Abstract
Infection with Thelazia nematodes results in eye disease in wild and domestic animals. The aim of the present study was to describe the occurrence of Thelazia nematodes in European bison, and to subject the isolated parasites to molecular identification and phylogenetical analysis. The eyeballs of 18 European bison from the Bieszczady Mountains, culled due to dysfunctional vision, were collected for study. The conjunctival sacs, tear ducts, corneal surface and nictitating membrane were rinsed with a saline solution. Any obtained nematodes were isolated under a stereoscopic microscope, and then identified as T. gulosa or T. skrjabini by molecular analysis of partial cox1 sequences. The prevalence of infection with Thelazia spp. was found to be 61%, with a 95% confidence interval (CI 95%) of 39–80%. Thelazia skrjabini was isolated from 56% (CI 95% 34–75%) of examined animals; T. gulosa was significantly less common (p = 0.038) with the prevalence of infection reaching 22% (CI 95% 9–45%). Three European bison were cross-infected with both T. gulosa and T. skrjabini. Phylogenetic analysis found the obtained sequences to be similar to those of Thelazia species from domestic ungulates in Europe. Infection intensity ranged from 1 to 16 nematodes per individual (median of three nematodes), and was significantly higher in females (6 nematodes) than in males (1 nematode; p = 0.019). A tendency for seasonal occurrence of nematodes in European bison was also observed. Our study provides further information regarding the patterns of Thelazia transmission in European bison in Poland.
Similar content being viewed by others
Introduction
Infectious diseases contribute to a species being classified as endangered1 and can cause significant temporary or permanent declines in local populations2. Parasitic diseases in particular may have a substantial impact on wildlife population dynamics and represent a critical issue in the conservation of endangered species3. The recent changes in the climate appear to support parasite development and survival rates, as well as disease transmission and host susceptibility4,5; as such, the emergence of new parasitic diseases, and the re-emergence of previous ones, may soon represent a major threat to wildlife diversity. One recent example of a disease that existed previously in domestic ruminants but has recently begun to rapidly spread in wildlife is thelaziosis, which has been noted in populations of endangered European bison (Bison bonasus) in Poland, a middle European country6.
Nematodes of the genus Thelazia are known to cause eye diseases in wild and domestic animals worldwide7. Several Thelazia species specific to particular hosts have been identified in Europe, and ruminants may be exposed to T. gulosa, T. skrjabini, T. rhodesi and T. lacrymalis8. Thelazia gulosa is also considered a parasite of zoonotic potential and has recently been identified in humans in the United States of America9. Thelazia lacrymalis and T. rhodesi have also been isolated from the eyeballs of a horse7,10.
Adult nematodes localize in the conjunctival sac and tear ducts, under the nictitating membrane and on the cornea of infected animals6,7. The eye worms are transmitted by secretophagous non-biting flies from the genus Musca, which become infected with the first stage larvae while feeding on animal lacrimal secretions6,11. In the intermediate host, the first-stage larvae develop into the invasive third stage, migrate to the fly suckers and are passed to the conjunctival sac of the next definitive host11. The pathogenic effect of Thelazia nematodes derives from the mechanical irritation of the conjunctiva and cornea, as well as the toxic effect of parasitic metabolites. The infected animals suffer from acute conjunctivitis, often complicated by secondary bacterial infections leading to purulent eye inflammation, corneal opacity, and ulceration6,8.
In Poland, nematodes of the species T. gulosa and T. skrjabini were commonly detected in cattle in the 1960s and 1970s, especially in densely-populated herds12,13,14. Infection manifested itself only as transient blindness, probably due to regular deworming of livestock, and was of little concern6. However, the incidence of thelaziosis in European bison has been increasing in Poland since 2018. The animals suffer from lesions of the eyeballs, leading to visual impairment, blindness, and eventually death6. As such, thelaziosis is becoming a matter of veterinary concern in wild ruminants in Poland and warrants regular monitoring.
European bison is an endangered species that went extinct in the wild at the beginning of the 1900s; the population has been restored from captive individuals since the 1950s and eventually reintroduced to European forests15. Currently, over 7000 free-living European bison can be found worldwide, of which about 2300 (one-third of the world population) live in Poland. One of the largest populations in the country, comprising over 600 free-living individuals, inhabits the Polish part of the Bieszczady Mountains; the population itself is divided into eastern and western subpopulations16.
Currently, the European bison population is managed with the aim of preserving its genetic diversity and establishing new herds, which involves relocating free-living and captive animals between European countries17. As international transport of animals might favour the spread of some pathogens to new areas18, there is a particular need to monitor the occurrence of Thelazia species in European bison in Poland.
The aim of our study was to determine the prevalence and the species diversity of nematodes of the genus Thelazia in the free-living population of European bison in the Bieszczady Mountains in Poland, and to subject isolated parasites to molecular identification and phylogenetic analysis.
Results
Study population
The study population comprised 18 bison, nine males and eight females, aged from 6 to 18 years (median 14 years) (sex and age unknown for 1 bison). The median age of the males and females was 15 and 11 years, respectively, and did not differ significantly between sexes (p = 0.068). Males were significantly heavier (median 710 kg, range 350–800 kg) than females (median 400 kg, range 350–450 kg) (p = 0.009) (body weight unknown for 2 bison).
Anatomopathological analysis
The most common lesions were bilateral conjunctival congestion together with fibrin deposits and corneal opacity. Corneal ulceration, purulent ocular inflammation and atrophic eyeballs were less common. Changes led to vision disfunction or blindness in the animals, which resulted in their culling.
Indices of Thelazia infection
The overall prevalence of nematodes of the genus Thelazia was 61% (95% confidence interval (CI 95%) 39–80%; 11/18 bison) with the prevalence of T. skrjabini being significantly higher than of T. gulosa (p = 0.038) (Table 1). Co-infection with both Thelazia species was observed in three examined European bison, whereas seven were infected only with T. skrjabini and one was infected only with T. gulosa.
The number of nematodes detected in a single bison (i.e. the intensity of infection) ranged from 1 to 16, and this value did not differ significantly between animals infected with one Thelazia species and those with mixed infection (p = 0.375) (Table 1). The intensity of infection did not differ significantly between the left and right eyes of an infected bison (p = 0.859) and was not correlated with age (Spearman’s rank correlation coefficient (Rs) = − 0.18, p = 0.628) nor body weight (Rs = − 0.55, p = 0.098). The intensity of Thelazia infection was significantly higher in females than males (p = 0.019). The highest intensity of Thelazia spp. infection was observed in August and the lowest in May; however, no statistical analysis was feasible due to the low number of examined animals (Supplementary Fig. S1).
Molecular and phylogenetic analysis
The molecular analysis of the partial cox1 gen amplified from the isolated nematodes yielded two species: T. gulosa (OL362019) and T. skrjabini (OL362009).
The sequence of T. gulosa obtained from European bison was identical to that isolated from bovines in Italy (AJ544881). However, the obtained sequence of T. skrjabini did not demonstrate a 100% similarity with any submission in the GenBank library. Our obtained sequence is the first molecular record of the cox1 gene from T. skrjabini. Phylogenetic analysis of the cox1 sequences (Fig. 1), with Dirofilaria repens and D. immitis as an outgroup, revealed the presence of two clades. Both sequences obtained during the present study were included in the same clade, together with T. californiensis from a dog in the USA (MW055239), but in different subclades. Thelazia gulosa was grouped together with the isolate from the bovine in Italy (AJ544881) and T. skrjabini with T. lacrymalis from a horse in Romania (ON024373). The second clade included T. rhodesi isolated from cattle in Romania (MT511659) and sequences of T. callipaeda from a dog in Moldova (MN163032) and China (NC_018363).
Phylogenetic tree of Thelazia spp. based on cox1 partial sequences, constructed by Bayesian inference (BI) analysis using MrBayes version 3.2. The GTR+I+G model was chosen as the best-fitting nucleotide substitution model using JModelTest version 2.1.10 software45,46. Analysis was run for 1,000,000 generations, with 250,000 generations discarded as ‘burn-in’. Nodal support is indicated as Bayesian posterior probabilities. Sequences of Dirofilaria repens and D. immitis were used as the outgroup. Sequences from this study are in bold. The scale bars are proportional to the number of substitutions per site.
Discussion
Being highly exposed to the fly vectors of eyeworms, free-grazing animals such as bovids are considered especially vulnerable to Thelazia spp. infection8. As such, bovine thelaziosis, caused by nematodes of the species T. gulosa, T. rhodesi, and T. skrjabini, is the most commonly-reported animal thelaziosis19. Many studies concerning the occurrence of Thelazia nematodes in domestic cattle have been published in Europe, including the United Kingdom, Italy, Romania, Denmark, Ukraine, and Poland12,13,19,20,21,22,23,24; however, the reports of thelaziosis in wild ruminants are more limited. During the last century, a few asymptomatic Thelazia infections have been observed in European bison in Poland25,26. Since 2018, the number of clinical cases of thelaziosis has been begun to surge6. Not surprisingly, a high number of individuals were found to be infected with Thelazia spp. nematodes in our present study (60%), and all examined animals suffered from vision disfunction. It has been proposed that the disappearance of adult nematodes in eyes may resemble the self-cure phenomenon of gastrointestinal Strongyles in sheep21; however, little data is available to support this hypothesis.
Two species of Thelazia nematodes were isolated from the eyeballs of European bison in the Bieszczady Mountains, viz. T. skrjabini and T. gulosa, both considered typical of ruminants8. Although T. skrjabini has rarely been reported as a cause of bovine thelaziosis in Europe19, it was found to be significantly more prevalent in the studied European bison than T. gulosa.
Three European bison were cross-infected with both T. gulosa and T. skrjabini; this has previously been observed by other authors and is believed to be a consequence of the sympatric occurrence of the species in the environment27. Cross-infections might be also related to high host exposure and susceptibility to the parasite28; however the number of isolated Thelazia nematodes did not differ significantly between European bison with single-species and mixed infection.
Thelazia gulosa and T. skrjabini were placed in the same clade, as sister taxa, together with Thelazia nematodes from domestic ungulates in Europe (Fig. 1). This might indicate that domestic hosts act as possible sources of Thelazia infection for European bison in the Bieszczady Mountains6,29. The close phylogenetic relationship of European bison with domesticated bovids might increase the risk of cross‐species transmission of some parasites3, and possibly Thelazia nematodes. Currently, no data is available regarding bovine thelaziosis in Poland as it has not been monitored in cattle herds for the previous 50 years12,13,14. Thompson30 proposes that the lack of such monitoring programs in domestic hosts might account for an increased exposure of wildlife to parasites of domestic livestock. As such, it is possible that cattle may play a role in the transmission of Thelazia nematodes to free-living European bison.
Thelazia rhodesi, a common etiological factor of bovine thelaziosis8,19,31, was not found in any European bison in the present study. Indeed, phylogenetical analysis revealed that Thelazia nematodes isolated from the studied European bison were genetically distinct from T. rhodesi. The occurrence of T. rhodesi is expected to be restricted to countries with warmer climates than Poland, especially those in the southern part of Europe, such as Italy and Romania19,24.
Among the studied European bison, the females demonstrated significantly higher Thelazia nematode infection intensity than the males. Lower parasitic loads have also been observed in bison bulls by other authors, and it has been proposed that this difference probably results from the solitary lifestyle of the males: the females tend to live in groups, together with calves and subadults, which favours the spread of parasitic diseases4,32,33. In addition, females might be more susceptible to the infection due to immunosuppression caused by pregnancy or lactation34. In addition to reproductive status, host immunity may also vary in relation to stress or food availability35. It has been proposed that the nutritive status of the animals might influence their resistance to parasitosis36; indeed, studies indicate that greater food consumption favoured the immunocompetence of females and resulted in lower female-biased parasite infestation37. However, as several different mechanisms are responsible for gender-biased parasitism37, it is difficult to identify specific factors affecting higher Thelazia infection intensity in female European bison.
Eyeworm transmission requires the continuous presence of vectors in the environment38, and the occurrence of nematodes demonstrates seasonal variation dependent on the activity of the intermediate hosts6,39. Although the small number of examined animals in our study was too low to perform statistical analysis of seasonal variation, a higher intensity of Thelazia infection was observed in the summer compared to spring and autumn (Supplementary Fig. S1). Thus, the increasing transmission and infectivity of Thelazia spp. in European bison might be associated with climate variability and higher fly activity.
Two species of the genus Musca, viz. M. domestica and M. autumnalis, have so far been detected in the Bieszczady Mountains40,41, although no current data on their distribution is available. Both flies were also observed on large livestock farms in the adjacent region of Southern Poland42. It has been proposed that the widespread occurrence of M. domestica and M. autumnalis in the natural and semi-natural environment may result from them being synanthropic fly species connected with livestock husbandry40. Although it has been suggested that M. domestica may play a role in Thelazia transmission8, M. autumnalis is considered the main vector43 and might participate in passing the infection between sylvatic and synanthropic ruminant species. As there are no current data of Thelazia occurrence in other wild and domestic ruminants in Poland, it is important to begin regular eyeworm monitoring in areas known to be at risk of thelaziosis in European bison.
Thelazia spp. infections in wild ruminants in Europe are probably more widespread than previously assumed, and further studies are needed to identify the patterns of Thelazia transmission; this is particularly important given a zoonotic potential of the infection. Understanding the epidemiology of Thelazia spp. in European bison is essential for reducing the risk of infection, for example, by improving management strategies to avoid exposure to worms through the eyes. Future studies are needed to determine the occurrence of nematodes in the domestic cattle herds in Poland and to identify the role of Muscidae flies as vectors of bovine thelaziosis.
Materials and methods
Study area
The material was collected in the Baligród and Komańcza Forest Districts, in the Polish part of the Bieszczady Mountains, south-eastern Poland (Fig. 2). The area is protected by the Cisna-Wetlina Landscape Park and The East Carpathian Biosphere Reserve. The landscape of the Bieszczady Mountains is characterized by parallel, long mountain ranges running from the north-west to the south-east, with the height gradually increasing from west to east. The highest peak of this part of the Bieszczady Mountains is Tarnica (1346 m above sea level). The climate is temperate and warm, and predominantly continental. The mean annual temperature is 7.5 °C and the annual precipitation ranges from 800 to 1200 mm, with the highest rates in the summer and the lowest during winter. The slopes and valleys are covered by Carpathian beechwood complexes, with an admixture of sycamore and fir trees, and the streams are lined by alder forests. Beechwood forests grow up to 1150 m above sea level and lie directly alongside the mountain pastures44. Agriculture is dominated by breeding and grazing of sheep and cattle. Agricultural lands mostly consist of meadows and pastures.
Study area showing the locations of the Forest Districts: Komańcza (1) and Baligród (2) (Microsoft Paint 11.2208.6.0; www.microsoft.com).
Material collection and parasitological examination
Between May and October of 2019 and 2020 18 free-living European bison from the western subpopulation in the Baligród and Komańcza Forest Districts in the Bieszczady Mountains were culled due to apparent lesions on the surface of eyeballs. During the postmortem examination, age was estimated based on tooth development and wear, and the sex and body weight of the animals were recorded. The eyeballs were collected, together with adjacent tissues, transported at 4 °C to the laboratory, and examined.
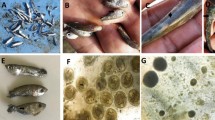
In the laboratory, the anatomopathological changes of the cornea and conjunctival sacs were subjected to macroscopic examination. Then, the conjunctival sacs, tear ducts, corneal surface and the nictitating membrane were rinsed with physiological solution. The eyeballs were dissected and their structures thoroughly rinsed. The decantated sediment was examined for the presence of nematodes under a stereoscopic microscope (Polskie Zakłady Optyczne, Poland) at × 40 magnification. Any obtained parasites were isolated and identified to the species level based on morphometrical features6. The nematodes were subsequently preserved in 70% alcohol and stored for further molecular analysis.
Molecular and phylogenetic procedures
To disrupt the surface of the nematodes, they were cut in half and mechanically ground with sterile sand (50 mg). DNA was isolated from nematode specimens with a commercial DNA Mini Kit (Syngen, Poland) according to the manufacturer’s protocol, with some modifications.
The DNA was amplified by PCR using primers designed in the Witold Stefański Institute of Parasitology, Polish Academy of Sciences: COIintF (5′-TGATTGGTGGTTTTGGTAA-3′) and COIintR (5′-ATAAGTACGAGTATCAATATC-3′). As a result, a 640 bp fragment of the mitochondrial cytochrome c oxidase subunit 1 gene (cox1) was obtained. The reactions were conducted in a 40 µl reaction mixture containing 2.0 µl of DNA template, 0.2 µl (1U) of Color Taq DNA Polymerase (EURx, Poland), 1 µl of dNTPs mix (10 mM), 0.5 µl of each primer (20 mM), 5 µl of 10× Polymerase buffer (pH 8.6, 25 mM MgCl2), and 30.8 µl of H2O. Nuclease-free water was added to the PCR mixture as a negative control. DNA amplification was performed using the DNA Engine T100 Thermal Cycler (BioRad, USA) according to the following program: denaturation at 94 °C for one minute, followed by 34 cycles of denaturation at 95 °C for 20 s, annealing at 56 °C for 20 s and extension at 72 °C for 40 s, with a final extension performed at 72 °C for 5 min.
The PCR products were visualized on a 1.2% agarose gel (Promega, USA) stained with SimplySafe (EURx, Poland) and a size-marked DNA Marker 100 bp LOAD DNA ladder (Syngen, Poland). Visualization was performed using ChemiDoc, MP Lab software (Imagine, BioRad, USA). The obtained PCR products were purified with the DNA clean-up Kit (Syngen, Poland). Following purification, the products were sequenced by a commercial facility (Genomed, Poland) and assembled using ContigExpress, Vector NTI Advance v.11.0 (Invitrogen Life Technologies, New York, NY, USA).
The obtained sequences were compared with those from GenBank in BLAST (NCBI, USA) and submitted to GenBank. A phylogenetic tree of Thelazia spp. based on cox1 partial sequences was constructed by Bayesian inference (BI) analysis using MrBayes version 3.2. The GTR+I+G model was chosen as the best-fitting nucleotide substitution model using JModelTest version 2.1.10 software45,46. The analysis was run for 1,000,000 generations, with 250,000 generations discarded as burn-in. The phylogenetic trees were visualized using the TreeView software (S&N Genealogy Supplies, UK).
Statistical analysis
Categorical variables were presented as counts and percentages, and compared between groups using the maximum likelihood G test, or Fisher’s exact test if the expected count in any cell of the contingency table was below 5. The 95% confidence intervals (CI 95%) for proportions were calculated using the Wilson’s score method47. Numerical variables were presented as the median and range, and compared between groups using the Mann–Whitney U test (unpaired groups) or the Wilcoxon’s signed rank test (paired groups). Correlations between two numerical variables were determined using the Spearman’s rank correlation coefficient (Rs). The significance level (α) was set at 0.05. Statistical analysis was performed in TIBCO Statistica 13.3 (TIBCO Software Inc., Palo Alto, CA).
Ethics declaration
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered with accordance to Directive 2010/63/EU of The European Parliament and of The Council of 22 September 2010 on the protection of animals used for scientific purposes. All European bison were legally culled on the basis of permission from the General Directorate for Environmental Protection in Poland. The decision of General Directorate for Environmental Protection was implemented by the Regional Head Office of National Forests in Krosno, which supervised animals sacrifice. European bison as wild animals, were culled by professional hunters with respect to the animals welfare and safety rules. The dissection of animals as well as collection of the material was performed in the field, after culling.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Smith, K. F., Sax, D. F. & Lafferty, K. D. Evidence for the role of infectious disease in species extinction and endangerment. Conserv. Biol. 20, 1349–1357 (2006).
Daszak, P., Cunningham, A. A. & Hyatt, A. D. Emerging infectious diseases of wildlife—Threats to biodiversity and human health. Science 287, 443–449 (2000).
Pedersen, A. B., Jones, K. E., Nunn, C. L. & Altizer, S. Infectious diseases and extinction risk in wild mammals. Conserv. Biol. 21, 1269–1279 (2007).
Kołodziej-Sobocińska, M. Factors affecting the spread of parasites in populations of wild European terrestrial mammals. Mamm. Res. 64, 301–318 (2019).
Borowik, T., Ratkiewicz, M., Maślanko, W., Duda, N. & Kowalczyk, R. Too hot to handle: Summer space use shift in a cold-adapted ungulate at the edge of its range. Landsc. Ecol. 35, 1341–1351 (2020).
Demiaszkiewicz, A. W. et al. The Nematodes Thelazia gulosa Railiet and Henry, 1910 and Thelazia skrjabini Erschov, 1928 as a cause of blindness in European Bison (Bison bonasus) in Poland. Acta Parasitol. 65, 963–968 (2020).
Anderson, R. C. (ed.) Nematode Parasites of Vertebrates. Their Development and Transmission (CAB International, 1992).
Otranto, D. & Traversa, D. Thelazia eyeworm: An original endo- and ecto-parasitic nematode. Trends Parasitol. 21, 1–4 (2005).
Bradbury, R. S. et al. A second case of human conjunctival infestation with Thelazia gulosa and a review of T. gulosa in North America. Clin. Infect. Dis. 70, 518–520 (2020).
Lyons, E. T., Drudge, J. H. & Tolliver, S. C. Thelazia lacrymalis in horses in Kentucky and observations on the face fly (Musca autumnalis) as a probable intermediate host. J. Parasitol. 62, 877–880 (1976).
Giangaspero, A., Traversa, D. & Otranto, D. Ecology of Thelazia spp. in cattle and their vectors in Italy. Parasitologia 46, 257–259 (2004).
Kostyra, J. The clinical picture and treatment of bovine thelaziosis (Przebieg i leczenie telazjozy u bydła, in Polish). Med. Weter. 16, 584–587 (1960).
Rosłan, J. Badania nad telazjozą bydła w Polsce. Wiad. Parazyt. 11, 73–79 (1965).
Kozakiewicz, B. Bovine thelaziosis in Żuławy (Telazjoza bydła na Żuławach, in Polish). Med. Weter. 27, 241–243 (1971).
Samojlik, T. (ed.) European Bison. The Nature Monograph (Springer, 2013).
Raczyński, J. (ed.) European Bison Pedigree Book (Księga rodowodowa żubrów, in Polish) 1–82 (Białowieski Park Narodowy, 2019).
Pucek, Z. et al. (eds) European Bison. Status Survey and Conservation Action Plan (IUCN/SSC Action Plans for the Conservation of Biological Diversity, 2004).
Buchmann, K., Christiansen, L.-L., Kania, P. W. & Thamsborg, S. M. Introduced European bison (Bison bonasus) in a confined forest district: A ten year parasitological survey. Int. J. Parasitol. Parasites Wildl. 18, 292–299 (2022).
Giangaspero, A., Otranto, D., Vovlas, N. & Puccini, V. Thelazia gulosa Railliet & Henry, 1910 and T. skrjabini Erschow, 1928 infection in southern Europe (Italy). Parasite 7, 327–329 (2000).
Klësov, M. D. Contribution to the question of the biology of two nematodes of the genus Thelazia Bosc, 1819, parasites of the eyes of cattle. Dokl. Akad. Nauk SSSR 75, 591–594 (1950).
Arbuckle, J. B. & Khalil, L. F. A survey of Thelazia worms in the eyelids of British cattle. Vet. Rec. 102, 207–210 (1978).
Tweedle, D. M., Fox, M. T., Gibbons, L. M. & Tennant, K. V. Change in the prevalence of Thelazia species in bovine eyes in England. Vet. Rec. 157, 555–556 (2005).
Kolstrup, N. Thelazia skrjabini in Danish cattle. Nord. Vet. Med. 26, 459–462 (1974).
Deak, G., Ionică, A. M., Oros, N. V., Gherman, C. M. & Mihalca, A. D. Thelazia rhodesi in a dairy farm in Romania and successful treatment using eprinomectin. Parasitol. Int. 80, 102183 (2021).
Dróżdż, J. A study on helminth and helminthiases in bison, Bison bonasus (L.) in Poland. Acta Parasitol. Pol. 9, 55–96 (1961).
Dróżdż, J., Demiaszkiewicz, A. W. & Lachowicz, J. The helminth fauna of free-ranging European bison, Bison bonasus (L.). Acta Parasitol. Pol. 34, 117–124 (1989).
Otranto, D., Tarsitano, E., Traversa, D., De Luca, F. & Giangaspero, A. Molecular epidemiological survey on the vectors of Thelazia gulosa, Thelazia rhodesi and Thelazia skrjabini (Spirurida: Thelaziidae). Parasitology 127, 365–373 (2003).
Viney, M. E. & Graham, A. L. Patterns and processes in parasite co-infection. Adv. Parasitol. 82, 321–369 (2013).
Demiaszkiewicz, A. W. & Kaczor, S. Thelaziasis in European bison in Bieszczady—A case report. Życie Weter. 90, 108–110 (2015).
Thompson, R. C. Parasite zoonoses and wildlife: One health, spillover and human activity. Int. J. Parasitol. 43, 1079–1088 (2013).
Naem, S. Thelazia rhodesi (Spirurida, Thelaziidae), bovine eyeworm: Morphological study by scanning electron microscopy. Parasitol. Res. 100, 855–860 (2007).
Krasińska, M. & Krasiński, Z. A. Composition, group size, and spatial distribution of European bison bulls in Białowieża Forest. Acta Theriol. 40, 1–21 (1995).
Krasińska, M., Krasiński, Z. A. & Bunevich, A. N. Factors affecting the variability in home range size and distribution in European bison in the Polish and Belarussian parts of the Białowieża Forest. Acta Theriol. 45, 321–334 (2000).
Lloyd, S. Effect of pregnancy and lactation upon infection. Vet. Immunol. Immunopathol. 4, 153–176 (1983).
Martin, L. B., Weil, Z. M. & Nelson, R. J. Seasonal changes in vertebrate immune activity: Mediation by physiological trade-offs. Philos. Trans. R. Soc. Lond. B Biol Sci. 363, 321–339 (2008).
Muturi, K. N. et al. The effect of dietary polyunsaturated fatty acids (PUFA) on infection with the nematodes Ostertagia ostertagi and Cooperia oncophora in calves. Vet. Parasitol. 129, 273–283 (2005).
Krasnov, B. R. & Matthee, S. Spatial variation in gender-biased parasitism: Host-related, parasite-related and environment-related effects. Parasitology 137, 1527–1536 (2010).
Dunn, A. M. (ed.) Veterinary Helminthology (William Heinemann Medical Books, 1978).
Khedri, J., Radfar, M. H., Borji, H. & Azizzadeh, M. Epidemiological survey of bovine thelaziosis in southeastern of Iran. Iran. J. Parasitol. 11, 221–225 (2016).
Draber-Mońko, A. Synanthropic Calyptrata in selected habitats in Poland. Wiad. Parazytol. 32, 411–418 (1986).
Draber-Mońko, A. Dipterans of the families Muscidae (Muscinae) and Scathophagidae (Diptera, Calyptrata) of the Świętokrzyski Region. Fragmenta Faunistica 36, 205–233 (1993).
Nosal, P., Kowal, J., Węglarz, A. & Wyrobisz-Papiewska, A. The occurrence and diversity of flies (Diptera) related to ruminant farming in southern Poland. Ann. Parasitol. 65, 357–363 (2019).
Cotuțiu, V. D. et al. Thelazia lacrymalis in horses from Romania: Epidemiology, morphology and phylogenetic analysis. Parasites Vectors 15, 425 (2022).
Nowosad, M. Outlines of climate of the Bieszczady National Park and its buffer zone in the light of previous studies. Roczniki Bieszczadzkie 4, 163–183 (1995).
Guindon, S. & Gascuel, O. A. Simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704 (2003).
Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 9, 77 (2012).
Altman, D. G. et al. (eds) Statistics with Confidence 2nd edn, 46–47 (BMJ Books, 2000).
Acknowledgements
The authors would like to thank Dr. Stanisław Kaczor (DVM) for performing the dissections of animals and assisting in material collection.
Author information
Authors and Affiliations
Contributions
K.F.H. was responsible for parasitological examination of eyeballs, molecular analysis of isolated nematodes as well as preparation of original draft. Z.L. designed starters for molecular analysis, supervised molecular part of the research and performed phylogenetical analysis. A.W.M. performed molecular and phylogenetical analysis. M.C. performed statistical analysis and prepared Supplementary Material. B.M. supervised the research team and take part in conceptualisation of the study. A.W.D. was responsible for parasitological examination of eyeballs, conceptualisation of the study and supervision of the research team. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Filip-Hutsch, K., Laskowski, Z., Myczka, A.W. et al. The occurrence and molecular identification of Thelazia spp. in European bison (Bison bonasus) in the Bieszczady Mountains. Sci Rep 12, 22508 (2022). https://doi.org/10.1038/s41598-022-27191-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-27191-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.