Abstract
Epigenetic influence plays a role in the association between exposure to air pollution and attention deficit hyperactivity disorder (ADHD); however, research regarding sulfur dioxide (SO2) is scarce. Herein, we investigate the associations between prenatal SO2 exposure and ADHD rating scale (ARS) at ages 4, 6 and 8 years repeatedly in a mother–child cohort (n = 329). Whole blood samples were obtained at ages 2 and 6 years, and genome-wide DNA methylation (DNAm) was analyzed for 51 children using the Illumina Infinium HumanMethylation BeadChip. We analyzed the associations between prenatal SO2 exposure and DNAm levels at ages 2 and 6, and further investigated the association between the DNAm and ARS at ages 4, 6 and 8. Prenatal SO2 exposure was associated with ADHD symptoms. From candidate gene analysis, DNAm levels at the 6 CpGs at age 2 were associated with prenatal SO2 exposure levels. Of the 6 CpGs, cg07583420 (INS-IGF2) was persistently linked with ARS at ages 4, 6 and 8. Epigenome-wide analysis showed that DNAm at 6733 CpG sites were associated with prenatal SO2 exposure, of which 58 CpGs involved in Notch signalling pathway were further associated with ARS at age 4, 6 and 8 years, persistently. DNAm at age 6 was not associated with prenatal SO2 exposure. Changes in DNAm levels associated with prenatal SO2 exposure during early childhood are associated with increases in ARS in later childhood.
Similar content being viewed by others
Introduction
Attention deficit hyperactivity disorder (ADHD) is a neurobehavioral disorder characterized by inattentiveness, hyperactivity, and impulsivity1. Children with ADHD have difficulties in learning, family relationships, and social interaction2. ADHD affects 5–7% of children and adolescents worldwide3,4. Furthermore, at least 5% of children do not meet the full diagnostic criteria despite exhibiting ADHD symptoms5. ADHD in children often persists into late adolescence and adulthood, which is a risk factor of other mental health issues, including antisocial behaviors, self-harm, and substance misuse5.
Although the etiology of ADHD is largely unknown and complex, it is characterized by numerous gene-environmental interactions6. Preconceptional, gestational, and perinatal conditions have been indicated to affect ADHD6. Maternal nutrition during pregnancy, pesticides and heavy metal exposure during gestation, premature birth have been reported as risk factors of ADHD in children6. Prenatal exposure to air pollution is also a potential risk factor for ADHD7,8. Previous studies have reported an association between children’s behavioral development and prenatal exposure to sulfur dioxide (SO2)9,10, one of the major gaseous pollutants derived from coal-fired power plants, smelters, and industrial emissions11. Although the relation between SO2 exposure and ADHD has not been well investigated, SO2 has been associated with various neurodevelopmental deficits, such as fine motor skills, executive function, and ADHD-related hospital admissions12,13.
Epigenetic processes such as DNA methylation (DNAm) have been proposed to underly the association between environmental exposures and ADHD14. A previous study has demonstrated that methylation differences of the growth factor-independent 1 transcriptional repressor (GFI1) region partially mediated the association between maternal smoking during pregnancy and ADHD symptoms at age 615. Another study found that long-term prenatal exposure to paracetamol (acetaminophen) is associated with DNAm differences in children diagnosed with ADHD16. Recent evidence suggests that air pollution may affect methylation through an oxidative stress pathway17,18. Air pollution-induced reactive oxygen species oxidize 5-hydroxymethylcytosine causing DNA demethylation19. It also leads to the hypomethylation of CpG cytosine residues by inhibiting the activity of methyltransferases via alteration in their sequence alignment to the corresponding base sequences of the DNA20. To date, no study has investigated the mediation effect of DNAm on the association between SO2 and ADHD.
In this study, we aimed to (1) examine the association between prenatal exposure to SO2 and ADHD symptoms at multiple ages during childhood and (2) investigate the effect of DNAm on the association between SO2 exposure and ADHD symptoms. We first analyzed the association between prenatal SO2 and ADHD symptoms at ages 4, 6 and 8, which are critical periods of symptom manifestation. Then, we identified CpG sites (CpGs) associated with both prenatal SO2 exposure and ADHD symptoms during childhood. We utilized both candidate gene analysis approach by targeting CpGs associated with ADHD symptoms, and an epigenome-wide association study. We also aimed to detect co-methylated CpGs from a module correlated with both prenatal SO2 exposure and ADHD symptoms using weighted gene co-metylation network analysis (WGCNA).
Results
General characteristics of study participants
There were significant differences in maternal smoking and SO2 exposure during the 1st trimester between the entire cohort and the sub-cohort (Table 1). Other covariates were not significantly different between the entire cohort and sub-cohort. The mean age of mothers at pregnancy was 31.3 years, most of the mothers were college graduates (70.5–71.7%), while 76.9–79.6% of mothers were exposed to environmental tobacco smoking (ETS) during pregnancy. Mean maternal IQ was 116–118. Mean maternal SO2 exposure during pregnancy was 0.0044–0.0060 ppm depending on the trimester. Among children, 51.0–53.5% were girls, 86.3–90.3% were singleton, 88.0–91.8% were born full-term. Mean postnatal SO2 exposure ranged from 0.052 to 0.058 ppm depending on the ages. Mean ADHD rating scale (ARS) was 5.96–6.96 at age 4, 5.87–6.04 at age 6, and 5.57–6.34 at age 8. In the sub-cohort, prenatal and postnatal SO2 exposure were not significantly different according to the trimester or ages (Fig. 1).
Selection of candidate CpGs
After the systematic review, a total of 22 studies were selected17,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41 (Table S1). A total of 597 CpGs were pooled from these studies (Table S2), and final 375 CpGs were selected for analysis. The detailed result of the systematic review and selection of candidate CpGs is presented in Supplementary Materials and Fig. S1.
Association between prenatal exposure to SO2 and ADHD symptoms
In the entire cohort (n = 329), one interquartile range (IQR) increase in prenatal SO2 exposure during the 1st trimester of pregnancy was significantly associated with 8.38% (95% CI 3.19, 13.83) increase in ARS at age 4; however, such association was not observed at age 6 or 8 (Table 2). The associations between prenatal SO2 exposure during the entire period or 2nd trimester of pregnancy and childhood ADHD symptoms at ages 4, 6 and 8 were not significant. Prenatal SO2 exposure during the 3rd trimester of pregnancy was not associated with ADHD symptoms at ages 4 or 6, but was negatively associated with ADHD symptoms at age 8.
Association between prenatal exposure to air pollution and DNAm
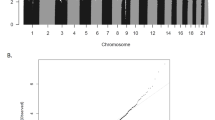
DNAm at age 2 at the candidate CpGs was not associated with prenatal SO2 exposure at the 1st–3rd, 1st, or 2nd trimester of pregnancy (Tables S3–S5). However, DNAm in children at age 2 at cg07583420 (INS-IGF2), cg20296524 (TARBP1), cg15705054 (PBXIP1), cg05075097 (INS-IGF2), and cg25163476 (INS-IGF2) were positively associated with an IQR increase in maternal SO2 exposure during the 3rd trimester of pregnancy (Table 3, Fig. 2A, Table S6). DNAm at cg05951817 (SLC6A4) was negatively associated with SO2 exposure during the 3rd trimester. DNAm at the 375 candidate CpGs in children at age 6 was not associated with prenatal SO2 exposure in any trimester (Tables S7–S10). We also conducted the epigenome-wide analysis for 326,898 CpGs to investigate the association between prenatal SO2 exposure and DNAm at age 2. We found that a total of 6,733 CpG sites were associated with prenatal exposure during the 3rd trimester with FDR-corrected p-value < 0.05 (Table S11, Fig. 2B), and the 6 CpGs listed above were included in the 6733 CpGs. Conversely, neither candidate gene analysis nor EWAS yielded significant CpGs within FDR-corrected p-value < 0.05 at age 6 (Fig. 2C).
The association between prenatal SO2 exposure, DNA methylation, and ADHD rating score. (A) The association between prenatal SO2 exopusre and DNA methyhlation. (B) Manhattan plot showing the association between prenatal SO2 exposure during the 3rd trimester and DNA methylation at age 2. The green dots indicate CpGs selected from literature review for a candidate gene analysis. The blue line is the threshold for FDR-corrected p-value < 0.05. The CpGs significantly associated with prenatal SO2 exposure from the candidate gene analysis were above the threshold line (cg07583420 (INS-IGF2), cg20296524 (TARBP1), cg15705054 (PBXIP1), cg05075097 (INS-IGF2), and cg25163476 (INS-IGF2)). (C) Manhattan plot showing the association between prenatal SO2 exposure during the 3rd trimester and DNA methylation at age 6. (D) The association between DNA methylation at the CpGs linked with prenatal SO2 exposure and the ADHD rating scale at ages 4, 6 and 8.
Association between postnatal SO2 exposure and DNAm
The associations between the postnatal SO2 exposures at age of 2 years and DNAm at age 2, or the association between the postnatal SO2 exposure at ages 2, 4 or 6 years and DNAm at age 6 were not significant (Tables S12–S15). The associations between the cumulative postnatal SO2 exposures from age 2 to 4 or from 2 to 6 and DNAm at age 6 were not significant (Tables S16,S17).
Association between DNAm and ADHD symptoms
Among the 6 CpGs with significant associations at age 2 with prenatal SO2 exposure, DNAm level at cg07583420 (INS-IGF2) were associated with 37.25% (95% CI 8.12, 74.22), 37.00% (95% CI 4.23, 80.08), and 34.82% (95% CI 5.44, 72.40) increase in ARS at ages 4, 6 and 8, respectively (Fig. 2D, Table S18).
Among 6733 CpGs from EWAS between prenatal SO2 exposure during the 3rd trimester and DNAm, 58 CpGs, 219 CpGs, and 2063 CpGs were associated with ARS at ages 4, 6, and 8 years, respectively (Tables S19–S21). Among these, DNAm at 58 CpGs was persistently associated with ARS at ages 4, 6 and 8 years (Fig. S2).
Mediation analysis
The indirect effect of prenatal exposure during the 3rd trimester on ARS in childhood through DNAm at cg07583420 (INS-IGF2) was positive and significant at ages 4 (p-value 0.028) and 6 (p-value 0.004) but not significant at age 8 (Table 4, Fig. 3). Direct effects, from prenatal exposure to childhood ARS, were marginally significant at ages 6 (p-value 0.060) and 8 (p-value < 0.001). Particularly at age 6, indirect effect, direct effect, and total effect (indirect effect + direct effect) were all positive and significant or marginally significant.
Identifying methylation quantitative trait loci (mQTL)
A total of 60 SNPs were found within 100 kb from either side from cg07583420 (INS-IGF2). Four SNPs showed significant association with the DNA methylation levels at cg07583420 (p-value < 0.05): rs139951739, rs6578246, rs17885785, and rs3213223. However, none was significant when corrected for multiple comparisons using FDR method (Table S22, Fig. S3).
Pathway enrichment analysis
Reactome pathways analysis showed that mostly NOTCH signalling was involved in genes annotated to the 58 CpGs associated with prenatal SO2 exposure during the 3rd trimester and ARS through 4, 6 and 8 years (Table S23, Figure S4).
WGCNA
A total of 375 CpGs were analyzed for co-methylation. Two modules were detected: grey (316 CpGs) and turquoise (59 CpGs) (Fig. 4A,B). The correlation between the grey module and prenatal exposure to SO2 during the 3rd trimester was not significant (Fig. 4C). CpGs from the grey modules were analyzed again for co-methylation, and the turquoise module was correlated with ARS at age 4 (Pearson’s coefficient correlation 0.38, p-value 0.003), and the grey module was correlated with ARS at age 8 (Pearson’s coefficient correlation 0.28, p-value 0.03) (Fig. 4D). CpGs from the final turquoise module were assessed, and the CpGs associated with SO2 exposure during the 3rd trimester of pregnancy including INS-IGF2, PBXIP1, and SLC6A4 (Table 3) were found from the turquoise module (Fig. 4E), suggesting that these CpGs are co-methylated in relation with ARS at age 4.
Weighted gene co-methylation network analysis of DNA methylation at CpGs for candidate gene analysis. (A) Dendrogram produced by hierarchical chlustering of samples. Modules of co-methylated CpGs are indicated by different color (grey and turquoise) beneath the dendrogram. (B) Network heatmap plot. Dendrograms from hierarchical clustering are corresponded to the color-coded modules (grey and turquoise). In the heatmap, bright yellow color indicate high co-expression inter-connectedness. (C) Correlating modules with exposure variables (prenatal SO2 exposure by trimester). (D) Correlating modules with outcome variable (ADHD rating scales at age 4, 6 and 8). (E) Network between CpGs from the turquoise module.
Discussion
To the best of our knowledge, this is the first study to report the epigenetic effect on the positive association between prenatal SO2 exposure and ADHD symptoms at ages 4, 6 and 8 years in a mother–child cohort. For a candidate gene analysis, we selected 375 CpGs from a systematic literature review of EWAS of ADHD. In the sub-cohort with available DNAm measurements at age 2, an IQR increase in prenatal SO2 exposure during the 3rd trimester was associated with an increased level of DNAm at CpGs of INS-IGF2, TARBP1, PBXIP1, and SLC6A4. Among these, DNAm level at cg07583420 (INS-IGF2) was positively associated with ARS at ages 4, 6 and 8, persistently (Fig. 5). When significant CpGs (n = 6733) from EWAS were tested for the association with ARS, 58 CpGs were persistently associated with ARS at ages 4, 6 and 8 years, which were involved in NOTCH signalling pathway. DNAm at age 6 was not associated with prenatal SO2 exposure. We further investigated the association between postnatal exposure to SO2 and DNAm by several different approaches, which showed no significant result. This implies that prenatal SO2 exposure may affect DNAm more significantly than postnatal SO2 exposure.
Prenatal environmental exposure may affect ADHD development through DNAm, which may lead to long-term phenotype changes42. The fact that DNAm was associated with prenatal SO2 at age 2 but not at age 6 may be related to the trajectory of global DNAm over the developmental stages, which fluctuates at an earlier stage of development and later stabilized43. This implies that a sensitive window period of DNAm may exist in which DNAm at earlier childhood is more susceptible to environmental triggers than later childhood44. For example, DNAm at several CpGs at age 7 was mostly predicted by early childhood adversity before age 345. To investigate the period of clinical manifestation of ADHD with respect to DNAm, we examined ADHD symptoms at ages 4, 6 and 8. Surprisingly, DNAm was associated with ADHD symptoms during the wide range of early childhood from ages 4 to 8 consistently rather than focal stage of childhood, suggesting that the effect of DNAm can extend to a long period.
DNAm is also under genetic control if mQTL is identified adjacent to cg07583420 (INS-IGF2). In the present study, we could not identify statistically significant mQTL, possibly due to the small sample size. Further studies with a larger sample size are therefore warranted to elucidate the complex interplay between genetic, epigenetic, and environmental factors in the etiology of ADHD in children.
Although ADHD is associated with various compounds of air pollution, studies on the relationship between SO2 and neurodevelopmental outcomes, like ADHD, are limited. SO2 exposure prenatally up to 12 months was negatively associated with fine motor scores at 18 months of age12, and SO2 exposure was related to poor executive function in 6–12-year-old children in a cross-sectional study in China12. Another study reported an association between short-term exposure to SO2 and ADHD-related hospital admission in adolescents aged 10–19 years13.
The biological mechanism underlying the association between SO2 exposure and ADHD remains unknown. Therefore, only a few hypothetical mechanisms can be inferred from previous studies. SO2 can increase lipid peroxidation in the brain and generate reactive oxygen species46, as well as various inflammatory cytokines. These reactive oxygen species and inflammatory cytokines can relocate to the central nervous system via systemic circulation, thereby inducing neuroinflammation. SO2 has also been reported to induce neurotoxicity through protein oxidation, DNA protein cross-links, apoptosis, and damage of cell constituting the central nervous systems including cerebral cortex neurons, glial cell and nerve fibers47,48.
Another possible link is the association between SO2 and other regional pollutants. The region close to power plants or industrial facilities using fossil fuel shows higher SO2 level49. A cohort study with 5193 children including 116 patients with ADHD reported that the proximity to an industrial estate in the study area was associated with an increased risk of ADHD, and suggested prenatal exposure to organochlorine compound released from the industrial estate as a potential explanation for the finding50.
Notably, the result of WGCNA showed that the CpGs (cg07583420 (INS-IGF2), cg15705054 (PBXIP1), cg05075097 (INS-IGF2), and cg25163476 (INS-IGF2)) associated with prenatal SO2 exposure were co-methylated, corresponding to the module simultaneously correlated with prenatal SO2 during the 3rd trimester and ARS at age 4. IGF2 plays an integral role in brain development after birth51. IGF2 is associated with developmental abnormalities in the structure and/or function of the cerebellum and the hippocampus52,53, both of which are associated with ADHD. Higher IGF2 methylation can predict ADHD symptoms in youth with conduct disorders33. In mice, IGF2 enhancer deletion disrupted levels of striatal dopamine, which has been suggested to be involved in the pathophysiology of ADHD54.
Notch signalling pathway plays an essential role in embryogenesis and organogenesis55, particularly in regulation of neurogenesis56, and is also known to be involved in schizophrenia and bipolar disorder57. In a mice model, damages in neural stem cells affected cognitive impairment during Pb exposure, which was dependent on the Notch pathway58. Prenatal exposure to ketamine in rats, which increased the expression level of Notch1, inhibited the proliferation and differentiation of neural stem cells in hippocampus and impaired neurocognitive function including learning and memory in adulthood56.
Our study presents certain limitations. First, the small sample size limits the statistical power of the results, hence, further replication of these results in larger population is warranted. ADHD symptoms were assessed by a parent-rated questionnaire on a continuum, rather than analyzed dichotomously according to a formal diagnosis by a specialist. Therefore, the results of this study may differ in a clinical sample. SO2 gaseous exposure could have greater temporal variability, therefore, the results should be interpreted cautiously. In addition, DNAm measured in the peripheral blood may not reflect the methylation signature in the brain, as epigenetic markers are tissue specific. In the absence of gene expression data, direct conclusions about the transcriptional consequences of the DNAm changes could not be made. Despite these limitations, our study also has notable merits. We employed a prospective study design, with prenatal exposure assessment, methylation profiles at age 2 and 6, and longitudinal neurocognitive outcome measurements at ages 4, 6 and 8. Investigation of the effect of methylation provided insight into the mechanism underlying the association between air pollution and ADHD. The longitudinal study design enabled identification of susceptible window period of DNAm changes in association with prenatal environmental exposure, which was earlier childhood in this study. We adjusted for potential covariates related to both the exposure and outcome, including both maternal and childhood characteristics.
Conclusion
In this study, we found that prenatal exposure to SO2 contributed to differential methylation at a CpG site located within INS-IGF2 at age 2, which, in turn, was associated with ADHD symptoms at ages 4, 6 and 8 years. These findings indicate that an epigenetic mechanism involving methylation could underlie the relationship between the toxicity of SO2 and neurodevelopment in children.
Methods
Study population
Study participants were selected from the Environment and Development of Children (EDC) Study, a prospective cohort study that investigated the environmental effects on growth and neurodevelopment59. Mothers participating in the Congenital Anomaly Study cohort were contacted during 2012–2015 after birth, and a total of 726 mother–child pairs were recruited for EDC study, followed by regular follow-up at 2-year intervals (i.e. 2, 4, 6 and 8 years). We collected epidemiological information, anthropometric characteristics, and neurocognitive outcomes biennially. ADHD symptoms were evaluated at ages 4, 6, and 8. Among these children, 329 had information on ADHD symptoms all at ages 4, 6 and 8.
In a sub-cohort, we selected 60 children at age 2 and analyzed DNAm from whole blood at age 2 and 6 repeatedly. Similar to the main EDC cohort, we examined neurocognitive functions including symptoms of ADHD at ages 4, 6 and 8. Among 60 children, 54 at age 4, 60 at age 6, and 57 at age 8 were assessed for ADHD symptoms. Those assessed at these ages were included in the main analysis (n = 51).
Ethical statements
The methods of this study were approved by the Institutional Review Board of Seoul National University College of Medicine (IRB No. 1201-010-392) and was conducted according to the guidelines and regulations of the Declaration of Helsinki. Informed consent was obtained from mothers according to the Institutional Review Board of Seoul National University College of Medicine (IRB No. 1201-010-392).
Measurement of air pollution
We used the levels of air pollution including district-specific monthly SO2 as the main exposure. The levels of SO2 were collected from the publicly available data from Air Korea (http://www.airkorea.or.kr/eng) from the Korea Ministry of Environment, which monitors the hourly air pollution concentration levels at 257 stations nationwide. We assigned air pollution levels to the various pregnancy stages, such as the 1st, 2nd, 3rd trimester, as a proxy of exposure to pregnant women based on the residential address. The SO2 levels were measured from the nearest monitoring station from the residential address of each participant. The nearest monitoring stations was assigned according to the Euclidean distance between each residential address and the closest monitoring station using ArcGIS.
DNAm analysis
Bisulfite sequencing and microarray
The Illumina Infinium HumanMethylation EPIC BeadChip (850 K) was used for the samples from the 2-year-olds, and the Illumina Infinium HumanMethylation 450 K BeadChip was used for the samples of the 6-year-olds (Illumina, San Diego, CA, USA). Detailed experimental procedures are presented in the Supplementary Materials.
Quality control and probe filtering
Array CpG probes which had a detection p-value > 0.05 in more than 25% of samples were filtered out. Filtered data were normalized using the Beta Mixture Quantile (BMIQ) method60 and corrected for batch effect using ComBat package in R. CpGs with at least one “not-available” (NA) values for the normalization were excluded, leaving 865,688 CpGs from the EPIC BeadChip (850 K) at age 2, and 460,960 CpGs from the 450 K BeadChip at age 6. Among these CpGs, we selected the overlapping 430,101 CpGs. After excluding SNP-associated CpGs (SNP distance ≤ 1 or SNP minor allele frequency ≥ 0.05), CpGs corresponded to non-CpG loci or the X or Y chromosomes, and CpGs at cross-reactive probes, and multimodal CpGs, 326,898 CpGs were finally selected for analysis (Fig. S1). We identified multimodal CpGs via the Dip test61 using diptest R package62 (Dip test’s p-value < 0.05).
Systematic review of literature and selection of candidate CpGs
We selected candidate CpGs through a systematic literature review. The detailed procedure of systematic review is shown in the Supplementary Materials.
SNP Genotyping
We used Axiom®2.0 Reagent Kit (Affymetrix Axiom®2.0 Assay User Guide) according to manufacturer’s protocol; the detailed procedure is provided in the Supplementary Materials. We used Korean Chip (K-CHIP) available from the K-CHIP consortium to produce Genotype data. K-CHIP was designed by the Center for Genome Science, Korea National Institute of Health, Korea (4845-301, 3000-3031).
ADHD symptom screening
To evaluate ADHD symptoms in children at ages 4, 6, and 8, the Korean version of the ARS IV (K-ARS) was completed by the parents63,64. K-ARS is a standardized screening tool for ADHD symptoms in Korean children65 and has shown validity and reliability66. K-ARS, which has also been used in relation to environmental exposure67, is composed of 18 questions corresponding to the diagnostic criteria of ADHD according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition. Nine questions evaluate inattention, and the other 9 are related to hyperactivity and impulsivity. Each item is rated from 0 to 3, with a total score ranging from 0 to 5468.
Covariates
Covariates were pooled from the literature review69,70,71. Covariates included maternal age at pregnancy, maternal educational level (middle school graduate, high school graduate, college graduate, or graduate school attendance), maternal smoking status during pregnancy (current smoker, ex-smoker, never-smoker), ETS during pregnancy (yes/no), child’s sex, gestational age (weeks), multiple births (singleton or twin/triplet), season of child’s birth, cell type fraction, and maternal IQ evaluated by the short version of the Korean Wechsler Adult Intelligence Scale72. Cell type distribution, defined as the fraction of CD8 + T cells, CD4 + T cells, natural killer (NK) cells, B cells, monocytes, and neutrophils, was estimated by using the adult leukocyte reference panel73 and Minfi R package74.
Statistical analysis
We investigated the association between trimester-specific prenatal exposure to SO2 (ppm) and ARS at ages 4, 6 and 8 in the cohort of 329 mother–child pairs by linear regression analysis by adjusting for maternal age at pregnancy, mother’s education levels, maternal smoking, maternal ETS, and season of child’s birth. Covariates were selected based on directed acyclic graph75 using the publicly available program (http://www.dagitty.net/) (Fig. S5).
In a sub-cohort (n = 51), we investigated the association between prenatal trimester-specific exposure to SO2 (ppm) and DNAm at the CpGs selected from the literature review (n = 375) at ages 2 and 6, using multivariable linear regression. We expressed the changes in DNAm per interquartile range (IQR) increase in SO2. The regression model for the association between prenatal SO2 and DNAm was adjusted for maternal age at pregnancy, mother’s educational level, maternal smoking, ETS during pregnancy, and season of the child’s birth (Fig. S6). To investigate the association between postnatal SO2 exposure and DNA methylation levels, we used a linear regression model for the average SO2 level during age 2 (12 months) and DNAm at age 2, and the average SO2 level during age 2, 4 and 6 each and DNAm at age 6, adjusted for mother’s age, postnatal ETS, postnatal mother’s smoking, and the season of birth. To evaluate the effects of cumulative SO2 exposure, we have analyzed the association between SO2 exposure at ages 2–4 years and 2–4–6 years and DNAm at age 6. SO2 exposure at ages 2–4 years was taken from the average of SO2 exposure at ages 2 and 4 years, and SO2 exposure at 2–4-6 years was taken from the average of SO2 exposures at age 2, 4 and 6 years. Benjamini–Hochberg method76 was used to correct the effects of multiple comparisons by using FDR. We also performed EWAS for 326,898 CpGs at ages 2 and 6 with prenatal SO2 exposure by trimester, adjusted by the same covariates.
Furthermore, we investigated the association between an increase in DNAm at prenatal SO2 exposure-associated CpGs at ages 2 and ARS at ages 4, 6, and 8, after adjusting for mother’s age at pregnancy, mother’s education level, maternal smoking, ETS during pregnancy, season of the child’s birth, maternal IQ, the child’s sex, preterm birth, and multiple births (Fig. S7).
WGCNA was used to detect co-methylated modules (clusters of CpGs) using WGCNA R package77,78. Using beta-value of methylation at the CpGs from candidate gene analysis, weighted co-methylation networks (module) were identified. For each module, hierarchical clustering was performed for all samples, and the dendrogram were grouped into modules. Then the correlation was tested between the modules and trimester-specific SO2 exposure during pregnancy, using Pearson correlation coefficient. We selected the module with a significant correlation with exposure variables, then pooled CpGs corresponding to the module of interest. Next, the methylation levels at these CpGs were used to detect co-methylation module, and the correlation between the modules and ADHD symptom scores at ages 4, 6 and 8 were then analyzed. The networks between CpGs were visualized using Cytoscape ver 3.8.2. (https://cytoscape.org/).
We investigated the mediation effect of DNAm at the significant CpGs on the association between prenatal SO2 and ARS in childhood using Mediation R package79. Indirect effect indicates the effect of prenatal exposure to SO2 affecting ARS through DNAm, whereas direct effect refers to the effect of prenatal SO2 exposure directly affecting ARS, not through DNAm. The total effect is defined as the sum of indirect and direct effects.
To identify mQTL, we searched for SNPs positioned within 100 kb window to either side of the significant CpGs, then analyzed the correlation between DNA methylation levels at the CpGs and SNP genotypes. The p-value for the correlation between genotype and DNAm levels was calculated using the Kruskal–Wallis test. Multiple comparisons were corrected via FDR.
For functional enrichment analysis, Gene Ontology (GO) terms and Reactome cell signaling pathways were identified using topGO R package80 and ReactomePA R package81.
Data availability
The data that support the findings of this study are available from Environment and Development of Children (EDC) cohort. The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADHD:
-
Attention deficit hyperactivity disorder
- ARS:
-
ADHD rating scale
- DNAm:
-
DNA methylation
- ETS:
-
Environmental tobacco smoking
- EWAS:
-
Epigenome-wide association study
- FDR:
-
False discovery rate
- GFI1 :
-
Growth factor-independent 1 transcriptional repressor
- GO:
-
Gene ontology
- IQR:
-
Interquartile range
- NK:
-
Natural killer
References
APA. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington. VA: American Psychiatric Publishing. (2013).
Biederman, J. et al. Functional impairments in adults with self-reports of diagnosed ADHD: A controlled study of 1001 adults in the community. J. Clin. Psychiatry 67, 524–540. https://doi.org/10.4088/jcp.v67n0403 (2006).
Polanczyk, G., de Lima, M. S., Horta, B. L., Biederman, J. & Rohde, L. A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry 164, 942–948. https://doi.org/10.1176/ajp.2007.164.6.942 (2007).
Willcutt, E. G. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics 9, 490–499. https://doi.org/10.1007/s13311-012-0135-8 (2012).
Sayal, K., Prasad, V., Daley, D., Ford, T. & Coghill, D. ADHD in children and young people: Prevalence, care pathways, and service provision. Lancet Psychiatry 5, 175–186. https://doi.org/10.1016/S2215-0366(17)30167-0 (2018).
Nunez-Jaramillo, L., Herrera-Solis, A. & Herrera-Morales, W. V. ADHD: Reviewing the causes and evaluating solutions. J. Pers. Med. https://doi.org/10.3390/jpm11030166 (2021).
Oudin, A. et al. Prenatal exposure to air pollution as a potential risk factor for autism and ADHD. Environ. Int. 133, 105149. https://doi.org/10.1016/j.envint.2019.105149 (2019).
Thygesen, M. et al. Exposure to air pollution in early childhood and the association with attention-deficit hyperactivity disorder. Environ. Res. 183, 108930. https://doi.org/10.1016/j.envres.2019.108930 (2020).
Yorifuji, T., Kashima, S., Higa Diez, M., Kado, Y. & Sanada, S. Prenatal exposure to traffic-related air pollution and child behavioral development milestone delays in Japan. Epidemiology 27, 57–65 (2016).
Yorifuji, T. et al. Prenatal exposure to outdoor air pollution and child behavioral problems at school age in Japan. Environ. Int. 99, 192–198. https://doi.org/10.1016/j.envint.2016.11.016 (2017).
Chen, T. M., Gokhale, J., Shofer, S. & Kuschner, W. G. Outdoor air pollution: Nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am. J. Med. Sci. 333, 249–256. https://doi.org/10.1097/MAJ.0b013e31803b900f (2007).
Lin, C. C. et al. Multilevel analysis of air pollution and early childhood neurobehavioral development. Int. J. Environ. Res. Public Health 11, 6827–6841. https://doi.org/10.3390/ijerph110706827 (2014).
Park, J. et al. Association between short-term air pollution exposure and attention-deficit/hyperactivity disorder-related hospital admissions among adolescents: A nationwide time-series study. Environ. Pollut. 266, 115369. https://doi.org/10.1016/j.envpol.2020.115369 (2020).
Hamza, M. et al. Epigenetics and ADHD: Toward an integrative approach of the disorder pathogenesis. J. Atten. Disord. 23, 655–664. https://doi.org/10.1177/1087054717696769 (2019).
Miyake, K. et al. DNA methylation of GFI1 as a mediator of the association between prenatal smoking exposure and ADHD symptoms at 6 years: The Hokkaido Study on Environment and Children’s Health. Clin. Epigenetics. 13, 74. https://doi.org/10.1186/s13148-021-01063-z (2021).
Gervin, K., Nordeng, H., Ystrom, E., Reichborn-Kjennerud, T. & Lyle, R. Long-term prenatal exposure to paracetamol is associated with DNA methylation differences in children diagnosed with ADHD. Clin. Epigenetics 9, 77. https://doi.org/10.1186/s13148-017-0376-9 (2017).
Shukla, A. et al. Air pollution associated epigenetic modifications: Transgenerational inheritance and underlying molecular mechanisms. Sci. Total Environ. 656, 760–777. https://doi.org/10.1016/j.scitotenv.2018.11.381 (2019).
Wu, Y. et al. Air pollution and DNA methylation in adults: A systematic review and meta-analysis of observational studies. Environ. Pollut. 284, 117152. https://doi.org/10.1016/j.envpol.2021.117152 (2021).
Menezo, Y. J., Silvestris, E., Dale, B. & Elder, K. Oxidative stress and alterations in DNA methylation: Two sides of the same coin in reproduction. Reprod. Biomed. Online 33, 668–683. https://doi.org/10.1016/j.rbmo.2016.09.006 (2016).
Baccarelli, A. et al. Rapid DNA methylation changes after exposure to traffic particles. Am. J. Respir. Crit. Care Med. 179, 572–578. https://doi.org/10.1164/rccm.200807-1097OC (2009).
Adriani, W. et al. Potential for diagnosis versus therapy monitoring of attention deficit hyperactivity disorder: A new epigenetic biomarker interacting with both genotype and auto-immunity. Eur. Child Adolesc. Psychiatry 27, 241–252. https://doi.org/10.1007/s00787-017-1040-9 (2018).
Chen, Y. C. et al. Neuroanatomic, epigenetic and genetic differences in monozygotic twins discordant for attention deficit hyperactivity disorder. Mol. Psychiatry 23, 683–690. https://doi.org/10.1038/mp.2017.45 (2018).
Dadds, M. R., Schollar-Root, O., Lenroot, R., Moul, C. & Hawes, D. J. Epigenetic regulation of the DRD4 gene and dimensions of attention-deficit/hyperactivity disorder in children. Eur. Child Adolesc. Psychiatry 25, 1081–1089. https://doi.org/10.1007/s00787-016-0828-3 (2016).
Goodman, S. J. et al. Obsessive-compulsive disorder and attention-deficit/hyperactivity disorder: Distinct associations with DNA methylation and genetic variation. J. Neurodev. Disord. https://doi.org/10.1186/s11689-020-09324-3 (2020).
Hammerschlag, A. R., Byrne, E. M., Bartels, M., Wray, N. R. & Middeldorp, C. M. Refining attention-deficit/hyperactivity disorder and autism spectrum disorder genetic loci by integrating summary data from genome-wide association, gene expression, and DNA Methylation studies. Biol. Psychiatry 88, 470–479. https://doi.org/10.1016/j.biopsych.2020.05.002 (2020).
Heinrich, H. et al. Attention, cognitive control and motivation in ADHD: Linking event-related brain potentials and DNA methylation patterns in boys at early school age. Sci. Rep. 7, 3823. https://doi.org/10.1038/s41598-017-03326-3 (2017).
Li, S. C. et al. DNA methylation in LIME1 and SPTBN2 genes is associated with attention deficit in children. Children-Basel https://doi.org/10.3390/children8020092 (2021).
Meijer, M. et al. Genome-wide DNA methylation patterns in persistent attention-deficit/hyperactivity disorder and in association with impulsive and callous traits. Front. Genet. https://doi.org/10.3389/fgene.2020.00016 (2020).
Miyake, K. et al. DNA methylation of GFI1 as a mediator of the association between prenatal smoking exposure and ADHD symptoms at 6 years: The Hokkaido Study on Environment and Children’s Health. Clin. Epigenetics https://doi.org/10.1186/s13148-021-01063-z (2021).
Mooney, M. A. et al. Large epigenome-wide association study of childhood ADHD identifies peripheral DNA methylation associated with disease and polygenic risk burden. Transl. Psychiatry https://doi.org/10.1038/s41398-020-0710-4 (2020).
Neumann, A. et al. Association between DNA methylation and ADHD symptoms from birth to school age: A prospective meta-analysis. Transl. Psychiatry 10, 398. https://doi.org/10.1038/s41398-020-01058-z (2020).
Park, S. et al. Associations between serotonin transporter gene (SLC6A4) methylation and clinical characteristics and cortical thickness in children with ADHD. Psychol. Med. 45, 3009–3017. https://doi.org/10.1017/S003329171500094X (2015).
Rijlaarsdam, J. et al. Prenatal unhealthy diet, insulin-like growth factor 2 gene (IGF2) methylation, and attention deficit hyperactivity disorder symptoms in youth with early-onset conduct problems. J. Child Psychol. Psychiatry 58, 19–27. https://doi.org/10.1111/jcpp.12589 (2017).
Rovira, P. et al. Epigenome-wide association study of attention-deficit/hyperactivity disorder in adults. Transl. Psychiatry https://doi.org/10.1038/s41398-020-0860-4 (2020).
Sengupta, S. M., Smith, A. K., Grizenko, N. & Joober, R. Locus-specific DNA methylation changes and phenotypic variability in children with attention-deficit hyperactivity disorder. Psychiatry Res. 256, 298–304. https://doi.org/10.1016/j.psychres.2017.06.048 (2017).
van Dongen, J. et al. Epigenome-wide association study of attention-deficit/hyperactivity disorder symptoms in adults. Biol. Psychiatry 86, 599–607. https://doi.org/10.1016/j.biopsych.2019.02.016 (2019).
van Mil, N. H. et al. DNA methylation profiles at birth and child ADHD symptoms. J. Psychiatr. Res. 49, 51–59. https://doi.org/10.1016/j.jpsychires.2013.10.017 (2014).
Walton, E. et al. Epigenetic profiling of ADHD symptoms trajectories: A prospective, methylome-wide study. Mol. Psychiatry 22, 250–256. https://doi.org/10.1038/mp.2016.85 (2017).
Weiß, A. L. et al. DNA methylation associated with persistent ADHD suggests TARBP1 as novel candidate. Neuropharmacology https://doi.org/10.1016/j.neuropharm.2020.108370 (2021).
Wilmot, B. et al. Methylomic analysis of salivary DNA in childhood ADHD identifies altered DNA methylation in VIPR2. J. Child Psychol. Psychiatry 57, 152–160. https://doi.org/10.1111/jcpp.12457 (2016).
Xu, Y. et al. Multiple epigenetic factors predict the attention deficit/hyperactivity disorder among the Chinese Han children. J. Psychiatr. Res. 64, 40–50. https://doi.org/10.1016/j.jpsychires.2015.03.006 (2015).
Mill, J. & Petronis, A. Pre- and peri-natal environmental risks for attention-deficit hyperactivity disorder (ADHD): The potential role of epigenetic processes in mediating susceptibility. J. Child Psychol. Psychiatry 49, 1020–1030. https://doi.org/10.1111/j.1469-7610.2008.01909.x (2008).
Perez, R. F. et al. Longitudinal genome-wide DNA methylation analysis uncovers persistent early-life DNA methylation changes. J. Transl. Med. 17, 15. https://doi.org/10.1186/s12967-018-1751-9 (2019).
Choi, Y. J. et al. Effect of prenatal bisphenol A exposure on early childhood body mass index through epigenetic influence on the insulin-like growth factor 2 receptor (IGF2R) gene. Environ. Int. 143, 105929. https://doi.org/10.1016/j.envint.2020.105929 (2020).
Dunn, E. C. et al. Sensitive periods for the effect of childhood adversity on DNA methylation: Results from a prospective. Longitudinal Study. Biol. Psychiatry. 85, 838–849. https://doi.org/10.1016/j.biopsych.2018.12.023 (2019).
Meng, Z. Oxidative damage of sulfur dioxide on various organs of mice: Sulfur dioxide is a systemic oxidative damage agent. Inhal. Toxicol. 15, 181–195. https://doi.org/10.1080/08958370304476 (2003).
Meng, Z. & Liu, Y. Cell morphological ultrastructural changes in various organs from mice exposed by inhalation to sulfur dioxide. Inhal. Toxicol. 19, 543–551. https://doi.org/10.1080/08958370701271373 (2007).
Sang, N., Hou, L., Yun, Y. & Li, G. SO(2) inhalation induces protein oxidation, DNA-protein crosslinks and apoptosis in rat hippocampus. Ecotoxicol. Environ. Saf. 72, 879–884. https://doi.org/10.1016/j.ecoenv.2008.07.007 (2009).
Ray, S. & Kim, K.-H. The pollution status of sulfur dioxide in major urban areas of Korea between 1989 and 2010. Atmos. Res. 147–148, 101–110. https://doi.org/10.1016/j.atmosres.2014.05.011 (2014).
Saez, M., Barcelo, M. A., Farrerons, M. & Lopez-Casasnovas, G. The association between exposure to environmental factors and the occurrence of attention-deficit/hyperactivity disorder (ADHD). A population-based retrospective cohort study. Environ. Res. 166, 205–214. https://doi.org/10.1016/j.envres.2018.05.009 (2018).
Pidsley, R., Dempster, E., Troakes, C., Al-Sarraj, S. & Mill, J. Epigenetic and genetic variation at the IGF2/H19 imprinting control region on 11p15.5 is associated with cerebellum weight. Epigenetics 7, 155–163. https://doi.org/10.4161/epi.7.2.18910 (2012).
Chen, D. Y. et al. A critical role for IGF-II in memory consolidation and enhancement. Nature 469, 491–497. https://doi.org/10.1038/nature09667 (2011).
Ouchi, Y. et al. Reduced adult hippocampal neurogenesis and working memory deficits in the Dgcr8-deficient mouse model of 22q11.2 deletion-associated schizophrenia can be rescued by IGF2. J. Neurosci. 33, 9408–9419. https://doi.org/10.1523/JNEUROSCI.2700-12.2013 (2013).
Pai, S. et al. Differential methylation of enhancer at IGF2 is associated with abnormal dopamine synthesis in major psychosis. Nat. Commun. 10, 2046. https://doi.org/10.1038/s41467-019-09786-7 (2019).
Zhou, W. et al. BLOS2 negatively regulates Notch signaling during neural and hematopoietic stem and progenitor cell development. eLife 5, e18108. https://doi.org/10.7554/eLife.18108 (2016).
Huang, H. et al. Neonatal anesthesia by ketamine in neonatal rats inhibits the proliferation and differentiation of hippocampal neural stem cells and decreases neurocognitive function in adulthood via inhibition of the notch1 signaling pathway. Mol. Neurobiol. 58, 6272–6289. https://doi.org/10.1007/s12035-021-02550-3 (2021).
Ohi, K. et al. Schizophrenia risk variants in ITIH4 and CALN1 regulate gene expression in the dorsolateral prefrontal cortex. Psychiatr. Genet. 26, 142–143 (2016).
Sun, L. et al. Lead exposure induced neural stem cells death via notch signaling pathway and gut-brain axis. Oxid. Med. Cell. Longev. 2022, 7676872. https://doi.org/10.1155/2022/7676872 (2022).
Kim, K. N. et al. Cohort profile: The environment and development of children (EDC) study: A prospective children’s cohort. Int. J. Epidemiol. 47, 1049–1050f. https://doi.org/10.1093/ije/dyy070 (2018).
Teschendorff, A. E. et al. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 29, 189–196. https://doi.org/10.1093/bioinformatics/bts680 (2013).
Hartigan, J. A. & Hartigan, P. M. The dip test of unimodality. Ann. Stat. 13, 70–84 (1985).
Maechler, M. Package ‘diptest’. (2021). https://cran.r-project.org/web/packages/diptest/diptest.pdf.
Dupaul, G. J., Power, T. J., Anastopoulos, A. D. & Reid, R. The ADHD Rating Scale—IV: Checklists, Norms and Clinical Interpretation (New York Guilford Press, 1998).
Pappas, D. ADHD rating scale-IV: Checklists, norms, and clinical interpretation. J. Psychoeduc. Assess. 24, 172–178. https://doi.org/10.1177/0734282905285792 (2016).
Kim, J.-W. et al. The child behavior checklist together with the ADHD rating scale can diagnose ADHD in Korean community-based samples. Can. J. Psychiatry 50, 802–805 (2005).
So, Y., Noh, J., Kim, Y., Ko, S. & Koh, Y. The reliability and validity of Korean parent and teacher ADHD rating scale. J. Korean Neuropsychiatr. Assoc. 41, 283–289 (2002).
Hong, S. B. et al. Environmental lead exposure and attention deficit/hyperactivity disorder symptom domains in a community sample of South Korean school-age children. Environ. Health Perspect. 123, 271–276. https://doi.org/10.1289/ehp.1307420 (2015).
Kim, M.A.-O. et al. Prevalence of attention-deficit/hyperactivity disorder and its comorbidity among Korean children in a community population. J. Korean Med. Sci. 32, 401–406 (2017).
Aghaei, M., Janjani, H., Yousefian, F., Jamal, A. & Yunesian, M. Association between ambient gaseous and particulate air pollutants and attention deficit hyperactivity disorder (ADHD) in children; a systematic review. Environ. Res. 173, 135–156. https://doi.org/10.1016/j.envres.2019.03.030 (2019).
Alfano, R. et al. The impact of air pollution on our epigenome: How far is the evidence? (A systematic review). Curr. Environ. Health Rep. 5, 544–578. https://doi.org/10.1007/s40572-018-0218-8 (2018).
Dall’Aglio, L. et al. The role of epigenetic modifications in neurodevelopmental disorders: A systematic review. Neurosci. Biobehav. Rev. 94, 17–30. https://doi.org/10.1016/j.neubiorev.2018.07.011 (2018).
Lim, Y. R., Lee, W. K., Lee, W. H. & Park, J. W. The study on the accuracy and validity of Korean Wechsler intelligence scale short forms : A comparison of the WAR07 subtest vs Doppelt subtest. Korean J. Clin. Psychol. 19, 563–574 (2000).
Houseman, E. A. et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinform. 13, 86. https://doi.org/10.1186/1471-2105-13-86 (2012).
Aryee, M. J. et al. Minfi: A flexible and comprehensive bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30, 1363–1369. https://doi.org/10.1093/bioinformatics/btu049 (2014).
Greenland, S., Pearl, J. & Robins, J. M. Causal diagrams for epidemiologic research. Epidemiology 10, 37–48 (1999).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Statist. Soc. B. 57, 289–300 (1995).
Langfelder, P. & Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 9, 559. https://doi.org/10.1186/1471-2105-9-559 (2008).
Davies, M. N. et al. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol. 13, R43. https://doi.org/10.1186/gb-2012-13-6-r43 (2012).
Tingley, D., Yamamoto, T., Hirose, K., Keele, L. & Imai, K. Mediation: RPackage for causal mediation analysis. J. Stat. Softw. https://doi.org/10.18637/jss.v059.i05 (2014).
Alexa, A. & Rahnenfuhrer, J. o. Gene set enrichment analysis with topGO. (2016). http://www.mpi-sb.mpg.de/∼alexa.
Yu, G. & He, Q. Y. ReactomePA: An R/Bioconductor package for reactome pathway analysis and visualization. Mol. Biosyst. 12, 477–479. https://doi.org/10.1039/c5mb00663e (2016).
Acknowledgements
The authors thank Jin-A Park, Ji-Young Lee, Yumi Choi, and Hyun-ji Lee for their assistance with data collection, and Macrogen for DNAm analysis (https://www.macrogen.com/en). The authors also thank Hyungdong Jin at Macrogen for technical help.
Funding
This study was supported by grants from the Environmental Health Center, funded by the Korean Ministry of Environment, an R&D Research program funded by the Ministry of Food and Drug Safety of Korea (#18162MFDS121), and the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education (2018R1D1A1B07043446), the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean Government (MSIT) (2019M3E5D1A01069345), and Helsefonden (21-B-0063).
Author information
Authors and Affiliations
Contributions
Y.J.C., J.I.K., Y.H.L. and Y.C.H. conceived and designed the study. Y.C.H., B.J.K., C.H.S., Y.A.L., Y.H.L. acquired funding and established the data. Y.J.C., J.C., S.M. and K.S.L. performed the data analysis. Y.J.C., J.C., J.I.K., D.W.L., S.J.P. drafted the original manuscript. J.I.K. and Y.H.L. supervised and reviewed the writing. Z.K., Y.C.H., B.N.K., C.H.S. and Y.A.L. made critical revisions of the manuscript for important intellectual contents. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, YJ., Cho, J., Hong, YC. et al. DNA methylation is associated with prenatal exposure to sulfur dioxide and childhood attention-deficit hyperactivity disorder symptoms. Sci Rep 13, 3501 (2023). https://doi.org/10.1038/s41598-023-29843-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29843-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.