Abstract
New insecticides have recently been produced to help control pyrethroid-resistant malaria vectors including the pyrrole, chlorfenapyr. Monitoring the susceptibility of mosquito populations against this new product and potential cross-resistance with current insecticides is vital for better resistance management. In this study, we assessed the resistance status of the major malaria vectors Anopheles gambiae and Anopheles funestus to chlorfenapyr across Africa and explored potential cross-resistance with known pyrethroid resistance markers. Efficacy of chlorfenapyr 100 µg/ml against An. gambiae and An. funestus from five Cameroonian locations, the Democratic Republic of Congo, Ghana, Uganda, and Malawi was assessed using CDC bottle assays. Synergist assays were performed with PBO (4%), DEM (8%) and DEF (0.25%) and several pyrethroid-resistant markers were genotyped in both species to assess potential cross-resistance between pyrethroids and chlorfenapyr. Resistance to chlorfenapyr was detected in An. gambiae populations from DRC (Kinshasa) (mortality rate: 64.3 ± 7.1%) Ghana (Obuasi) (65.9 ± 7.4%), Cameroon (Mangoum; 75.2 ± 7.7% and Nkolondom; 86.1 ± 7.4). In contrast, all An. funestus populations were fully susceptible. A negative association was observed between the L1014F-kdr mutation and chlorfenapyr resistance with a greater frequency of homozygote resistant mosquitoes among the dead mosquitoes after exposure compared to alive (OR 0.5; P = 0.02) whereas no association was found between GSTe2 (I114T in An. gambiae; L119F in An. funestus) and resistance to chlorfenapyr. A significant increase of mortality to chlorfenapyr 10 µg/ml was observed in An. funestus after to PBO, DEM and DEF whereas a trend for a decreased mortality was observed in An. gambiae after PBO pre-exposure. This study reveals a greater risk of chlorfenapyr resistance in An. gambiae populations than in An. funestus. However, the higher susceptibility in kdr-resistant mosquitoes points to higher efficacy of chlorfenapyr against the widespread kdr-based pyrethroid resistance.
Similar content being viewed by others
Introduction
Malaria is a major public health problem in sub-Saharan Africa. Although important gains have been achieved in reducing malaria burden since 20001, pyrethroids resistance has emerged as a major obstacle to the global fight against the disease2. To mitigate the risk, recent novel non-pyrethroid insecticides or repurposed ones from agriculture have been developed to control malaria vectors3,4. These new molecules have unique toxicity mechanisms, relying mainly on mosquito physiology not on “usual” neurological or simple detoxification pathways. Among these products, there is chlorfenapyr, a new insecticide class (pyrrole) acting by disrupting respiratory pathways and proton gradients through the uncoupling of oxidative phosphorylation in mitochondria5 to exert mosquito mortality6. Chlorfenapyr inhibits the production of energy in mosquito’s mitochondria which consequently affects crucial and vital functions until eventual death5. This mode of action on an insect’s metabolism is particularly relevant for the control of vectors harboring metabolic insecticide resistance mechanisms such as cytochrome P450, glutathione S-transferases as increased metabolic activity increases the activation of the toxin and increase mosquito mortality6. This insecticide generally presents delayed toxicity when insects are inactive or constrained to a cage as these new physiological insecticides depend on the mosquito metabolism to act. This makes their evaluation very challenging with conventional neuro-toxic tests like WHO cone bioassay7,8. As this insecticide is bio activated by metabolic enzymes, evaluating the effect of metabolic-based pyrethroid resistance on the efficacy of chlorfenapyr could help improve vector control.
Compared to target-site resistance, metabolic detoxification is very common in mosquitoes and considered to be more likely to cause control failure9. The first phase of metabolic resistance involves the cytochrome P450 (CYP) enzyme system and other detoxifying metabolic enzymes that catalyse the oxidation reaction of insecticides; the second stage involves uridine diphosphate (UDP) glucosyltransferases (UGTs), which form various conjugated metabolites through conjugation; finally, in the third phase, the ATP-binding cassette transporters (ABC transporters) are involved in eliminating the second-stage metabolites from the cells10. In An. funestus, metabolic resistance to pyrethroids is mainly driven by CYP6s and CYP9s genes family such as CYP6P9a, CYP6P9b, CYP6K1, CYP4M7, CYP325A, CYP9J11, CYP9K1, CYP6P5, CYP6P4a/b and GSTs such as GSTe211,12,13,14. In An. gambiae, this is mainly driven by CYP6P3, CYP6P4, CYP6M2, and GSTe215,16. Only limited molecular diagnostic tools are currently available to detect metabolic resistance in field mosquitoes preventing to assess its impact on the efficacy of vector control tools. It was shown for example in An. funestus from southern Africa that the CYP6AA1 had the ability to metabolise various types of pyrethroids17, while the overexpression of CYP6Z1 is associated with cross-resistance against pyrethroids and carbamates18. In An. gambiae, overexpression of CYP6P3 has been shown to be responsible for cross-resistance between pyrethroids and carbamates, and CYP6M2 is linked to cross-resistance of pyrethroids and organophosphates11,13,19. Moreover, it was shown that the CYP9K1 can metabolise deltamethrin and pyriproxyfen20. If such positive cross-resistance is seen with chlorfenapyr that will reduce the effectiveness of chlorfenapyr but if there is a negative cross–resistance, this will be very interesting as pyrethroid-resistant mosquitoes will be more vulnerable to the new active ingredient. Previous studies suggest that PBO has antagonistic effect on the toxicity of chlorfenapyr against Culex quinquefasciatus and An. stephensi mainly because it is bioactivated by cytochrome P450s and by blocking these enzymes, chlorfenapyr is not metabolised to its toxic form but just excreted. However, there is no clear evidence of negative association between P450-based or GSTs-based pyrethroid resistance and chlorfenapyr in mosquitoes probably due to the absence of DNA-based marker for metabolic resistance. This should be a critical step before the implementation of chlorfenapyr-based tool in the field. In this study, we comparatively assessed the susceptibility status of the major malaria vectors An. gambiae and An. funestus to chlorfenapyr across Africa and explored potential cross-resistance with known pyrethroid molecular markers.
Methods
Study sites
Mosquitoes were collected in five agricultural settings in Cameron (Mangoum, Nkolondom, Njombe-pendja, Mibellon and Elende) from May to July 2021, in one locality in 2021 from DR Congo (Ndjili Brasserie, a suburb of Kinshasa: 4° 19′ 39″ S, 15° 18′ 48″ E), Uganda (Mayuge: 0° 23′ 10.8′′ N, 33° 37′ 16.5′′ E); Ghana (Atatem: 5° 56′ N, 1° 37′ W) and Malawi (Chikwawa: (16° 1′ S; 34° 47′ E). In Cameroon, immature stages of An. gambiae were collected from the breeding site using the dipping method whereas adult An. funestus and An. gambiae from other countries were collected using electric aspirators. Two-five days old virgin females (F1 from field collected F0 or emerging F0 from collected larvae) were used for the bioassays.
Molecular identification
Members of the An. gambiae complex were identified by the SINE-PCR26,27 after genomic DNA extraction with the Livak method25 whereas those from An. funestus group were analysed using cocktail PCR described by Koekemoer et al.21.
Insecticide formulation
Technical-grade active ingredients was supplied by Sigma (PESTANAL®, analytical standard, Sigma-Aldrich, Dorset, United Kingdom). Stock solution was prepared by diluting the active ingredient in acetone or absolute ethanol, and storing in 50 ml falcon tubes, wrapped in aluminum foil, and at 4 °C. Working solutions were prepared using 1 ml of the stock solution. Field collected mosquitoes from the various sites were exposed to the insecticide in CDC bottle assay.
Determination of susceptibility to chlorfenapyr
Approximately 24 h after coating bottles with insecticide, 25 female (2–5 days old) were exposed to chlorfenapyr 100 µg/ml for 1 h and the knocked-down mosquitoes were recorded at the end of the 60 min (Kd-60) exposure period. After recording the Kd-60 mosquitoes were gently aspirated from the bottle into clean paper cups and provided with 10% sugar solution soaked in cotton wool during the recovery period and the mortality was recorded 24 h, 48 h and 72 h post-exposure. In addition to the testing with the diagnostic dose of 100 µg/ml reported in literature22, ranges of insecticide concentrations (0, 10, 20, 30, 40, 50, and 100 µg/ml) were tested on the susceptible lab strain Kisumu and the LC50 obtained was used for comparison of susceptibility profile between different populations of An. funestus. Time exposure was performed also against field anopheles mosquitoes using the diagnostic dose of chlorfenapyr (100 µg/ml) in order to compare the LT50 between different populations.
Evaluating the potential antagonistic/synergistic effect of piperonyl butoxide (PBO), di-ethyl maleate (DEM) and s,s,s-tri-butylphosphorotrithioate (DEF) on susceptibility to chlorfenapyr
To evaluate whether the inhibition of some enzyme systems could reduce susceptibility to chlorfenapyr, mosquitoes were pre-exposed to inhibitors of P450s (4% PBO), GSTs (8% DEM) or esterases (0.25% DEF) for 1 h, followed by 1 h exposure to chlorfenapyr (10 µg/ml for An. funestus and 100 µg/ml for An gambiae). Mortality was recorded 24 h, 48 h and 72 h after exposure and the differences in mortalities between inhibited and non-inhibited experiments were compared using a Chi-square test.
Genotyping of pyrethroid markers for potential cross-resistance with chlorfenapyr
To assess the potential cross-resistance between chlorfenapyr and pyrethroids in An. gambiae, we crossed the highly resistant field strain from Nkolondom with the fully susceptible laboratory strain Kisumu. This hybrid (F4) strain were exposed to sublethal dose of chlorfenapyr to select the dead and alive mosquitoes. These mosquitoes were genotyped for the L1014F target-site knockdown resistance (Kdrw) and I114T-GSTe2 mutation all associated with DDT/pyrethroid resistance in An. gambiae using a Taqman (Santa Clara, CA, USA) method as previously described23,24. In An. funestus, the L119F-GSTe2 mutation was genotyped in mosquitoes from Elende using the AS-PCR method as described recently25.
Odds ratio and Fisher exact test were used to establish statistical significance of any association between this DDT/pyrethroid resistance markers and the ability of mosquitoes to survive chlorfenapyr exposure.
Results
Molecular identification of mosquitoes tested
PCR assays revealed that all the An. gambiae s.l tested from Mangoum (44/44), Nkolondom (50/50), and Congo (60/60) were An. gambiae. Those collected in Njombe were mainly An. coluzzii (60/60). The An. gambiae sl population from Uganda was a mix with 83% An. gambiae and 17% An. arabiensis whereas those from Ghana were 60% (39/65) An. gambiae and 40% (39/65) An. coluzzii. Molecular identification of a subset (n = 50) of F0 females An. funestus s.l. from Mibellon revealed that 45 (90%) were An. funestus s.s., 3 (6%) were identified as hybrid An. funestus/An. rivulorum-like and 2 (4%) were An. rivulorum-like. Concerning the An. funestus collected from Elende, 100% (72/72) were An. funestus s.s. The Malawi An. funestus populations were at 91.1% An. funestus s.s and the remaining was other species of the group (4.54% An. parensis, 1.77% An. rivulorum) and hybrids (2.33%).
Diagnostic dosage of chlorfenapyr using the susceptible lab strain Kisumu
Figure 1 summarises the mortality of Kisumu after exposure to chlorfenapyr. The LC50 and LC99 for both insecticides are represented on the same figure. No knock down was observed irrespective of the solvent used. The LC50 was approximately 10 µg/ml 24 h post-exposure whereas the LC99 within the 24 h was obtained with 50 µg/ml.
Assessment of diagnostic dose of chlorfenapyr using the susceptible lab strain Kisumu. Percentage mortality (24 h, 48 h and 72 h) of the susceptible lab strain Kisumu after exposure to chlorfenapyr (with acetone as solvent). LC50 represents the concentration able to kill 50% of mosquitoes and LC99 the concentration able to kill 99%.
Exposure of the field collected samples to chlorfenapyr 10 µg/ml (the LC50 obtained with Kisumu) revealed lower mortality in all the field samples compared to Kisumu (Fig. 2) expect in Njombe-penja (81.3 ± 3.1%) where the difference was not significant (χ2 = 3.9; P > 0.05). Compared to Kisumu (69.2% ± 10.6%), the mortality rate after 72 h to chlorfenapyr 10 µg/ml was lower (13.3 ± 3.1) in An. gambiae from Nkolondom (χ2 = 64.4; P < 0.0001) and 20.0 ± 5.0 in An. gambiae from Mangoum (χ2 = 48.7; P < 0.0001). A slight increase in mortality rate was observed in An. gambiae from Mayuge (37.04 ± 18.7) and An. funestus from Mibellon (35.5 ± 7.9) and Elende (45.8 ± 13.4). All this showed that An. gambiae from Mangoum and Nkolondom which presented the lowest mortality (Fig. 2) are the less susceptible and that these populations are more likely to develop chlorfenapyr resistance.
Susceptibility profile to chlorfenapyr 100 µg using acetone and absolute ethanol as solvents
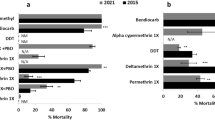
As the dose of 100 µg is the one recommended for routine evalutation of susceptibility to chlorfenapyr in malaria vectors in the field, we charaterised 10 populations including An. gambiae and An. funestus using both acetone and absolute ethanol. The mortality rate at this dose for the two solvents is summarised in the Table S1 showing a resistance trend in most of the An. gambiae populations and slight high efficacy of acetone compared to absolute ethanol. Most of the An. funestus populations were susceptible to this dose of chlorfenapyr when diluted in acetone but with ethanol some population (Njombe penja and Elende) showed a slightly reduced mortality rate, although not significant (Fig. 3A). In An. gambiae, varying levels of susceptibility was observed for the two solvents used (Fig. 3B; Table S1) with slightly reduced mortality rate observed with absolute ethanol as solvent compared to acetone. An. gambiae from Nkolondom, DR Congo and Mangoum had the lowest mortality rate with chlorfenapyr 100 µg/ml diluted in absolute ethanol with mortality rates of 58.2 ± 18.4%, 64.3 ± 7.1%, and 65.7 ± 18.5% respectively follow by An. gambiae from Mibellon with 75.6 ± 8.2% mortility (Fig. 3B). When using acetone as solvent, An. gambiae from Ghana had the lowest mortality (65.9 ± 7.4%) rate followed by Mangoum (75.2 ± 7.7%) and Nkolondom (86.1 ± 7.4) (Fig. 3B). Unfortunately, the low sample size did not allow the testing with acetone in Congo DRC. All these results show that An. gambiae populations are more likely to develop chlorfenapyr resistance compared to An. funestus. Also, the results show that both absolute ethanol and acetone are comparable solvents for chlorfenapyr and An. gambiae from Congo DRC, Ghana, Mangoum (CMR) and Nkolondom (CMR) were found as the most resistant populations.
Time-course exposure to chlorfenapyr 100 µg/ml
In addition to the previous tests, we performed bioassays with An. funestus (Mibellon) and An. gambiae (Mibellon and Nkolondom) to chlorfenapyr 100 µg/ml varying the exposure time (15 min, 30 min, 45 min and 60 min). This test revealed that the LT90 is reacheable within the 30 min of exposure for An. funestus whereas the LT50 was obtained with 15 min exposure time (Fig. 4). In An. gambiae from the same location (Mibellon), the LT90 was obtained only after 60 min exposure and the LT50 after 30 min exposure. In Nkolondom An gambiae, the strength of resistance was higher with LT90 not obtained within the 60 min exposure period and the LT50 above 45 min of exposure. All this results confirm a greater risk of chlorfenapyr resistance in An. gambiae populations than in An. funestus.
Effect of piperonyl butoxide (PBO), di-ethyl maleate (DEM) and s,s,s-tri-butylphosphorotrithioate (DEF) on susceptibility to chlorfenapyr
With the greater susceptibility observed with all the An. funestus populations, we performed bioassays with the inhibitors of P450s (PBO), GSTs (DEM) and ESTs (DEF) to assess the impact of these enzyme systems on the susceptibility to chlorfenapyr. Bioassays conducted on An. funestus from Mibellon and Elende revealed a significant recovery of susceptibility to chlorfenapyr 10 µg/ml in both populations tested for all the three inhibitors (Fig. 5). However, the restoration of susceptibility was higher in An. funestus from Elende compared to those from Mibellon. In An. funestus from Elende, the mortality rate obtained with chlorfenapyr 10 µg/ml (45.8 ± 13.1%) increased significantly after pre-exposure to PBO (χ2 = 22.9; P < 0.0001), DEM (χ2 = 30.2; P < 0.0001) and DEF (χ2 = 16.5; P < 0.0001) as summarised in Fig. 5. In An. gambiae populations where resistance was observed, pre-exposure to PBO revealed a slight reduced level of mortality to the same dose of chlorfenpyr (10 µg) but with no significant difference for Mibellon (χ2 = 1; P = 0.2) and Nkolondom (χ2 = 1; P < 0.5). The same trend was observed with chlorfenapyr100 µg (χ2 = 0.2; P = 0.6). This is indicative of a limited effect of P450s on chlorfenapyr potency in An gambiae.
Results of tests with PBO, DEM and DEF. Effect of pre-exposure to inhibitors PBO, DEM and DEF against chlorfenpayr 10 μg/ml for An. funestus (from Mibellon and Elende) and An. gambiae (from Mibellon and Nkolondom) and 100 μg/ml for An. gambiae from Nkolondom. Results are average of percentage mortalities from four-five replicates each. The bars represent the standard error on the mean (SEM), * indicate the level of significance and NS represents no significant difference.
Association between pyrethroid resistant markers and susceptibility to chlorfenapyr
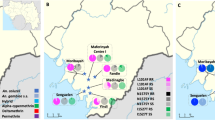
Mosquitoes with the 1014F-resistant allele were more susceptible to chorfenapyr exposure indicating a negative cross-resistance between the L1014F-Kdr_w marker and chlorfenapyr resistance. The distribution of L1014F-Kdr_w genotypes in alive hybrid Nkolondom/Kisumu (F4) after exposure with a sub-lethal of chlorfenapyr (10 µg/ml) was as follows: 8.7% (4/46) homozygous resistant (1014F/F), 69.6% (32/46) heterozygotes (L1014F-RS) and 21.7% (10/46) homozygous susceptible (L/L1014) (Fig. 6A,B). In the dead mosquitoes, 28.9% (13/45) were homozygous resistant (1014F/F), 53.3% (24/45) were heterozygotes (L1014F-RS) whereas 17.4% (8/45) were homozygous susceptible (L/L1014). A significant difference was observed in the distribution of L1014F-Kdr_w genotypes between alive and dead mosquitoes (χ2 = 13.3; P < 0.001) with high proportion of RR in the dead compared to the alive (χ2 = 5.9; P = 0.01). However, no difference was found in the genotype frequency of SS in the alive compared to the dead (χ2 = 0.3; P = 0.5) same for the heterozygote (χ2 = 2.5; P = 0.1). At the allelic level, the frequency of the 1014F allele was low in the dead compared to alive (χ2 = 3.6; P = 0.05). Assessment of the odds ratio revealed that mosquitoes with the RR genotype have less chance to survive compared to RS (OR 0.2; CI 0.09–0.5; P = 0.0004) and SS (OR 0.2; CI 0.09–0.6; P = 0.007). No association was found between the I114T-GSTe2 mutation and chlorfenapyr resistance in An. gambiae (Fig. 6C,D). For this marker, no significant difference was observed in the genotype distribution between alive and dead ((χ2 = 0.2; P = 0.9). This was confirmed by the absence of correlation between RR and SS (OR 1.1; CI 0.4–2.8; P = 0.5) as presented in Table 1.
Association between pyrethroids resistance markers and resistance to chlorfenapyr in An. gambiae. Distribution of the L1014F-Kdr_w genotypes (A) and alleles (B) among the dead and alive mosquitoes after exposure to chlorfenapyr and (C) and (D) for I114T-GSTe2. R represents the resistant allele while S represent the susceptible allele.
In An. funestus from Elende, analysis of the association between the L119F-GSTe2 mutation and the ability to survive chlorfenapyr exposure revealed that mosquitoes with the homozygote resistant genotype (RR) had a significant ability to survive compared to SS (OR 2.8 CI 1.3–7.3; P = 0.02) (Fig. 7A,B; Table 1).
Discussion
The present study assessed the susceptibility status of the major malaria vectors An. gambiae and An. funestus to chlorfenapyr across Africa and explored for the first time potential cross-resistance with known pyrethroid molecular markers.
Vector populations exhibit a variable resistance profile across Africa
Most of the An. gambiae populations tested were resistant to chlorfenapyr (100 µg AI/bottle) while all the An. funestus were susceptible to this insecticide. The susceptibility to chlorfenapyr observed in this study particularly in An. funestus is reported in many other african countries and support the choice of this insecticide for vector control. Large-scale susceptibility testing of this insecticide using the interim discriminating concentration of 100 µg AI/bottle as done in this study against thousands of An. gambiae s.l. in 16 countries demonstrated vector susceptibility, including mosquitoes with multiple resistance mechanisms to pyrethroids7. Other studies in Faranah Prefecture of Guinea with Chlorfenapyr 100 µg AI/bottle revealed 100% mortality with wild An. gambiae, while in the Agréby-Tiassa Region of south-east Côte d’Ivoire, the same concentration induced only 95.5% mortality indicating a reduced suceptibility26,27. In this study, when using the concentration of 100 µg AI/bottle, we detected well-established resistance levels in An. gambiae populations from Cameroon, DR Congo and Ghana showing that this species could rapidly develop chlorfenapyr resistance compared to An. funestus. Such ability to develop chlorfenapyr resistance could be associated to agricultural practices as all the An. gambiae tested were collected from agricultural settings. An. gambiae larvae tend to breed in small temporal water bodies such as puddles and ditches which can be easily contaminated by agricultural pesticides selecting the resistant mosquitoes. However, there is still no evidence that chlorfenapyr is actively been used in these agricultural sites. One cannot also exclude a possible cross-resistance with other pesticides used by farmers. Overall, more investigations are needed to establish the potential source of selection for chlorfenapyr resistance in such populations of malaria vectors. The significant reduced susceptibility level observed to chlorfenapyr in the three localities would be among the first reports of tolerance to this insecticide in malaria vectors. Such reduced susceptibility would suggest that field populations of malaria vectors, notably from An. gambiae, may already harbour some genetic factors to withstand exposure to this insecticide and this calls for the design of suitable resistance management plan to anticipate on such resistance. Full susceptibility observed in An. funestus populations indicates that chlorfenapyr-based tools could help controlling this species as reported with IG2 in several countries such as Benin and Burkina Faso and Ivory Coast where this net induced 71% and 80% and 87% mortality in An. gambiae and An. funestus respectively. This high susceptibility of An. funestus could also explain the highest efficacy of Interceptor G2 net in the randomised-control trial in Tanzania a region where this species was predominant (94.5%)28.
PBO displays synergistic effect with chlorfenapyr in An. funestus but potential antagonistic effect in An. gambiae from Cameroon
Because of the significant resistance noticed in some An. gambiae populations and the susceptibility observed with all the An. funestus populations, we performed bioassays with the inhibitors of P450s (PBO), GSTs (DEM) and ESTs (DEF) to identify the possible enzyme systems impacting susceptibility to chlorfenapyr as it was reported that metabolic activity increases the bioactivation of this insecticide increasing mosquito mortality6. In An. gambiae, PBO-prexposure slightly reduced the mortality of An. gambiae to chlorfenapyr 10 µg/ml and 100 µg/ml showing potential antagonistic effect of this P450-inhibitor. Suprisingly, tests conducted on An. funestus from Mibellon and Elende revealed a significant increase of susceptibility to chlorfenapyr 10 µg/ml in all the two populations tested for all the three inhibitors. This indicates that CYPs may affect detoxification of chlorfenapyr as observed in silkworms for BmCYP4C129, but further studies are needed to investigate the exact role of CYPs in the processing of this insecticide. A trend of antagonism seeing with PBO on An. gambiae was reported in potentiation studies conducted with susceptible and resistant strains of An. stephensi with 15% PBO30. The same antagonistic effect was also reported in insecticide-susceptible Ae. aegypti by Paul et al.31. Monooxygenases metabolize chlorfenapyr to its active toxic insecticide form5, and was demonstrated in Tetranychus urticae, where PBO antagonized 2.3-fold toxicity of chlorfenapyr32. This antagonistic effect shows that chlorfenapyr can be activated by cytochrome P450 monooxygenases, and uncoupled oxidative phosphorylation in mitochondria and could therefore improve the efficacy of chlorfenapyr in areas of high P450-based resistance. The contrasting pattern between An. funestus and An. gambiae could indicate a complexity in mechanisms conferring chlorfenapyr resistance.
Negative association between L1014F-kdr marker and chlorfenapyr resistance
Understanding the cross-resistance pattern conferring resistance to a particular candidate insecticide is critical to formulate strategies for resistance management33. The high levels of susceptibility observed in An. funestus field populations to chlorfenapyr indicates a potential lack of cross-resistance with pyrethroid insecticides for the tested populations. This was confirmed for the I114T and L119F-GSTe2 (DDT/pyrethroid resistant marker) mutations for which no correlation was seen with the ability to survive chlorfenapyr exposure. This can be justified by the fact that chlorfenapyr do not have a particular target site like other insecticides. Indeed, chlorfenapyr is considered as pro-insecticide that is activated by oxidase enzymes suggesting a potential for negative cross-resistance30 as observed for the kdr in this study. Mosquitoes bearing the 1014F-resistant allele were more vulnerable to chlorfenapyr as observed previously with clothianidin34 showing that this insecticide could help controlling kdr-resistant mosquitoes. However, resistance was only detected in An. gambiae populations (where the kdr is mostly fixed) showing that mechanisms developed for chlorfenapyr resistance in An. gambiae are not linked to the kdr and need to be investigated. It is also possible that the correlation between kdr and resistance to chlorfenapyr here could be caused by a linkage of kdr and metabolic resistance and not necessarily a direct action of kdr as the chlorfenapyr does not have a particular target. Tests with synergist of pyrethroids increased the level of suscceptibility to chlorfenapyr showing potentially that some detoxification enzymes belonging to cytochrome P450s, GSTs or esterases could also affect chlorfenapyr efficacy as seeing for BmCYP4C1 in silkworms29. However, more studies are needed to establish the level of association between pyrethroid resistance and chlorfenapyr efficacy in major malaria vectors or to characterise the molecular basis of chlorfenapyr tolerance observed in An. gambiae. This will allow to predict the potential for cross-resistance and spread of chlorfenapyr resistance and help guiding National Malaria Control Programmes in the selection of insecticides for ITNs and IRS.
Conclusions
This study showed that although the kdr-resistant mosquitoes were more susceptible to chlorfenapyr with limited role of GSTe2-based resistant markers, An. gambiae is more likely to develop chlorfenapyr resistance compared to An. funestus. This indicates that chlorfenapyr resistance needs to be well monitored and mechanisms driving resistance to this new insecticide class elucidated at the early stage to help prolong the effectiveness of chlorfenapyr-based tools such as interceptor G2.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Bhatt, S. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015).
Hemingway, J. et al. Country-level operational implementation of the Global Plan for Insecticide Resistance Management. Proc. Natl. Acad. Sci. USA 110, 9397–9402. https://doi.org/10.1073/pnas.1307656110 (2013).
WHO. List of WHO Prequalified Vector Control Products (World Health Organization, 2019).
Ngufor, C. et al. Chlorfenapyr (a pyrrole insecticide) applied alone or as a mixture with alpha-cypermethrin for indoor residual spraying against pyrethroid resistant Anopheles gambiae sl: An experimental hut study in Cove. Benin. PLoS One 11, e0162210 (2016).
Black, B. C., Hollingworth, R. M., Ahammadsahib, K. I., Kukel, C. D. & Donovan, S. Insecticidal action and mitochondrial uncoupling activity of AC-303,630 and related halogenated pyrroles. Pestic. Biochem. Physiol. 50, 115–128 (1994).
Hunt, D. A. & Treacy, M. Insecticides with Novel Modes of Action 138–151 (Springer, 1998).
Oxborough, R. M. et al. Determination of the discriminating concentration of chlorfenapyr (pyrrole) and Anopheles gambiae sensu lato susceptibility testing in preparation for distribution of Interceptor® G2 insecticide-treated nets. Malar. J. 20, 1–10 (2021).
Oxborough, R. M. et al. The activity of the pyrrole insecticide chlorfenapyr in mosquito bioassay: Towards a more rational testing and screening of non-neurotoxic insecticides for malaria vector control. Malar. J. 14, 1–11 (2015).
Hemingway, J. The role of vector control in stopping the transmission of malaria: Threats and opportunities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130431. https://doi.org/10.1098/rstb.2013.0431 (2014).
Ye, M. et al. The role of insect cytochrome P450s in mediating insecticide resistance. Agriculture 12, 53 (2022).
Edi, C. V. et al. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 10, e1004236. https://doi.org/10.1371/journal.pgen.1004236 (2014).
Ibrahim, S. S. et al. Allelic variation of cytochrome P450s drives resistance to bednet insecticides in a major malaria vector. PLoS Genet. 11, e1005618. https://doi.org/10.1371/journal.pgen.1005618 (2015).
Mitchell, S. N. et al. Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proc. Natl. Acad. Sci. USA 109, 6147–6152. https://doi.org/10.1073/pnas.1203452109 (2012).
Riveron, J. M. et al. Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus. Proc. Natl. Acad. Sci. USA 110, 252–257. https://doi.org/10.1073/pnas.1216705110 (2013).
Fossog Tene, B. et al. Resistance to DDT in an urban setting: Common mechanisms implicated in both M and S forms of Anopheles gambiae in the city of Yaounde Cameroon. PLoS One 8, e61408. https://doi.org/10.1371/journal.pone.0061408 (2013).
Edi, A. V. C. et al. First detection of N1575Y mutation in pyrethroid resistant Anopheles gambiae in Southern Cote d’Ivoire. Wellcome Open Res. 2, 71. https://doi.org/10.12688/wellcomeopenres.12246.1 (2017).
Ibrahim, S. S. et al. Pyrethroid resistance in the major malaria vector Anopheles funestus is exacerbated by overexpression and overactivity of the P450 CYP6AA1 across Africa. Genes 9, 140 (2018).
Ibrahim, S. S., Ndula, M., Riveron, J. M., Irving, H. & Wondji, C. S. The P450 CYP 6Z1 confers carbamate/pyrethroid cross-resistance in a major African malaria vector beside a novel carbamate-insensitive N485I acetylcholinesterase-1 mutation. Mol. Ecol. 25, 3436–3452 (2016).
Stevenson, B. J. et al. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: Sequential metabolism of deltamethrin revealed. Insect Biochem. Mol. Biol. 41, 492–502. https://doi.org/10.1016/j.ibmb.2011.02.003 (2011).
Vontas, J. et al. Rapid selection of a pyrethroid metabolic enzyme CYP9K1 by operational malaria control activities. Proc. Natl. Acad. Sci. USA 115, 4619–4624. https://doi.org/10.1073/pnas.1719663115 (2018).
Koekemoer, L., Kamau, L., Hunt, R. & Coetzee, M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am. J. Trop. Med. Hyg. 66, 804–811 (2002).
Oxborough, R. M. et al. Determination of the discriminating concentration of chlorfenapyr (pyrrole) and Anopheles gambiae sl susceptibility testing in preparation for distribution of Interceptor® G2 insecticide-treated nets (2021).
Bass, C., Williamson, M. S., Wilding, C. S., Donnelly, M. J. & Field, L. M. Identification of the main malaria vectors in the Anopheles gambiae species complex using a TaqMan real-time PCR assay. Malar. J. 6, 1–9 (2007).
Mitchell, S. N. et al. Metabolic and target-site mechanisms combine to confer strong DDT resistance in Anopheles gambiae. PLoS One 9, e92662 (2014).
Tchouakui, M. et al. Fitness costs of the glutathione S-transferase epsilon 2 (L119F-GSTe2) mediated metabolic resistance to insecticides in the major african malaria vector anopheles funestus. Genes 9, 25 (2018).
Stica, C. et al. Characterizing the molecular and metabolic mechanisms of insecticide resistance in Anopheles gambiae in Faranah, Guinea. Malar. J. 18, 1–15 (2019).
Meiwald, A. et al. Reduced long-lasting insecticidal net efficacy and pyrethroid insecticide resistance are associated with over-expression of CYP6P4, CYP6P3 and CYP6Z1 in populations of Anopheles coluzzii from South-East Cote d Ivoire. bioRxiv 20, 20 (2020).
Mosha, J. F. et al. Effectiveness and cost-effectiveness against malaria of three types of dual-active-ingredient long-lasting insecticidal nets (LLINs) compared with pyrethroid-only LLINs in Tanzania: A four-arm, cluster-randomised trial. Lancet 399, 1227–1241 (2022).
Shao, Y. et al. Transcriptional response of detoxifying enzyme genes in Bombyx mori under chlorfenapyr exposure. Pestic. Biochem. Physiol. 177, 104899. https://doi.org/10.1016/j.pestbp.2021.104899 (2021).
Raghavendra, K. et al. Chlorfenapyr: A new insecticide with novel mode of action can control pyrethroid resistant malaria vectors. Malar. J. 10, 1–7 (2011).
Paul, A., Harrington, L. C. & Scott, J. G. Evaluation of novel insecticides for control of dengue vector Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 43, 55–60 (2006).
Van Leeuwen, T., Stillatus, V. & Tirry, L. Genetic analysis and cross-resistance spectrum of a laboratory-selected chlorfenapyr resistant strain of two-spotted spider mite (Acari: Tetranychidae). Exp. Appl. Acarol. 32, 249–261 (2004).
Stumpf, N. & Nauen, R. Cross-resistance, inheritance, and biochemistry of mitochondrial electron transport inhibitor-acaricide resistance in Tetranychus urticae (Acari: Tetranychidae). J. Econ. Entomol. 94, 1577–1583 (2001).
Tchouakui, M. et al. Comparative study of the effect of solvents on the efficacy of neonicotinoid insecticides against malaria vector populations across Africa (2022).
Funding
This work was supported by the Renewal Wellcome Trust Senior Research Fellowship in Biomedical Sciences (217188/Z/19/Z) and the BMGF Grant (INV-006003) awarded to CSW.
Author information
Authors and Affiliations
Contributions
C.S.W. conceived and designed the study; M.T., L.M.J.M., B.D.M, D.N.N., H.R.T. collected the samples with the help of J.K., F.W., E.Z.M. and M.O., A.O.; M.T., T.A., and H.R.T., maintained the strain in the insectary and performed bioassays; M.T. and T.A. performed the molecular analyses; M.T., and C.S.W. analyzed the data; M.T. wrote the manuscript with contributions from C.S.W. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tchouakui, M., Assatse, T., Tazokong, H.R. et al. Detection of a reduced susceptibility to chlorfenapyr in the malaria vector Anopheles gambiae contrasts with full susceptibility in Anopheles funestus across Africa. Sci Rep 13, 2363 (2023). https://doi.org/10.1038/s41598-023-29605-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29605-w
This article is cited by
-
Can the performance of pyrethroid-chlorfenapyr nets be reduced when combined with pyrethroid-piperonyl butoxide (PBO) nets?
Malaria Journal (2023)
-
High efficacy of chlorfenapyr-based net Interceptor® G2 against pyrethroid-resistant malaria vectors from Cameroon
Infectious Diseases of Poverty (2023)
-
Sub-lethal exposure to chlorfenapyr reduces the probability of developing Plasmodium falciparum parasites in surviving Anopheles mosquitoes
Parasites & Vectors (2023)
-
Insecticidal roof barriers mounted on untreated bed nets can be as effective against Anopheles gambiae as regular insecticide-treated bed nets
Scientific Reports (2023)
-
Chlorfenapyr metabolism by mosquito P450s associated with pyrethroid resistance identifies potential activation markers
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.