, E. I. Calcium efflux is essential for bacterial survival in the eukaryotic host. Mol. Microbiol 70, 435–444. https://doi.org/10.1111/j.1365-2958.2008.06425.x (2008).
Rizzuto, R. et al. Calcium and apoptosis: Facts and hypotheses. Oncogene 22, 8619–8627. https://doi.org/10.1038/sj.onc.1207105 (2003).
McShan, D., Ray, P. C. & Yu, H. Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 22, 116–127. https://doi.org/10.1016/j.jfda.2014.01.010 (2014).
Brieger, K., Schiavone, S., Miller, F. J. Jr. & Krause, K. H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 142, w13659. https://doi.org/10.4414/smw.2012.13659 (2012).
Knorre, D. A. & Severin, F. F. Longevity and mitochondrial membrane potential. Biochemistry (Mosc) 77, 793–794. https://doi.org/10.1134/S0006297912070127 (2012).
Jeong, S. Y. & Seol, D. W. The role of mitochondria in apoptosis. BMB Rep. 41, 11–22. https://doi.org/10.5483/bmbrep.2008.41.1.011 (2008).
Zoski, C. Vol. 1 934 (Elsevier Science, 2006).
Peralta-Ruiz, Y. et al. Colletotrichum gloesporioides inhibition using chitosan-Ruta graveolens L essential oil coatings: Studies in vitro and in situ on Carica papaya fruit. Int. J. Food Microbiol. 326, 108649. https://doi.org/10.1016/j.ijfoodmicro.2020.108649 (2020).
Liu, L., Trimarchi, J. R., Smith, P. J. & Keefe, D. L. Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell 1, 40–46. https://doi.org/10.1046/j.1474-9728.2002.00004.x (2002).
Author information
Authors and Affiliations
Contributions
L.S. wrote the main manuscript text, experimental project section, and make characterization of nanomaterials and biochemistry interpretation and laboratory. J.B.M. complete an experimental section on microbiology. D.P. make an experimental section on microbiology. L.G. microbiology characterization. L.V. confocal experimental section. M.R.C. and A.P. reviewed the manuscript. A.A. project experimental section. C.C.L. project experimental section. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Molina Hernandez, J.B., Scotti, L., Valbonetti, L. et al. Effect of membrane depolarization against Aspergillus niger GM31 resistant by ultra nanoclusters characterized by Ag2+ and Ag3+ oxidation state. Sci Rep 13, 2716 (2023). https://doi.org/10.1038/s41598-023-29918-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29918-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.