Abstract
Outbreaks of Asynapta groverae, an invasive mycophagous gall midge, in South Korea have been repeatedly reported since the first occurrence in 2008. This species is a nuisance to residents owing to its mass emergence from newly built and furnished apartments. Here, the levels of genetic diversity, divergence, and structure of invasive A. groverae populations were investigated to understand their ability to survive in novel locations. Population genetic analyses were performed on seven invasive populations, including the first outbreak, sporadically emerged, and two laboratory-isolated (quarantined) populations, using the mitochondrial COI sequences and the ten novel microsatellite markers developed in this study. Non-indigenous A. groverae managed to maintain their populations for 12 years despite decreased genetic polymorphisms resulting from multiple incidences of founder effects by a small number of colonists. Additionally, the advantageous sustainability of A. groverae in the particle boards from which they emerge suggests that human-mediated dispersal is plausible, which may allow for the successful spread or invasion of A. groverae to new locations. This study is one of the few examples to demonstrate that an insect species successfully invaded new regions despite exhibiting decreased genetic diversity that was maintained for a decade. These findings indicate that the high genetic diversity of the initial founding population and asexual reproduction would contribute to the successful invasion of A. groverae in novel environments.
Similar content being viewed by others
Introduction
Globally, invasive species in non-native ranges are among the leading threats to biodiversity, ecosystems, and industries1,2,3,4,5. Recent climate change events, including global warming, have facilitated the survival and adaptation of newly invaded populations to novel local habitats, which were previously not the ecological niche of the invasive species6,7. Additionally, globalization in recent years has increased human activities (such as trade and travel), which have further contributed to the spread of invasive species8,9. After the invasion, alien species may either not survive owing to unfit environmental conditions or successfully colonize new habitats and expand. Established populations of alien species can be a threat to the community if they exhibit predation and/or competition with endemic species, leading to the disturbance of the whole ecosystem. Several cases of invasive species-induced ecological and economic damages have been reported. The identification of the processes involved in the establishment, dispersal route, and spread of alien species is challenging10,11.
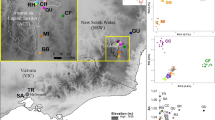
The first outbreak of Asynapta groverae Jiang and Bu (Porricondylinae, Cecidomyiidae), a mycophagous gall midge, in South Korea was recorded in 200812 (see Fig. 1). This small gall midge was first described in Madhya Pradesh, India as Dicerura indica13. The small gall midge was later renamed Asynapta indica by recombining the genus name but subsequently named as Asynapta groverae by Jiang and Bu14. The first outbreak of A. groverae in newly furnished apartments in South Korea was reported in 200812,15. Since 2008, sporadic outbreaks have been reported across the country, especially during the spring and summer seasons16 (Fig. 2). Asynapta groverae, which was not previously recorded in this region, is the only known species of the genus Asynapta in South Korea. Globally, 49 Asynapta species have been recorded12,17. The first morphological description of the larval and pupal stages of A. groverae has been recently published16.
Larva (A)12, pupal case (B), and an adult male (C) of Asynapta groverae.
Limited studies have reported the outbreak pattern and ecological characteristics of A. groverae12,15. The adult lifespan of A. groverae is 3–4 days, that is, no longer than one week, whereas the larval period is approximately 2–3 weeks long. Thus, the generation time from egg to adult is approximately 3–4 weeks under optimal environmental conditions, such as temperature and humidity15. The sole source of A. groverae outbreaks in Korea is the particle boards, which are used for manufacturing furniture and are a place of inhabitation and emergence for larvae and adults (Fig. 2). Particle boards are manufactured from lumber waste products and used for the construction of new furniture for apartments. Additionally, particle boards can be manufactured using mycelium-based composites, which are synthetic construction materials that use natural fungal growth. Thus, particle boards are susceptible to infections from all fungi18. Most A. groverae individuals were found on the fungus-infected part of the particle boards. The larvae of A. groverae feed on fungi12,15. Thus, particle boards are a rich source of fungal food for A. groverae larvae. Outbreaks of A. groverae in the form of adults, larvae, and pupae from newly built and furnished apartments are a nuisance to residents (Fig. 2)15 owing to the increased amount of insect biomass during the emerging period in indoor conditions. However, A. groverae do not serve as allergens or pathogens to humans15. Complete eradication of the A. groverae has not been successful as a few individuals were found even after one month post-pest control15.
Although continuous outbreaks of A. groverae have been reported during the last decade12, the ecological characteristics (such as voltinism, generation time, and life history), origin, and dispersal route of A. groverae have not been elucidated. Furthermore, the A. groverae genetic attributes, such as the level of genetic diversity, population differentiation and structure, and temporal stability of gene pools, have not been reported. The genetic diversity level and population structure are strongly associated with the successful colonization of invasive species into novel habitats19,20,21,22. Moreover, the genetic diversity of alien species is low during the initial invasion as the invasion is mediated by a few colonists, representing a small fraction of the source population (founder effect)23,24. Small and isolated populations are prone to genetic drift owing to the bottleneck or founder effects25. A previous study reported that a genetic bottleneck facilitated the successful invasion of Linepithema humile, an Argentine ant species26. The loss of genetic diversity due to a population bottleneck in the invasive Argentine ant population decreases intraspecific aggression by forming interspecifically dominant supercolonies26. In contrast, a relatively high genetic diversity of the founder population has been reported in cases of simultaneous or independent introduction of multiple source populations27,28. For example, the high genetic diversity of the invading wasp population resulted in successful invasion into the introduced ranges29. Decreased or elevated genetic variations in the introduced population could lead to the successful invasion of a broad suite of taxa according to circumstances.
Population genetic and phylogeographic analyses can reveal invasion processes, such as the origin, invasive history, introductory routes, dispersal/expansion patterns, and temporal and spatial genetic variation of invasive populations, which provide essential information for effective and efficient invasive species monitoring and control30,31,32. For example, population genetic analyses of the pinewood nematode Bursaphelenchus xylophilus revealed several characteristics of invasive processes from the native range of North America to Europe33. Low levels of genetic polymorphisms detected among the introduced populations of B. xylophilus suggested a single event of invasion during the invasion process, which contributed to a small effective population size33. Moreover, a large effect of genetic drift in invasive populations was suggested based on the strong genetic structure of native populations33. Population dynamics of introduced species, such as range expansion patterns34, invasive population size19, and ecological niche20, have been recognized in diverse taxa.
Microsatellites, which are single sequence repeats, have been commonly used in population genetic studies on various insect taxa to identify genotypic diversity35, population genetic differentiation36, biological invasion37,38,39, host range expansion40, sexual selection41, ecological characteristics42, and speciation43. However, most microsatellites serve as species-specific markers. Hence, cross-species amplification of markers is limited to the genus level in most cases44,45. Most microsatellite markers have been developed for economic damage-causing species belonging to the family Cecidomyiidae46,47,48,49,50. Currently, microsatellite markers have not been identified for Asynapta species.
This study, for the first time, identified genome-wide microsatellites for Asynapta species and investigated the genetic diversity and structure of seven invasive A. groverae populations, including first reported (2008), sporadically emerged (2018–2020), and two laboratory-isolated populations. Population genetic analyses were also performed using the mitochondrial COI sequences. The analysis of spatio-temporal genetic patterns for 12 years since the first outbreak will provide useful insights into the successful invasion of A. groverae in South Korea from a population genetics perspective.
Results
mtDNA diversity, haplotype network, and population structure
Among the 121 A. groverae specimens from seven populations, the sequence of COI (648 bp) (Table 1; Fig. 3) had only 13 polymorphic nucleotide positions (nine parsimonious informative sites and four singletons). In total, 12 haplotypes (H1–H12) with low levels of genetic divergence were identified among the 121 A. groverae temporal samples collected in 2008 and between 2018 and 2020 (GenBank accession numbers OK561689–OK561809) (Table 2). The NH values ranged from 1 to 6 for each population. AG04 exhibited the highest NH value (six), whereas AG06 (an isolated population originating from the AG02 population) exhibited an NH value of 1 (Table 3). The overall h and π values for all seven populations were 0.465 and 0.002, respectively (Table 3).
A haplotype network for COI sequences from seven A. groverae populations exhibited a star-like pattern. The most common haplotype (H1) was in the center of the network surrounded by other haplotypes (Fig. 4). The major haplotype (H1, 73% of all specimens) located in the most internal position in the network (presumably ancestral) was identified from the specimens collected from all seven populations, including AG01 (specimen from the first outbreak population in 2008 in South Korea). The haplotypes at the edge of the network with only a few mutational steps (< 4) separated from the major haplotypes were observed in the individuals from the populations collected between 2018 and 2020.
Haplotype network of COI sequences from seven populations of Asynapta groverae. The area of the circle is proportional to the individual numbers of the respective haplotype. Numbers shown in parentheses represent the number of individuals belonging to a haplotype. Each line in the network represents a single mutational step between haplotypes irrespective of its length. Different colors denote different populations.
Of the 21 tests for pairwise FST values calculated among the seven populations, six exhibited significant mitochondrial genetic differentiation (− 0.004 to 0.351) (Table 4). The highest value of genetic differentiation was identified during the comparison between a laboratory-reared population (AG06) and a recently collected population in 2019 (AG04). Comparison of AG01 with AG03 and AG02 revealed no genetic differentiation (Table 4). The samples were collected in different years (10-year difference) from geographically close sampling sites (Fig. 3). Moderate levels of genetic differentiation (FST > 0.1) were mostly observed during comparisons with the AG04 population (Table 4).
AMOVA revealed the lack of significant genetic structures between the following population pairs: temporal grouping, the first outbreak population in 2008 and sporadically emerged populations between 2018 and 2020; spatial grouping, north and south populations; habitat grouping, natural and isolated populations. Most of the variance occurred among populations irrespective of the group (FST) (Table S1).
Development and characterization of novel microsatellite markers
High-throughput sequencing generated 76,509,371 reads with 1,887,386 scaffolds containing di-, tri-, and tetra-nucleotide repeat motifs (average length = 173 bp). The number of perfect microsatellite sequences, which were suitable for primer design, was 106,753 containing 72,317 di- (68%), 32,214 tri- (30%), and 1819 tetra-nucleotides (2%) repeat motifs. Of the 20 initially screened markers, 10 polymorphic microsatellite markers were successfully amplified with stable and reproducible amplicon patterns and distinct peaks in capillary electrophoresis (Table 2). The number of alleles for each locus ranged from 9 to 31. The mean polymorphic information content (PIC) across loci was 0.7856, representing highly polymorphic markers. The highest PICs (0.949) were observed at the AG_156 locus (Table 2). HO per locus ranged from 0.264 to 0.864 (mean = 0.8053), whereas HE ranged from 0.588 to 0.955 (mean = 0.5939). In total, 181 alleles (average = 18) were amplified from these markers within and across the populations and used for subsequent population genetic analysis.
Microsatellite diversity and population structure
The within-population genetic diversity was relatively high with the HE values in the range of 0.653–0.827. The highest HE and HO values were observed in the AG04 population (Table 3). The mean NA per population was 8.4 (range: 5.1–9.9) (Table 3). The AR level ranged from 4.418 to 7.099. The lowest AR was observed in one of the laboratory populations (AG06), which was similar to the observations with the COI locus. All populations, except AG05 and AG07, significantly deviated from the HWE (Table 3). The level of inbreeding (FIS) ranged from 0.138 to 0.355. The highest FIS value was observed in AG01, which was obtained from the first outbreak population in 2008.
All pairwise FST values calculated among the seven populations were significant (p < 0.01) and were in the range of 0.008–0.141 after Bonferroni correction (Table 4). These findings suggest that the invaded populations were genetically differentiated at the microsatellite loci analyzed.
STRUCTURE analysis of ten microsatellite genotypes represented the three most likely distinct genetic clusters (K = 3; Δ K = 200.35) from seven A. groverae populations (Fig. 5). The bar plot reveals that seven populations were barely admixed although a proportion of all three genetic clusters was identified in all populations (Fig. 5). The first outbreak population in 2008 (AG01) and other populations collected from 2018 to 2019 (AG02, 03, 04) were predominantly represented by a specific genetic cluster (in green). The lack of significant genetic differentiation among the four populations was further supported using FCA51 (Supplement Information, Fig. S1). Both AG05 (rearing population) and AG07 (collected in 2020 from Gijang, located distantly from the first outbreak site) exhibited similar structures (in blue). The weak genetic structure between AG05 and AG07 was further supported using FCA and FST values (Tables 4 and S1). AG06, the other isolated population, was the only population in which most individuals were assigned to the other genetic cluster (in red).
Population genetic structure of the seven Asynapta groverae populations determined using a Bayesian population assignment test with STRUCTURE based on ten microsatellite loci. All individuals are shown along the X-axis. The Y-axis denotes the probability of that individual belonging to each of the genetic clusters. The most likely number of genetic clusters after Delta K Evannos’ correction is 3 (K = 3). The population of all individuals is indicated below the plot.
AMOVA of microsatellites revealed the absence of genetic structures for all tested groupings (similar to observations with the COI locus) (Supplementary Information, Table S1). Significant variance was observed only within populations (Table S1).
Discussion
This study analyzed the spatio-temporal genetic population structure and genetic diversity levels in the invasive populations of A. groverae, a mycophagous gall midge, in South Korea using mitochondrial COI sequences and a novel set of ten nuclear microsatellite markers. Population genetic analyses were performed to gain insights into the successful invasion and persistence of A. groverae over the last 12 years from an ecological/evolutionary genetic perspective. The status of A. groverae as an invasive species in South Korea should be carefully verified before examining its origin, dispersal route, and population dynamics. Invasive species can be referred to as “a species that arrives (often with human assistance) in a habitat it had not previously occupied, then establishes population and spreads autonomously”52. India is reported to be the original habitat of A. groverae. The first outbreak of A. groverae was recorded in Korea in the Songdo region in 2008. Accurate species identification based on a combined analysis of morphological and genetic data is critical to study newly invasive species53. Specimens of larvae, pupa, and adults were carefully examined in the current study based on the various taxonomical keys following the first description of this species14, our previous studies on A. groverae12,16, other taxonomical investigations on asynaptine species54,55 and Cecidomyiidae56 (Supplementary Information, Figs. S2 and S3). Taxonomical examinations on the specimens in the different developmental stages further supported that A. groverae has never been reported in Korea before 2008. Unfortunately, however, molecular-based species identification (i.e. DNA barcoding) could not be applicable for A. groverae since only a single COI sequence is available from GenBank database for the genus Asynapta and its species was not even identified (Asynapta sp.)57.
Since 2008, several sporadic outbreaks (emergence) have been reported during the last 12 years. Furthermore, field investigations using sticky traps around the sites where outbreaks occurred and manufacturers of the particle boards were located did not reveal any evidence of A. groverae habitation in Korea15. All outbreaks occurred only in newly built apartments with new furniture manufactured from particle boards. No outbreaks from other habitats have been reported in Korea15. Although the possibility of the previous existence of A. groverae Jiang and Bu (Diptera: Cecidomyiidae) in Korea cannot be completely ruled out, this small gall midge can be considered a recently colonized invasive species through anthropogenic activities, such as trade and transport of wood materials from the native range for its dispersal.
The level of genetic polymorphisms in this invasive gall midge population in Korea was higher than that in other invasive cecidomyiids28. Obolodiplosis robiniae, a North American gall midge species, was first detected in 2004 and has spread extensively throughout China. The mean HE value of 22 invasive populations (20 individuals per population) at 14 microsatellite loci was 0.5346 in China (invasive population), whereas that of US native populations was 0.660628. Although a direct comparison between the two members of cecidomyiids is not possible, the HE value of A. groverae ranged from 0.653 to 0.827, which is markedly higher than that of O. robiniae. Low genetic diversity is often expected in the initial phase of invasion as limited numbers of colonists have typically been introduced as a founding population23,24. However, relatively high genetic polymorphisms in the founding population (AG01) of A. groverae may contribute to overcoming the genetic hurdle of early invasive processes28.
Invasive colonies/populations of this non-indigenous gall midge species have not flourished or completely disappeared over the last 12 years in Korea even though decreased genetic diversity is known to be detrimental to the persistence and adaptation of populations in novel environments58,59,60. The findings of this study demonstrated that the gene pool of the populations has not markedly changed for more than 10 years. The initial levels of HR and AR in AG02 and AG03 (collected in 2018) were maintained or slightly decreased when compared with those in AG01 (collected in 2008). In addition to decreased levels of genetic diversity, the level of genetic divergence was low as evidenced by some mutational steps from the ancestral haplotypes (Fig. 4). Only three genetic clusters were identified for all populations (Fig. 5) and no temporal genetic differentiation was detected using AMOVA (Supplementary Information, Table S1). However, the invasive populations exhibited detectable genetic differentiation, especially at microsatellites. This suggests that they are genetically divergent owing to the genetic drift effects during independent invasion events within South Korea. Outbreaks have been consistently reported in 2011, 2012, and 2014–2018 across the country since 200828.
Thus, the repeated successful invasion of this species for 12 years is an area of active research. The simplest explanation involves repeated multiple invasions (introductions) of A. groverae from the source population. The increased migration rate of the introduced population by multiple repeated invasions would mitigate decreased genetic diversity and inbreeding, which can contribute to successful invasion61. In particular, the genetic diversity of the AG04 population was slightly higher than that of the founding population (AG01). Wood materials used to produce particle boards, which are the source of all known outbreaks and A. groverae habitat, were imported from several countries in East Asia15. Multiple populations of A. groverae with a small number of individuals may have been repeatedly introduced from their native ranges during the last decades. If founding individuals have multiple source populations, increased genetic diversity and marked genetic divergence can be expected27,28. However, genetic polymorphisms have not increased in succeeding A. groverae populations, except AG04, in this study. The haplotypes found in 2019 and 2020 were not markedly different from those found in 2008 (Fig. 4). The genetic divergence levels in distantly located populations (AG04 and AG 07 populations collected in 2019 and 2020, respectively) were lower than those in the first reported population (AG01). Thus, repeated introductions of A. groverae to Korea from abroad might be a less likely hypothesis.
Alternatively, a founding population of A. groverae, which was introduced before or around 2008, has been maintained as a single invasion source during the last 12 years. The ability of A. groverae to survive and continue its life cycles on particle boards would facilitate the spread and persistence of the population. Particle boards can be stored in one place and distributed to manufacture furniture in newly built apartments in different regions. This study, for the first time, reported that the life cycle of A. groverae continued from the particle board even after one year in a separate box under laboratory conditions (Figs. 1, 2). Thus, this study is one of the few examples that demonstrated that invasive populations can be maintained as small populations with diminished genetic diversity for a prolonged duration without additional invasion events. This indicates that increasing genetic variation from multiple invasions and the marked population growth of invasive species in a new ecosystem might not always be a priori conditions for the successful colonization of non-native species into novel environments. This phenomenon has been argued as a ‘genetic dilemma’ in invasive species in which bottlenecked populations become invasive species despite having a low level of genetic diversity and low adaptive ability to new environments59. Introduced populations with low genetic variation can overcome their reduced evolutionary potential and reproductive fitness by asexual or self-fertilizing mode of reproduction, which leads to a high reproductive rate and removes deleterious alleles that cause inbreeding depression61,62,63. Although the reproductive strategy in A. groverae was not identified in this study, A. groverae may become a successful invader if asexual reproduction is possible in this species, irrespective of the level of genetic variability that would affect evolutionary potential and reproductive success.
Paedogenesis, which is parthenogenetic reproduction at the larval or pupal stages, has been reported in several Cecidomyiidae species since its first discovery in Miastor metraloas by Wagner64,65,66. Laval paedogenic reproduction has been identified in Heteropeza pygmaea Winnertz and Mycophila speyeri (Barnes) of Cecidomyiidae64,67,68,69. A recent molecular phylogeny analysis of Cecidomyiidae revealed that these two lineages appear to be basal to Asynaptini (to which A. groverae belongs)70. Paedogenesis, which is the larval reproductive life cycle, is also reported to be related to the optimal use of food patches, which leads to a short generation time in insects71. If fungal food resources are sufficient, larvae can continue their paedogenetic life. The larvae will start developing and undergoing metamorphosis under unfavorable conditions and subsequently fly away in search of new fungal sources72,73. Most A. groverae larvae and adults were found at the fungi-rich part in the particle boards in the outbreak population, as well as in the two isolated populations (AG05 and 06) after one year in the sealed box. Thus, particle boards can provide a rich fungal food source for A. groverae and ensure asexual reproduction with high propagation rates, which would allow for the continuous establishment of invading populations.
Although a particular genotype (shown in green in Fig. 5) was predominant in the source population (AG02), the two reared populations (AG05 and AG06) were almost assigned to the other two genotypes (shown as blue and red, respectively). Furthermore, all seven populations were almost individually assigned to one genetic group. These results indicate that the settlement of the newly invaded populations is a stochastic event involving a random process. Most A. groverae outbreak populations were small and isolated from particle boards. Many Cecidomyiidae species are poor flyers, and their long-distance dispersal must be assisted by other external factors, such as wind74,75,76. Thus, settlements of A. groverae are formed by multiple founder events and the resulting genetic drift effects of decreased diversity.
The isolated populations emerging from the AG02 population may suggest that the dispersal of A. groverae through the transportation of particle boards is plausible. In this study, particle boards in which A. groverae emerged in 2018 from one site (AG02) were collected and maintained for one year in a separate box at room temperature without any treatment. A. groverae could survive as larvae or eggs on the particle boards and successfully reproduce even after one year, which indicates the high sustainability of A. groverae. The remarkable ability of A. groverae to survive in the particle boards may further explain the reason for the sporadic appearance of A. groverae during the last 12 years, as well as for the non-decline of the population due to fitness reduction resulting from decreased genetic diversity and inbreeding. Similar to AG05, the most recent naturally emerging population (AG07) from the Gijang region also formed one genetic cluster (shown in blue). Thus, if particle boards function as a potential vehicle for dispersing A. groverae populations into new environments, human activity-mediated dispersal could result in founder events or bottlenecking during the invasion processes of this species.
The findings of this study provide useful insights into the successful colonization of the recently introduced A. groverae species and establishment of multiple invasive populations from the founding population during the early invasion processes. A combination of high genetic diversity within the founding population and the potential capability of asexual reproduction enabled this insect to become a successful invader. Furthermore, this study suggests that successful invasions and the subsequent dispersal of invasive species can be facilitated by anthropogenic activities, which can contribute to the sustainability of invasive species in new environments.
Methods
Sampling
Five populations of A. groverae adults were collected from newly furnished apartments in 2008, 2019, and 2020 (Table 1; Figs. 1, 2, 3). All specimens were carefully examined with bright-field and phase contrast microscopy (Olympus BX50, Japan). Species was diagnosed by various morphological key characteristics such as antenna and antero-ventral papillae present, spatula absent for larvae, and vein of wing, the number of flagellomeres, shape of genitalia, and pattern of seta under gonostylus claw for adult male and vein of wing, the number of flagellomeres, and shape of abdomen terminalia for adult female12,14,16,56 (Figs. S2, S3). The AG01 population was collected in 2008 when the first outbreak of A. groverae was reported in South Korea. These specimens were stored in the KU collection at Korea University. Other populations were collected from recent outbreaks from the particle boards of the newly furnished apartments in 2018 and 2019 (Fig. 3). However, the AG05 and AG06 populations were isolated (quarantined) at the laboratory at Korea University to test for the possibility of particle boards as a dispersal route of A. groverae (Fig. 3). Two isolated (quarantined) populations, originating from AG02, were collected in 2018. The particle boards from which the AG02 population was collected were maintained in a separate box in the laboratory for one year at room temperature. Separate particle boards were maintained for the AG05 and AG06 populations. New individuals that emerged from each population (each particle board) in 2019 were used for this study. All the specimens were preserved in 100% ethanol until analysis.
Mitochondrial DNA sequencing
Total genomic DNA was isolated from the thorax of the specimens using the DNeasy blood & tissue kit (Qiagen, USA). The mitochondrial DNA (mtDNA) COI sequences (648 bp) were amplified using the universal primers LCO1490 (5′-GGT CAA ATC ATA AAG ATA TTG G-3′) and HCO2198 (5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′)77. Polymerase chain reaction (PCR) was performed in a 20-μL reaction volume under the following conditions: an initial denaturation at 94 ℃ for 1 min, followed by 35 cycles of 94 ℃ for 30 s, 50–52 ℃ for 30 s, and 72 ℃ for 1–2 min; and a final extension step at 72 ℃ for 7 min. The amplicons were visualized on 1.5% agarose gels using UV light, purified using exonuclease I and shrimp alkaline phosphatase (New England BioLabs, USA), and sequenced using an ABI PRISM 3130xl Genetic Analyzer (Applied Biosystems, USA) by Macrogen INC Sequencing (Korea). COI sequences of A. groverae obtained in this study were deposited in GenBank (accession no: OK561689 and OK561809).
Development of novel microsatellite markers
Total genomic DNA was extracted from the thorax of adults (AG05) using the DNeasy blood & tissue kit (Qiagen, USA), following the manufacturer’s instructions. The integrity, concentration, and purity of the DNA were assessed using a Bioanalyzer 2100 (Agilent, USA). The sheared genomic DNA was processed to construct an Illumina paired-end library using a NxSeq® EZ-UltraLow DNA library kit. Next, paired-end reads (150 bp) were generated using the Illumina NextSeq500 platform (Illumina, USA). For assembly, sequencing errors were removed using the error correction module of SOAPec (ver. 2.02)78. Sequencing adaptors were trimmed using Skewer (ver. 0.2.2)79. Genome assembly was performed using SOAPdenovo2 (ver. 2.04-r240). To identify reliable assemblies, short reads were remapped to assembled sequences using GapCloser (ver. 1.12). Only assembled scaffolds were retained for microsatellite marker identification. In total, 1,887,386 scaffolds with an average length of 173 bp were obtained. Microsatellite sequences with 2–6 repeat motifs were identified using MISA80. The candidate loci were validated using PCR with primers designed using Primer 381. The primers were designed based on the following parameters: length, 20–26 nucleotides; annealing temperature, 51–54 ℃; product size, 100–300 bp. A set of 20 candidate microsatellite primer pairs was initially tested for polymorphisms. Among these, ten microsatellite markers were chosen. The GenBank accession numbers for the ten loci are listed in Table 2.
Microsatellite genotyping
One of each primer pair for the novel microsatellite markers was labeled with the fluorescent dye 5′-FAM. PCR was performed in a 20-μL reaction volume containing 30 ng DNA, 10 mM dNTPs, 100 nM of each primer, and 5 U HANLAB Taq polymerase (HANLAB, Korea) in an ABI 2720 Thermocycler (Applied Biosystems, USA). The PCR conditions were as follows: an initial denaturation at 95 ℃ for 2 min, followed by 35 cycles of 95 ℃ for 20 s, 50 ℃ for 30 s, and 72 ℃ for 30 s, and a final extension step at 72 ℃ for 5 min. The amplicons were electrophoresed on an ABI 3730x automated DNA sequencer and fragment sizes were analyzed using the GENEMAPPER software v. 5. (Applied Biosystems, USA).
Population genetic analyses
Mitochondrial diversity indices, including the number of haplotypes (NH), haplotype richness (HR), haplotype diversity (h), and nucleotide diversity (π), were estimated for each population and the entire pooled population using AREQUIN v.3.5.82. HR was estimated to correct for unequal sample size using the refraction method with CONTRIB v1.0283. The haplotype network was obtained using HAPSTAR v0.784. Microsatellite diversity was assessed based on the mean number of alleles per locus (NA), allelic richness (AR), expected (HE) and observed heterozygosity (HO), and observed inbreeding coefficient (FIS) using GENEPOP v4.085 and FSTAT v.2.9.3.286. The presence of null alleles was assessed using MICROCHECKER v. 2.2.387 with 1000 randomizations at 95% confidence level. Multi-locus tests for Hardy-Weinberg equilibrium (HWE) and null allele frequency at each locus were estimated using GENEPOP v4.0. To examine population differentiation, exact tests for population differentiation and the calculation of population pairwise FST values were performed for both markers using GENEPOP v4.0. Pairwise population comparisons were performed for significance using Bonferroni correction. Temporal and spatial genetic structures of A. groverae among the seven populations were assessed using hierarchical analysis of molecular variance (AMOVA) implemented in ARLEQUIN v.3.5. Seven populations were assigned to two temporal groups (collected in 2008 (AG01) and 2018–2020 (AG02–07)). Two biogeographic regions (north (N: AG01, 02, 03, 05, 06) and south (S: AG04, 07)) were assigned according to geographical distance irrespective of the collection year. Additionally, two isolated (quarantined) populations were assigned to the isolated group (I: AG05, 06) and natural population (N: AG01, 02, 03, 04, 07). The total molecular variance was partitioned among groups (Fct = ‘inter-group’ genetic variation), populations within groups (Fsc = ‘intra-group’ genetic variation), and within populations (Fst = ‘inter-population’) (Supplementary Information, Table S1).
The population genetic structures of A. groverae populations were examined using the Bayesian clustering algorithms implemented in STRUCTURE (v. 2.3.1)88 under a model of admixed ancestry among populations and correlated allele frequencies. Likelihood scores were calculated using the genetic clusters for each K value from 1 to 7 with 100,000 burn-in steps, followed by 1,000,000 Markov chain Monte Carlo iterations. Subsequently, the most likely K value was estimated using the web-based tool Structure Harvester (http://taylor0.biology.ucla.edu/structureHarvester/) based on the rate of change in the log probability data between successive K values. Furthermore, factorial correspondence analysis (FCA) based on genetic relationships among individuals with multi-locus genotypes was performed using GENETIX v4.5.289
Data availability
The datasets generated and/or analysed during the current study are available in GenBank under the accession numbers OK545742-OK545751, OK561689–OK561809 (https://ncbi.nlm.nih.gov/nuccore).
References
Hobbs, R. J. (ed.) Invasive Species in a Changing World (Island press, 2000).
Marbuah, G., Gren, I. M. & McKie, B. Economics of harmful invasive species: A review. Diversity 6, 500–523. https://doi.org/10.3390/d6030500 (2014).
Doherty, T. S., Glen, A. S., Nimmo, D. G., Ritchie, E. G. & Dickman, C. R. Invasive predators and global biodiversity loss. PNAS 113, 11261–11265. https://doi.org/10.1073/pnas.1602480113 (2016).
David, P. et al. Impacts of invasive species on food webs: A review of empirical data. Adv. Ecol. Res. 56, 1–60. https://doi.org/10.1016/bs.aecr.2016.10.001 (2017).
Roy, H. E. et al. Developing a list of invasive alien species likely to threaten biodiversity and ecosystems in the European Union. Glob. Change Biol. 25, 1032–1048. https://doi.org/10.1111/gcb.14527 (2019).
Walther, G. R. et al. Alien species in a warmer world: Risks and opportunities. Trends Ecol. Evol. 24, 686–693. https://doi.org/10.1016/j.tree.2009.06.008 (2009).
Peyton, J. et al. Horizon scanning for invasive alien species with the potential to threaten biodiversity and human health on a Mediterranean island. Biol. Invasions 21, 2107–2125. https://doi.org/10.1007/s10530-019-01961-7 (2019).
Meyerson, L. A. & Mooney, H. A. Invasive alien species in an era of globalization. Front. Ecol. Environ. 5, 199–208. https://doi.org/10.1890/1540-9295(2007)5[199:IASIAE]2.0.CO;2 (2007).
Hulme, P. E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 46, 10–18. https://doi.org/10.1111/j.1365-2664.2008.01600.x (2009).
Lodge, D. M. Biol Invasions: Lessons for ecology. Trends Ecol. Evol. 8, 133–137. https://doi.org/10.1016/0169-5347(93)90025-K (1993).
Keller, S. R. & Taylor, D. R. History, chance and adaptation during biological invasion: Separating stochastic phenotypic evolution from response to selection. Ecol. Lett. 11, 852–866. https://doi.org/10.1111/j.1461-0248.2008.01188.x (2008).
Ham, D., Kim, W. G., Lee, H., Choi, D. S. & Bae, Y. J. New Korean record of the mycophagous gall midge Asynapta groverae (Diptera: Cecidomyiidae) with its outbreak situation and ecological notes. Newsl. Entomol. Soc. Korea. 11, 25–30 (2018) (in Korean).
Grover, P. Studies on gall-midges from India XXXIV. On the study of Indian Porricondylini. Cecidologia Indica 6, 1–38 (1971).
Jiang, Y. X. & Bu, W. J. A newly recorded gall midge genus (Diptera, Cecidomyiidae) with a species, Asynapta groverae Jiang et Bu, nom. Nov. from China. Acta. Zootax. Sinica. 29, 786–789 (2004).
Bae, Y. J. Research report on the outbreak of the cecidomyiids (Diptera: Cecidomyiidae) from the Well-county apartment area in Songdo, Incheon. Incheon Metropolitan Development Corporation, Incheon 171 (2009) (in Korean).
Ham, D. & Bae, Y. J. Description of immature stages of Asynapta groverae (Diptera: Cecidomyiidae). Bull. Entomol. Res. 34, 103–107 (2018).
Gagné, R. J. & Jaschhof, M. A Catalog of the Cecidomyiidae (Diptera) of the World. 5th Edition, Digital, 121–124 (2021).
Jones, M., Mautner, A., Luenco, S., Bismarck, A. & John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 187, 108397. https://doi.org/10.1016/j.matdes.2019.108397 (2020).
Ross, K. G. & Shoemaker, D. D. Estimation of the number of founders of an invasive pest insect population: The fire ant Solenopsis invicta in the USA. Proc. R. Soc. B-Biol. Sci. 275, 2231–2240. https://doi.org/10.1098/rspb.2008.0412 (2008).
Brandt, M., Van Wlgenburg, E. & Tsutsui, N. D. Global-scale analyses of chemical ecology and population genetics in the invasive Argentine ant. Mol. Ecol. 18, 997–1005. https://doi.org/10.1111/j.1365-294X.2008.04056.x (2009).
Amouroux, P., Normand, F., Nibouche, S. & Delatte, H. Invasive mango blossom gall midge, Procontarinia mangiferae (Felt) (Diptera: Cecidomyiidae) in Reunion Island: Ecological plasticity, permanent and structured populations. Biol. Invasions 15, 1677–1693. https://doi.org/10.1007/s10530-012-0400-0 (2013).
Horst, C. P. & Lau, J. A. Genetic variation in invasive species response to direct and indirect species interactions. Biol. Invasions 17, 651–659. https://doi.org/10.1007/s10530-014-0756-4 (2015).
Nei, M., Maruyama, T. & Chakraborty, R. The bottleneck effect and genetic variability in populations. Evolution 29, 1–10. https://doi.org/10.2307/2407137 (1975).
Tsutsui, N. D. & Suarez, A. V. The colony structure and population biology of invasive ants. Conserv. Biol. 17, 48–58. https://doi.org/10.1046/j.1523-1739.2003.02018.x (2003).
Freeland, J. Molecular markers in ecology. In (eds Freeland, J., Pertersen, S. & Kirk, H.) Oxford 31–62 (2011).
Tsutsui, N. D., Suarez, A. V., Holway, D. A. & Case, T. J. Reduced genetic variation and the success of an invasive species. PNAS 97, 5948–5953. https://doi.org/10.1073/pnas.100110397 (2000).
Davis, M. A. Invasion Biology (Oxford University Press, 2009).
Yao, Y. X. et al. Genetic variation may have promoted the successful colonization of the invasive gall midge, Obolodiplosis robiniae, in China. Front. Genet. 11, 387. https://doi.org/10.3389/fgene.2020.00387 (2020).
Johnson, R. N. & Starks, P. T. A surprising level of genetic diversity in an invasive wasp: Polistes dominulus in the northeastern United States. Ann. Entomol. Soc. Am. 97, 732–737. https://doi.org/10.1603/0013-8746(2004)097[0732:ASLOGD]2.0.CO;2 (2004).
Roderick, G. K. Geographic structure of insect populations: Gene flow, phylogeography, and their uses. Annu. Rev. Entomol. 41, 325–352. https://doi.org/10.1146/annurev.en.41.010196.001545 (1996).
Puillandre, N. et al. Genetic bottleneck in invasive species: The potato tuber moth adds to the list. Biol. Invasions 10, 319–333. https://doi.org/10.1007/s10530-007-9132-y (2008).
Zhan, A., Macisaac, H. J. & Cristescu, M. E. Invasion genetics of the Ciona intestinalis species complex: From regional endemism to global homogeneity. Mol. Ecol. 19, 4678–4694. https://doi.org/10.1111/j.1365-294X.2010.04837.x (2010).
Mallez, S. et al. Worldwide invasion routes of the pinewood nematode: What can we infer from population genetics analyses?. Biol. Invasions 17(4), 1199–1213. https://doi.org/10.1007/s10530-014-0788-9 (2015).
Tsutsui, N. D. & Case, T. J. Population genetics and colony structure of the Argentine ant (Linepithema humile) in its native and introduced ranges. Evolution 55, 976–985. https://doi.org/10.1111/j.0014-3820.2001.tb00614.x (2001).
Kim, H., Hoelmer, K. A. & Lee, S. Population genetics of the soybean aphid in North America and East Asia: Test for introduction between native and introduced populations. Biol. Invasions 19, 597–614. https://doi.org/10.1007/s10530-016-1299-7 (2017).
Chen, M. H. & Dorn, S. Microsatellites reveal genetic differentiation among populations in an insect species with high genetic variability in dispersal, the codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae). Bull. Entomol. Res. 100, 75–85 (2010).
Zygouridis, N. E., Augustinos, A. A., Zalom, F. G. & Mathiopoulos, K. D. Analysis of olive fly invasion in California based on microsatellite markers. Heredity 102, 402–412. https://doi.org/10.1038/hdy.2008.125 (2009).
Lesieur, V. et al. The rapid spread of Leptoglossus occidentalis in Europe: A bridgehead invasion. J. Pest Sci. 92, 189–200. https://doi.org/10.1007/s10340-018-0993-x (2019).
Mutitu, E. K. et al. Reconstructing early routes of invasion of the bronze bug Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae): Cities as bridgeheads for global pest invasions. Biol. Invasions 22, 2325–2338. https://doi.org/10.1007/s10530-020-02258-w (2020).
Peccoud, J. et al. Host range expansion of an introduced insect pest through multiple colonizations of specialized clones. Mol. Ecol. 17(21), 4608–4618. https://doi.org/10.1111/j.1365-294X.2008.03949.x (2008).
Eyer, P. A., Moran, M. N., Blumenfeld, A. J. & Vargo, E. L. Development of a set of microsatellite markers to investigate sexually antagonistic selection in the invasive ant Nylanderia fulva. Insects 12, 643. https://doi.org/10.3390/insects12070643 (2021).
Schauer, B., Bong, J., Popp, C., Obermaier, E. & Feldhaar, H. Dispersal limitation of saproxylic insects in a managed forest? A population genetics approach. Basic Appl. Ecol. 32, 26–38. https://doi.org/10.1016/j.baae.2018.01.005 (2018).
Bereczki, J., Póliska, S., Váradi, A. & Tóth, J. P. Incipient sympatric speciation via host race formation in Phengaris arion (Lepidoptera: Lycaenidae). Org. Divers. Evol. 20, 63–76. https://doi.org/10.1007/s13127-019-00418-y (2020).
Selkoe, K. A. & Toonen, R. J. Microsatellites for ecologists: A practical guide to using and evaluating microsatellite markers. Ecol. Lett. 9, 615–629. https://doi.org/10.1111/j.1461-0248.2006.00889.x (2006).
Miah, G. et al. A review of microsatellite markers and their applications in rice breeding programs to improve blast disease resistance. Int. J. Mol. Sci. 14, 22499–22528. https://doi.org/10.3390/ijms141122499 (2013).
Lloyd, C. J., Norton, A. P., Hufbauer, R. A., Bogdanowicz, S. M. & Nissen, S. J. Microsatellite isolation from the gall midge Spurgia capitigena (Diptera: Cecidomyiidae), a biological control agent of leafy spurge. Mol. Ecol. Notes 4, 605–607. https://doi.org/10.1111/j.1471-8286.2004.00751.x (2004).
Bentur, J. S. et al. Isolation and characterization of microsatellite loci in the Asian rice gall midge (Orseolia oryzae) (Diptera: Cecidomyiidae). Int. J. Mol. Sci. 12, 755–772. https://doi.org/10.3390/ijms12010755 (2011).
Hinomoto, N., Higaki, T., Abe, J., Yamane, M. & Yano, E. Development and characterization of 21 polymorphic microsatellite loci in the aphidophagous gall midge, Aphidoletes aphidimyza (Diptera: Cecidomyiidae). Appl. Entomol. Zool. 47, 165–171. https://doi.org/10.1007/s13355-012-0104-z (2012).
Mezghani-Khemakhem, M. et al. Development of new polymorphic microsatellite loci for the barley stem gall midge, Mayetiola hordei (Diptera: Cecidomyiidae) from an Enriched Library. Int. J. Mol. Sci. 13, 14446–14450. https://doi.org/10.3390/ijms131114446 (2012).
Kim, H. et al. Development and characterization of 12 microsatellite loci from the blueberry gall midge Dasineura oxycoccana (Diptera: Cecidomyiidae). Appl. Entomol. Zool. 50, 415–418. https://doi.org/10.1007/s13355-015-0335-x (2015).
Benzécri, J. P. Construction d’une classification ascendante hiérarchique par la recherche en chaîne des voisins réciproques. Cahiers de l’analyse des données. 7, 209–218 (1982).
Simberloff, D. Invasive species. In Conservation Biology for all (eds Sodhi, N. S. & Ehrlich, P. R.) 131–152 (Oxford University Press, 2010).
Keum, E. et al. Morphological, genetic and symptomatic identification of an invasive jujube pest in Korea, Dasineura jujubifolia Jiao & Bu (Diptera: Cecidomyiidae). J. Asia Pac. Entomol. 101935, 2002. https://doi.org/10.1016/j.aspen.2022.101935 (2022).
Jaschhof, M. & Jaschhof, C. New and rarely found species of asynaptine Porricondylinae (Diptera: Cecidomyiidae) in northern Europe. Zootaxa https://doi.org/10.12651/JSR.2019.8.2.238 (2019).
Yuxia, J. & Wenjun, B. A newly recorded gall midge genus (Diptera, cecidomyiidae) with a species, Asynapta groverae Jiang et bu. nom. Nov. from China. Dong wu fen lei xue bao = Acta Zootaxonomica Sinica 29, 786–789 (2004).
Mamaev, M. & Krivosheina, N. P. The Larvae of the Gall Miges (CRC Press, 1992).
Dorchin, N., Harris, K. M. & Stireman, J. O. III. Phylogeny of the gall midges (Diptera, Cecidomyiidae, Cecidomyiinae): Systematics, evolution of feeding modes and diversification rates. Mol. Phylogenet. Evol. 140, 106602. https://doi.org/10.1016/j.ympev.2019.106602 (2019).
Gilpin, M. E. Minimal viable populations: Processes of species extinction. Conserv. Biol. Sci. Scarcity Divers. (1986).
Frankham, R., Ballou, J. D. & Briscoe, D. A. Introduction to Conservation Genetics (Cambridge University Press, 2002).
Hedrick, P. W. Genetic polymorphism in heterogeneous environments: The age of genomics. Annu. Rev. Ecol. Syst. 37, 67–93. https://doi.org/10.1146/annurev.ecolsys.37.091305.110132 (2006).
Kolbe, J. J. et al. Genetic variation increases during biological invasion by a Cuban lizard. Nature 431, 177–181. https://doi.org/10.1038/nature02807 (2004).
Frankham, R. Resolving the genetic paradox in invasive species. Heredity 94, 385–385. https://doi.org/10.1038/sj.hdy.6800634 (2005).
Sakai, A. K. et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 32, 305–332. https://doi.org/10.1146/annurev.ecolsys.32.081501.114037 (2001).
Wagner, N. P. Parthenogenesis in the larva of insects. Sci. Mem. Kasan Univ. 1, 25–111 (1862) (in Russian).
Meinert, F. Miastor metraloas: yderlige oplysning om den af Prof. Nic. Wagner nyligt beskneune insektlarva, som formerer sig ved spinedannelse. Naturhistorisk Tidsskrqt R3(3), 37–43 (1864).
Wyatt, I. J. Pupal paedogenesis in the Cecidomyiidae (Diptera). II. Proceedings of the Royal Entomological Society of London. J. Entomol. Ser. A-Gen. 38, 136–144. https://doi.org/10.1111/j.1365-3032.1963.tb00768.x (1963).
Wyatt, I. J. Immature stages of Lestremiinae (Diptera: Cecidomyiidae) infesting cultivated mushrooms. Trans. R. Entomol. Soc. Lond. 116, 15–27. https://doi.org/10.1111/j.1365-2311.1964.tb00823.x (1964).
Panelius, I. J. A revision of the European gall midges of the subfamily Porricondylinae (Diptera: Itonididae). Acta Zool. Fenn. 13, 1–157 (1965).
Schüpbach, P. M. & Camenzind, R. Germ cell lineage and follicle formation in paedogenetic development of Mycophila speyeri Barnes (Diptera: Cecidomyiidae). Int. J. Insect Morphol. Embryol. 12, 211–223. https://doi.org/10.1016/0020-7322(83)90018-1 (1983).
Sikora, T., Jaschhof, M., Mantič, M., Kaspřák, D. & Ševčík, J. Considerable congruence, enlightening conflict: molecular analysis largely supports morphology-based hypotheses on Cecidomyiidae (Diptera) phylogeny. Zool. J. Linn. Soc. 185, 98–110. https://doi.org/10.1093/zoolinnean/zly029 (2019).
Gould, S. J. Ontogeny and Phylogeny (Harvard University Press, 1985).
Went, D. F. Paedogenesis in the dipteran insect Heteropeza pygmaea: An interpretation. Int. J. Invertebr. Reprod. 1, 21–30. https://doi.org/10.1080/01651269.1979.10553296 (1979).
Hodin, J. & Riddiford, L. M. Parallel alterations in the timing of ovarian ecdysone receptor and ultraspiracle expression characterize the independent evolution of larval reproduction in two species of gall midges (Diptera: Cecidomyiidae). Dev. Genes Evol. 210, 358–372. https://doi.org/10.1007/s004270000079 (2000).
Olfert, O., Elliott, R. H. & Hartley, S. In Ecological Impacts of Non-native Invertebrates and Fungi on Terrestrial Ecosystems (eds Langor, D. W. & Sweeney, J.) 127–133 (Springer, 2008). https://doi.org/10.1007/978-1-4020-9680-8_9.
Miao, J. et al. Long-distance wind-borne dispersal of Sitodiplosis mosellana Géhin (Diptera: Cecidomyiidae) in Northern China. J. Insect Behav. 26, 120–129. https://doi.org/10.1007/s10905-012-9346-4 (2013).
Hao, Y. N. et al. Flight performance of the orange wheat blossom midge (Diptera: Cecidomyiidae). J. Econ. Entomol. 106, 2043–2047. https://doi.org/10.1603/EC13218 (2013).
Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299 (1994).
Luo, R. et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. GigaScience 1, 2047-217X-1–18. https://doi.org/10.1186/2047-217X-1-18 (2012).
Jiang, H., Lei, R., Ding, S. W. & Zhu, S. Skewer: A fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinform. 15, 1–12. https://doi.org/10.1186/1471-2105-15-182 (2014).
Beier, S., Thiel, T., Münch, T., Scholz, U. & Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 33, 2583–2585. https://doi.org/10.1093/bioinformatics/btx198 (2017).
Rozen, S. & Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. In Bioinformatics Methods and Protocols. Methods in Molecular Biology™ Vol. 132 (eds Misener, S. & Krawetz, S. A.) (Humana Press, 2000). https://doi.org/10.1385/1-59259-192-2:365.
Excoffier, L. & Lischer, H. E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567. https://doi.org/10.1111/j.1755-0998.2010.02847.x (2010).
Petit, R. J., Mousadik, A. E. & Pons, O. Identifying populations for conservation on the basis of genetic markers. Conserv. Biol. 12, 844–855. https://doi.org/10.1111/j.1523-1739.1998.96489.x (1998).
Teacher, A. G. F. & Griffiths, D. J. HapStar: Automated haplotype network layout and visualization. Mol. Ecol. Resour. 11, 151–153. https://doi.org/10.1111/j.1755-0998.2010.02890.x (2011).
Rousset, F. Genepop’007: A complete re-implementation of the genepop software for Windows and Linux. Mol. Ecol. Resour. 8, 103–106. https://doi.org/10.1111/j.1471-8286.2007.01931.x (2008).
Goudet, J. FSTAT, a program to estimate and test gene diversity and fixation indices (version 2.9.3). http://www2.unil.ch/popgen/softwares/fstat.htm (2001).
Van Oosterhout, C. V., Hutchinson, W. F., Wills, D. P. M. & Shipley, P. MICROCHECKER v. 2.2.3. (2006).
Evanno, G., Regnaut, S. & Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 14, 2611–2620. https://doi.org/10.1111/j.1365-294X.2005.02553.x (2005).
Belkhir, K., Borsa, P., Chikhi, L., Raufaste, N. & Bonhomme, F. GENETIX 4.05, logiciel sous Windows TM pour la génétique des populations. Montpellier, France: Laboratoire Génome, Populations, Interactions, CNRS UMR 5000, Université de Montpellier II (2004).
Acknowledgements
This work was supported by a grant from the National Institute of Biological Resources (NIBR), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR202211101). Additionally, this research was funded by a grant (NRF-2020R1C1C1007098) from the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT, and Future Planning, to J.H.K. We appreciate Mathias Jaschhof’s help for accurate species identification (Station Linné, Sweden). We also acknowledge the support provided by the members of the Biodiversity and Ecology Laboratory of Korea University, Chang-Ho Lim, and Ki-Hyun Kim for their assistance with sampling.
Author information
Authors and Affiliations
Contributions
Conceptualization and study design: J.H.K., Y.J.B. Sampling: D.H., S.H.P., J.H.K, J.M.H. Molecular experiments: J.H.K., D.H. Data analysis: J.H.K., D.H. Writing—original draft preparation: J.H.K., D.H., Y.J.B. Review and editing: J.H.K., D.H., Y.J.B., J.M.H., S.P., M.J.B. Project Administration: S.P., M.J.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, J.H., Ham, D., Park, S.H. et al. Population genetic structure of a recent insect invasion: a gall midge, Asynapta groverae (Diptera: Cecidomyiidae) in South Korea since the first outbreak in 2008. Sci Rep 13, 2812 (2023). https://doi.org/10.1038/s41598-023-29782-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29782-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.