Abstract
A new analytical quality by design-assisted HPLC–UV approach is presented, for the first time, for the concurrent determination of cetirizine (CTZ) and azelastine (AZE) in raw materials, commercial eye drops and aqueous humor. The two drugs are co-administered as eye drops in severe ocular allergies. A 23 full factorial design was adopted for the chromatographic optimization to ensure the best analytical performance and reliability, as well as to save time, effort and solvent consumption. The parameters, including pH, acetonitrile ratio, and flow rate, were selected as independent factors. The responses analyzed were resolution and tailing of peaks. The separation was achieved through isocratic elution on C8 column with mobile phase made up of acetonitrile: 0.3% triethylamine of pH 5 (60:40 v/v) at a flow rate of 1.2 mL min−1 and detection at 216 nm. The elution time was less than 6 min. The approach was fully validated in accordance with International Council for Harmonization (ICH) guidelines. Good linearity was achieved over the concentration ranges of 1.0–30 and 0.5–10 µg mL−1 with limits of detection of 0.310 and 0.158 µg mL−1 and limits of quantification of 0.940 and 0.479 µg mL−1 for CTZ and AZE, respectively, with correlation coefficients of 0.9998. The intra- and inter-day precisions were lower than 2%. The good sensitivity of the approach permits the analysis of CTZ and AZE in spiked aqueous humor with mean percentage recoveries of 100.93 ± 1.42 and 100.11 ± 1.55, respectively. The statistical comparison between results of the developed method and the comparison method revealed no differences, indicating the accuracy of the method.
Similar content being viewed by others
Introduction
Quality by design has emerged as a cutting-edge systematic approach that produces scientifically sound results. The application of design of experiments (DOE) in chromatographic separation has several merits in optimization and establishment of a robust and rugged method. It is an efficient tool for optimization of the experimental condition to obtain maximum information with lower number of experiments than univariate procedures1, 2. This matches the current direction for developing green analytical approaches with less investment, effort and solvents. DOE tests multiple factors simultaneously to obtain a certain response and interaction between them3.
Ocular allergy is one of the most common ocular surface diseases that has emerged in the last several decades4. It affects approximately 40% of the population globally5. It reduces the patient’s productivity at work as well as his overall quality of life4. Ocular allergy disease is the inflammation of the conjunctiva, which is caused by an immune system reaction mediated by immunoglobulin-E6. It is caused by mold, pollen and environmental stimuli leading to watery and swollen eye, itching and chemosis7. Because of the widespread and multifactorial causes of ocular allergy, a combination of therapies is often required to treat the associated signs and symptoms.
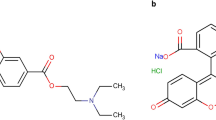
CTZ is [(4-chlorophenyl) phenylmethyl]piperazin-1-yl] ethoxy] acetic acid (Fig. 1a)8. It is a second-generation antihistamine which is widely used in the comprehensive management of allergic conjunctivitis9. BP8 stated non-aqueous potentiometric titration with sodium hydroxide, whilst USP10 described high performance liquid chromatography (HPLC) procedure for CTZ estimation. Various analytical techniques have been developed for the determination of CTZ either in pharmaceutical preparations, human plasma or aqueous humor as HPLC11,12,13,14,15,16, capillary electrophoresis17, 18, spectrofluorimetry19,20,21,22,23 and spectrophotometry24, 25.
AZE is 4-(4-chlorobenzyl)-2- [-1-methylhexahydro-1H-azepin-4-yl] phthalazin-1(2H)-one (Fig. 1b)8. It is a mast cell stabilizer applied topically to treat the symptoms of allergic conjunctivitis26. It was determined by non aqueous titration with 0.1 N perchloric acid potentiometrically in both BP8 and USP10. Different articles have been reported in the literature for the determination of AZE either in dosage form, human plasma or aqueous humor. These articles include: HPLC27,28,29,30,31, HPTLC32, 33, capillary electrophoresis34, electrochemical analysis35, 36, NMR37, spectrofluorimetry22, 38,39,40 and spectrophotometry41,42,43.
Topical antihistamines and mast cell stabilizers are frequently co-administered for the relief of ocular allergies44. CTZ as an antihistamine blocks H1 receptors, providing immediate relief before AZE starts to inhibit mast cell degranulation and histamine release. So, adding AZE can increase the efficacy of CTZ44.
Reviewing the literature showed that only one research was conducted in our previous lab for the simultaneous quantification of CTZ and AZE using synchronous spectrofluorimetry22. It has narrow linearity ranges for both drugs (0.1–2.0 µg mL−1 for both). Sulfuric acid was used in an attempt to improve the sensitivity. On the other hand, the proposed approach avoids the analysis in such a corrosive environment (pH less than 2). The reported HPLC11 for CTZ assay used gradient elution and mass spectrometry, which is not available in most laboratories due to its high cost. Our procedure had a shorter retention time for both drugs than the reported HPLC procedures 12, 13, 16, 27, 30. The developed HPLC procedure, whose mobile phase is made up of only two components, is much simpler than other documented HPLC procedures14, 15, 30, 31, whose mobile phases are made up of three or more components, therefore requiring more time for preconditioning. Also, our procedure had a lower LOQ for both drugs than the documented procedures13,14,15,16, 28, 29. All of these previous methods were developed by changing one factor at a time, which has assured to be time-consuming, expensive, does not fix errors, and may yield unpredictable responses45. Up till now, there has been no described HPLC procedure for the quantification of either CTZ or AZE alone in aqueous humor or the simultaneous quantification of both.
HPLC is currently considered the most widely used analytical technique in the pharmaceutical industry and in the analysis of pharmaceuticals in aqueous humor due to its availability, reproducibility and diversity of applications46. The goal of HPLC drug analysis is to verify the identity of a drug and provide quantitative results, as well as to control the progress of disease therapy47. It is characterized by ease of automation, permitting high throughput analysis of numerous samples over a short time, making it suitable for clinical assessment. It rendered the procedure more specific and reproducible than the fluorimetric one.
Accordingly, a new, rapid, selective, accurate and cost-effective analytical HPLC method is developed utilizing a full factorial design for the simultaneous estimation of CTZ and AZE in synthetic mixtures, single ophthalmic formulations and aqueous humor. Being co-administered makes their analysis in aqueous humor clinically important, which is why we conducted this study. The results obtained were auspicious with wider linearity ranges than the previously documented approaches and adequate sensitivity for both drugs.
Eye drops must be administered in the right dose to ensure optimal efficacy and minimize side effects for the patient. Novelty of the suggested HPLC method originates from the simultaneous analysis of both drugs in a complex matrix as aqueous humor for therapeutic drug monitoring, which had never been previously studied. There is a lack of chromatographic techniques for the determination of CTZ in ophthalmic formulations. This research paper includes a detailed investigation concerning their separation efficiency and quantification owing to their clinical impact, which in turn is expected to offer help to analysts caring to analyze the cited drugs. It is superior to the documented methods in completing the separation process in much less time, which in turn saves solvent consumption. It achieves a relatively high sensitivity with good peak shapes. It is also the first time to apply DOE (full factorial design) in the development and optimization of HPLC for either CTZ or AZE assay to give the best possible analytical performance rather than changing one factor at a time. With DOE, method developers are given a comprehensive understanding of the method thanks to specialized domain expertise. Knowledge of critical method parameters and design plots will ensure optimal performance of the method. Changing one factor at a time frequently leads to nonrobust performance2. The DOE-based technique simultaneously permits the quick and effective development of reliable methods. This encourages our developed approach to be employed as an efficient, easy and robust analytical tool for routine high-throughput analysis required in research centers and quality control laboratories. Additionally, promoting the approach to be carried out in pharmacokinetic investigations.
Experimental
Apparatus and software
A Knauer Azura® chromatograph with 20 µL loop and a Rheodyne injection valve was utilized in the chromatographic separation. It was equipped with P 6.1 L pump, 2.1 L UV detector and D 2.1 S degasser (Berlin, Germany). A consort NV P-901 pH meter (Belgium) was utilized for pH adjustment. DOE was performed with the assistance of Minitab® Statistical Software (release 16, State College, PA, USA).
Materials and reagents
-
CTZ (99.95% purity as certified) and AZE (99.80% purity as certified) bulk drugs were obtained from Apex and European Egyptian Pharmaceuticals Industry, respectively.
-
Cetirizine® (1% of CTZ) and Azelast® (0.05% of AZE) eye drops are products of Pharo Pharma (batch no. 5669002) and The Tenth of Ramadan for Pharmaceuticals (batch no. 202792), respectively. Both are purchased from the local pharmacy.
-
All the organic solvents utilized in the study were HPLC grade. Acetonitrile and ethanol were attained from Sigma Aldrich (Germany). Methanol was obtained from Tedia (USA).
-
TEA and Phosphoric acid were gotten from Sigma Aldrich and RiedeldeHäen, Honeywell Research Chemicals (Germany), respectively.
Standard solutions
CTZ and AZE stock solutions (100 µg mL−1 of each) were made individually in acetonitrile. Working standard solutions of CTZ and AZE were made individually by dilution of the stock solutions with acetonitrile to yield a concentration of 20 µg mL−1 of both analytes. Afterwards, dilution was made with the mobile phase to prepare solutions within the concentration ranges (1.0–30.0 µg mL−1 for CTZ and 0.5–10.0 µg mL−1 for AZE). All the solutions were kept in the refrigerator and wrapped in aluminum foil.
Chromatographic conditions
The separation was accomplished using C8 column (Hypersil MOS, 5 µm particle size, 150 mm × 4.6 mm). The mobile phase made up of 0.3% TEA: acetonitrile (40:60 v/v, pH 5) and delivered at a flow rate of 1.2 ml min−1. UV detection was performed at 216 nm.
General procedures
Construction of the calibration curves
Calibration curves were constructed by dilution of each analyte individually from the corresponding stock and working solutions with the mobile phase into a set of 10.0 mL volumetric flasks. The obtained solutions have a concentration range of 1.0–30.0 µg mL−1 for CTZ and 0.5–10.0 µg mL−1 for AZE. Each solution was injected in triplicate under the optimum chromatographic conditions. The peak area against the ultimate concentration of each analyte (µg mL−1) was plotted. Then, the regression equations were derived.
Analysis of CTZ/AZE in laboratory prepared mixtures
A set of laboratory prepared mixtures of CTZ and AZE were made by transferring different aliquots of stock and working solutions of both drugs in different ratios into a set of 10.0 mL volumetric flasks. The described procedure under “Construction of the calibration curves” was adopted. The concentrations of each analyte were calculated with the aid of the corresponding regression equations.
Analysis of CTZ and AZE in ophthalmic formulations
For Cetirizine® (1.0% CTZ): The contents of five bottles of the formulation were mixed and aliquot (equivalent to 20.0 mg) was transferred into 100.0 mL volumetric flask and completed to the mark with acetonitrile. Further dilution was made to prepare working standard solution of 20.0 µg mL−1. CTZ was analysed as described under “Construction of the calibration curves”.
For Azelast® (0.05% AZE): The contents of five bottles were mixed and aliquot (equivalent to 1.0 mg) was transferred into 50.0 mL volumetric flask and completed to the mark with acetonitrile (20 µg mL−1). AZE was assayed as explained under “Construction of the calibration curves”.
Analysis of CTZ/AZE in aqueous humor
Artificial aqueous humor was prepared to mimic the chemical composition of the human aqueous humor48. One mL of it was moved into 10.0 mL volumetric flasks, followed by adding different aliquots of both CTZ and AZE stock and working solutions containing (10–300 and 5–100 µg of CTZ and AZE, respectively). Implement the procedure under “Construction of the calibration curves”.
Results and discussion
Method optimization
Optimization of HPLC method is a complex process that necessitates the simultaneous modification of numerous variables to achieve the desired separation. DOE has recently been employed in order to improve separation quality and reduce the number of trials during the optimization phase3. Preliminary experiments should be conducted before applying a factorial design to test the feasibility of the experimental design. These experiments include:
Choice of column
Three columns were tried in the experiment including:
-
1.
ShimPack CLC-cyanopropyl (150 × 4.6 mm, 5 µm).
-
2.
HyperClone™ ODS C18 (150 × 4.6 mm, 5 µm).
-
3.
HyperClone™ MOS C8 (150 × 4.6 mm, 5 µm).
The last one was the most suitable regarding analysis time and resolution of peaks. Overlapped peaks were obtained using the rest of the listed columns.
Selection of suitable wavelength
The studied compounds exhibited maxima in their spectra at 209 and 231 nm for CTZ and 216 nm for AZE in acetonitrile (Fig. 2). Therefore, 216 nm was selected as the suitable wavelength with good sensitivity for both compounds.
Mobile phase composition
pH
pKa of CTZ (strongest acidic is 3.58 and strongest basic is 7.7449) and pKa of AZE is 9.1649. CTZ was delayed as pH decreased due to ionization suppression of the carboxylate anion. On the other hand, AZE would be ionized at low pH values (polar) and less retained. Mobile phase pH range of 4.5–5.0 was chosen for factorial design. In the preliminary trials, upon decreasing pH below 4.5, the resolution between peaks decreased. At pH higher than 5.0, the retention time of AZE increased greatly, with lower sensitivity for both analytes. Log P values of CTZ and AZE are 2.98 and 4.04, respectively49. Consequently, CTZ is more polar and eluted the first.
Type and ratio of the organic solvent
In the first trials in the screening phase, different organic solvents were tested, including: methanol, ethanol and acetonitrile. Overlapped peaks were obtained upon using methanol. Ethanol led to longer retention times. Therefore, acetonitrile was selected as it gave the best sensitivity, retention times and resolution of peaks. Increasing ratio of more than 60% resulted in poor resolution, while decreasing ratio of less than 50% resulted in longer retention times. Accordingly, ratios between 50–60% of acetonitrile were used as the range for the factorial design.
Type of the aqueous component
First of all, different ionic strengths of phosphate buffer with varying pH values using 0.2 M phosphoric acid were tried. But, AZE peak still suffered from unacceptable tailing (AZE tailing factor 2.31). Several attempts were made to overcome this tailing. The aqueous phases tested were: 0.05 M phosphate buffer pH 5 adjusted with either 0.2 M acetic acid or 0.3% TEA, 0.05 M acetate buffer pH 5 adjusted with 0.2 M acetic acid and 0.3% TEA pH 5 adjusted with 0.2 M phosphoric acid. The last one solved the tailing problem of AZE peak. TEA interacts with the silanol group in the column at higher affinity, which protects its interaction with the nitrogen atom of the studied analyte50. As a result, it reduces peak tailing. Additionally, this aqueous phase has benefits, including easy preparation and the column does not require too much time for washing after analysis as it does not contain any salt components.
Flow rate
A flow rate in the range of 1.0–1.2 mL min−1 was chosen for the factorial design. This range provided the best separation in a reasonable time.
Internal standard selection
Different internal standards such as labetalol, aspirin, diazepam, furosemide, valsartan, mebeverine and linezolid were tried. None of the internal standards gave a well separated peak in a reasonable retention time. Labetalol and diazepam overlapped with AZE. Linezolid showed poor resolution with CTZ. Mebeverine eluted after 10.0 min with a distorted peak. The rest of the drugs eluted with the solvent front. So, the study was continued without utilizing internal standard.
Full factorial design
A full factorial design is a type of DOE (multivariate optimization). In this study, a 23 ull factorial design (two levels and three independent factors) was applied for the optimization of the chromatographic separation. From the previous experiments, it was concluded that three independent factors had an impact on the chromatographic performance including: pH of the eluent, percentage of acetonitrile and flow rate. The 23 full factorial design proposed eight experiments to determine the optimum conditions that gave the best values for the responses. The responses determined were: resolution between CTZ and AZE (Rs), tailing factor of CTZ peak and tailing factor of AZE peak.
Minitab response optimizer was utilized to recognize the lower, target and upper values for the three responses (Table 1). Accordingly, the optimal conditions for the input values and desirability values were measured1, 2. The response optimizer measures composite desirability (D), which estimates whether the responses are within acceptable limits. This value is used to ensure that optimal conditions are reached and it ranges from zero to one. The optimal conditions can then be found by maximizing the composite desirability. The optimization plot in Minitab shows the effect of each factor on the responses or composite desirability and the interaction between them to give the optimal conditions2.
Factors affecting Rs between CTZ and AZE
In accordance with the Pareto chart of the factor effects (Supplementary Fig. S1a online), the main effects plot (Supplementary Fig. S2a online) and the normal plot (Supplementary Fig. S3a online), %acetonitrile (B) has the strongest significant impact for a 95% confidence level and a positive proportion on Rs. Also, pH of the eluent (A) has a significant positive impact on it. According to the interaction plots (Supplementary Fig. S4a online), %acetonitrile has a positive correlation with Rs when it interacts with pH at low and high levels. The other parameters’ interactions have nearly no noticeable effect on Rs.
Factors affecting tailing of CTZ
From the Pareto chart and interaction plots (Supplementary Figs. S1b and S4b online), the triple interaction (pH-acetonitrile-flow rate) has the highest impact on the tailing of CTZ peak. From the main effects plot for tailing of CTZ (Supplementary Fig. S2b online), flow rate (C) has the most potential negative impact as a single factor. Also, it has a negative influence when it interacts with pH or with %acetonitrile, whether at high or low levels for both factors.
Factors affecting tailing of AZE
As illustrated from the Pareto charts (Supplementary Fig. S1c online), the main effects plots (Supplementary Fig. S2c online) and the normal plot (Supplementary Fig. S3c online), flow rate (C) has the strongest negative impact on tailing of AZE. However, it is not significant at a 95% confidence level. Also, pH and %acetonitrile affect it negatively. The interaction plot (Supplementary Fig. S4c online) shows that %acetonitrile has an inverse impact on tailing of AZE when it interacts with pH at low and high levels. Additionally, flow rate has an inverse correlation with tailing of AZE when it interacts with pH or with %acetonitrile either at low or high levels for both factors.
The outcomes were statistically analyzed. Generally, the lower the coefficient of variation value, the better the reliability of the experiment. Coefficient of variation values were 17.98, 8.08 and 7.68 for Rs, tailing of CTZ and tailing of AZE, respectively. Coefficient of determination (R2) is the proportion of the explained variance of the response. R2 values were 99.4%, 38.8% and 59.6% for Rs, tailing of CTZ and tailing of AZE, respectively.
Consequently, the optimal mobile phase was acetonitrile: 0.3% TEA (pH 5) in the ratio of (60:40, v/v) and the flow rate was 1.2 mL min−1 depending on DOE (Fig. 3). Figure 4 presents the chromatogram of CTZ and AZE mixture.
Validation of the proposed method
Validation of the analytical procedure was done in accordance with the ICH guidelines51 in order to ensure that it is appropriate for its intended use. The validation parameters were investigated as follows:
Linearity and range
Calibration graphs for CTZ and AZE analysis were constructed between the peak area of each analyte and its concentration (µg mL−1) (Fig. 5). The designed approach was rectilinear over the concentration ranges of 1.0–30 for CTZ and 0.5–10 µg mL−1 for AZE. The following linear regression equations were derived from linear analyses:
where C is the analyte concentration (µg mL−1). Analytical data for the results are listed in Table 2.
Limit of detection and limit of quantitation
LOD and LOQ were estimated as listed in Table 2 according to ICH criteria51, utilizing standard deviation of the intercept and the slope.
Accuracy
The accuracy was confirmed by the acceptable percentage of recoveries from the quantitative measurement of CTZ and AZE bulk materials and synthetic mixtures. These data were compared to the data of the comparison synchronous spectrofluorimetric method22 and t and F values were calculated as listed in Table 3. These values point out the agreement of the data obtained by both methods52.
Precision
To test the intraday precision, three different concentrations of each analyte were analysed three times on the same day. The same concentrations were measured on three separate days to assess the interday precision. As illustrated in Supplementary Table S1 online, low SD, %RSD and %error values assure intraday and interday precision.
Selectivity
Method selectivity was examined via the quantitative measurement of CTZ and AZE in aqueous humor without interference from its components. The high percentage of recoveries and low SD values for the analysis of both compounds in aqueous humor revealed the selectivity.
Specificity
Method specificity was determined by testing the cited analytes in their commercial ophthalmic formulations and detecting interferences from dosage excipients. It was found that these excipients did not affect the assay performance. The results of the estimation of CTZ and AZE in raw materials and pharmaceutical formulations did not differ significantly.
Robustness
The robustness was checked by deliberately varying the approach parameters, including pH (5.0 ± 0.1), acetonitrile ratio (60 ± 1%) and TEA concentration (0.3 ± 0.05). These variations did not have remarkable effect on the performance of the HPLC approach, indicating it is robust.
System suitability
System suitability is an important parameter for ensuring that the resolution and reproducibility of the chromatographic procedure are adequate for the determination. Suitability parameters of the adopted approach were estimated as listed in Supplementary Table S2 online. The values are within the accepted ranges regarding USP10.
Method applications
Analysis of CTZ/AZE in laboratory prepared mixtures
The suggested approach was used to estimate CTZ and AZE in laboratory prepared mixtures. Supplementary Table S3 online shows acceptable percentage of recoveries.
Analysis of CTZ/AZE in ophthalmic formulations
The developed method was effectively employed for the estimation of CTZ and AZE in their single eye drops. The data listed in Supplementary Table S4 online did not differ significantly from those attained from the comparison method22, as proved from t and F values52. No interference from excipients was detected.
Analysis of CTZ/AZE in aqueous humor
Estimation of both medications concurrently in aqueous humor was adopted due to the high sensitivity and selectivity of the method (Fig. 6). In aqueous humor, CTZ and AZE had average absolute recoveries and %RSD of 100.93 ± 1.42 and 100.11 ± 1.55, respectively, as demonstrated in Supplementary Table S5 online.
Conclusion
Eye drops must be administered at the correct dose to achieve optimal efficacy and minimal side effects for the patient. In severe ocular allergies, co-administration of CTZ and AZE eye drops may be recommended. As a result, it was critical to establish a simple, selective, accurate, precise and robust HPLC approach for the simultaneous analysis of CTZ and AZE in aqueous humor for therapeutic drug monitoring. Our HPLC–UV method was validated according to ICH guidelines and it shows improved resolution and sensitivity parameters and wider linearity ranges for both analytes. This is the first chromatographic procedure for concurrent separation of both drugs within a short analysis time (less than 6 min). The optimization of the chromatographic conditions was achieved by utilizing two level full factorial design, which reduces time and the number of trials. Furthermore, the approach was successfully applied in real life situations by estimating the cited drugs in their commercial eye drops and aqueous humor with good recoveries and without interference. The method is characterized by broad applicability, rapidity and adequate robustness. Accordingly, it can be easily applied in quality control laboratories and pharmacokinetic studies.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request (Walaa Nabil Abd-AlGhafar).
References
Saad, A. S., Ismail, N. S., Soliman, M. & Zaazaa, H. E. Validated stability-indicating RP-HPLC method for simultaneous determination of clorsulon and ivermectin employing plackett-burman experimental design for robustness testing. J. AOAC Int. 99, 571–578 (2016).
EL-Shorbagy, H. I., Elsebaei, F., Hammad, S. F. & El-Brashy, A. M. Optimization and modeling of a green dual detected RP-HPLC method by UV and fluorescence detectors using two level full factorial design for simultaneous determination of sofosbuvir and ledipasvir: Application to average content and uniformity of dosage unit testing. Microchem. J. 147, 374–392 (2019).
Candioti, L. V., De Zan, M. M., Cámara, M. S. & Goicoechea, H. C. Experimental design and multiple response optimization. using the desirability function in analytical methods development. Talanta 124, 123–138 (2014).
Miyazaki, D. et al. Epidemiological aspects of allergic conjunctivitis. Allergol. Int. 69, 487–495 (2020).
Ryder, EC, Benson, S 2021 Conjunctivitis. StatPearls, (StatPearls Publishing, Tampa).
Jeremy Ono, S. & Abelson, M. B. Allergic conjunctivitis: Update on pathophysiology and prospects for future treatment. J. Allergy Clin. Immunol. 115, 118–122 (2005).
Bielory, L. & Friedlaender, M. H. Allergic conjunctivitis. Immunol. Allergy Clin. North Am. 28, 43–58 (2008).
Pharmacopoeia B. London: Her majesty's stationary office; Electronic version. 2022.
Malhotra, R. P., Meier, E., Torkildsen, G., Gomes, P. J. & Jasek, M. C. Safety of cetirizine ophthalmic solution 0.24% for the treatment of allergic conjunctivitis in adult and pediatric subjects. Clin. Ophthalmol. 13, 403–413 (2019).
Pharmacopoeia 44 U. S., The National Formulary 39; The US Pharmacopoeial Convention; Rockville: MD. 2021.
Ma, M., Feng, F., Sheng, Y., Cui, S. & Liu, H. Development and evaluation of an efficient HPLC/MS/MS method for the simultaneous determination of pseudoephedrine and cetirizine in human plasma: Application to phase-I pharmacokinetic study. J. Chromatogr. B. 846, 105–111 (2007).
Souri, E., Hatami, A., Ravari, N. S., Alvandifar, F. & Tehrani, M. B. Validating a stability indicating HPLC method for kinetic study of cetirizine degradation in acidic and oxidative conditions. Iran. J. Pharm. Res. 12, 287–294 (2013).
Aly, F., EL-Enany, N., Elmansi, H. & Nabil, A. A. Validated reversed phase hplc method for simultaneous determination of the antihistaminic cetirizine and beta2-adrenergic agonist salbutamol in their co-formulated tablets. SM Anal. Bioanal. Technique 2, 1011 (2017).
Shamshad, H., Naz, A. & Mirza, A. Z. Reverse phase HPLC method for the simultaneous determination of cetirizine, verapamil/Diltiazem and amlodipine. Anal. Bioanal. Chem. Res. 8, 139–145 (2021).
Shamshad, H. & Mirza, A. Z. Application of RP-HPLC method for the simultaneous determination of cetirizine in the presence of quinolones. Future J. Pharm. Sci. 7, 1–6 (2021).
Shamshad, H., Sayqal, A., Zeb, Z. & Mirza, A. Z. Simultaneous determination of chloroquine and pyrimethamine with cetirizine in an active form and human serum by RP-HPLC. J. Chromatogr. Sci. 9, 923–927 (2021).
Uysal, U. D. & Tunçel, M. Validated capillary electrophoresis study for the determination of cetirizine in pharmaceutical forms. J. Liq. Chromatogr. Relat. Technol. 29, 1781–1792 (2006).
Javid, F. S., Shafaat, A. & Zarghi, A. Determination of cetirizine and its impurities in bulk and tablet formulation using a validated capillary zone electrophoretic method. J. Anal. Chem. 69, 442–447 (2014).
Ibrahim, F., El-Din, M. S., Eid, M. & Wahba, M. E. K. Spectrofluorimetric determination of some H1 receptor antagonist drugs in pharmaceutical formulations and biological fluids. Int. J. Pharm. Sci. Res. 2, 2056–2072 (2011).
Wei, X. L., Gong, Q., Wang, L. S. & Liao, Y. Determination of cetirizine dihydrochloride by anti-fluorescence quenching on rhodamine B-Sodium tetraphenylborate system. Guang Pu Xue Yu Guang Pu Fen Xi 31, 1596–1600 (2011).
El-Din, M. K. S., Ibrahim, F., Eid, M. I. & Wahba, M. E. K. Validated spectroflurimetric determination of some H1 receptor antagonist drugs in pharmaceutical preparations through charge transfer complexation. J. Fluoresc. 22, 175–191 (2012).
Abd-AlGhafar, W. N., Aly, F. A., Sheribah, Z. A. & Saad, S. Synchronous fluorescence as a green and selective method for the simultaneous determination of cetirizine and azelastine in aqueous humor. J. Fluoresc. 32, 1199–1210 (2022).
El-Kommos, M. E., El-Gizawy, S. M., Atia, N. N. & Hosny, N. M. Determination of some non-sedating antihistamines via their native fluorescence and derivation of some quantitative fluorescence intensity-structure relationships. J. Fluoresc. 25, 1695–1709 (2015).
Pourghazi, K., Khoshhesab, Z. M., Golpayeganizadeh, A., Shapouri, M. R. & Afrouzi, H. Spectrophotometric determination of cetirizine and montelukast in prepared formulations. Int. J. Pharm. Pharm. Sci. 3, 128–130 (2011).
El-Didamony, A. M. & Ramadan, G. M. Charge-transfer interaction between antihistamine antiallergic drugs, diphenhydramine, fexofenadine, cetirizine and two π-acceptors in pharmaceutical forms. SN Appl. Sci. 2, 1–14 (2020).
Lambiase, A., Micera, A. & Bonini, S. Multiple action agents and the eye: Do they really stabilize mast cells?. Curr. Opin. Allergy Clin. Immunol. 9, 454–465 (2009).
El-Shaheny, R. N. & Yamada, K. Stability study of the antihistamine drug azelastine HCl along with a kinetic investigation and the identification of new degradation products. Anal. Sci. 30, 691–697 (2014).
Hassouna, M., Abdelrahman, M. & Abdelfatah, M. Simultaneous determination of azelastine hydrochloride and benzalkonium chloride by RP-HPLC method in their ophthalmic solution. Anal. Sci. 1, 555–565 (2017).
Patel, S. & Pasha, T. Y. Stability-indicating high-performance liquid chromatography method for determination of antihistamine drug azelastine. Asian J. Pharm. Clin. Res. 11, 248–251 (2018).
El-Masry, A. A., Hammouda, M. E. A., El-Wasseef, D. R. & El-Ashry, S. M. Eco-friendly green liquid chromatographic determination of azelastine in the presence of its degradation products: Applications to degradation kinetics. J. AOAC Int. 102, 81–90 (2019).
El-Masry, A. A., Hammouda, M. E., El-Wasseef, D. R. & El-Ashry, S. M. Eco-friendly green liquid chromatographic separations of a novel combination of azelastine and fluticasone in the presence of their pharmaceutical dosage form additives. Curr. Anal. Chem. 16, 277–286 (2020).
Salama, N. N., Abdel-Razeq, S. A., Abdel-Atty, S. & El-Kosy, N. Development and validation of densitometry TLC stability indicating method for quantitative determination of azelastine hydrochloride and emedastine difumarate in their drug products. J. Pharm. Res. Int. 4, 79–92 (2014).
Patel, K. G., Patel, S. K. G., Shah, P. A., Tandel, D. B. & Gandhi, T. R. Development and validation of HPTLC method along with forced degradation study for the simultaneous estimation of azelastine hydrochloride and fluticasone propionate in nasal spray formulation using design of experiment approach. Indian J. Pharm. Educ. Res. 54, 155–165 (2020).
Abdelwahab, N. S., Farid, N. F., Elagawany, M. & Abdelmomen, E. H. Efficient UPLC and CE methods for the simultaneous determination of azelastine hydrochloride and its genotoxic impurity. Biomed. Chromatogr. 32, e4346 (2018).
Abdel-Razeq, S. A., Foaud, M. M., Salama, N. N., Abdel-Atty, S. & El- Kosy, N. Voltammetric determination of azelastine-HCl and emedastine dirumarate in micellar solution at glassy carbon and carbon paste electrodes. Sens. Electroanal. 6, 289–305 (2011).
Badran, O. M., Salem, M. Y. & Kelani, K. M. Application of membrane selective electrodes for the determination of azelastine hydrochloride in the presence of its alkaline degradant in eye drops and plasma. J. Anal. Bioanal. Electrochem. 5, 325–340 (2013).
El-Masry, A. A., El-Wasseef, D. R., Eid, M., Shehata, I. A. & Zeid, A. M. Quantitative proton nuclear magnetic resonance method for simultaneous analysis of fluticasone propionate and azelastine hydrochloride in nasal spray formulation. R. Soc. Open. Sci. 8, 210483 (2021).
El-Masry, A. A., Hammouda, M. F. A., El-Wasseef, D. R. & El-Ashry, S. M. Validated sensitive spectrofluorimetric method for determination of antihistaminic drug azelastine HCl in pure form and in pharmaceutical dosage forms: Application to stability study. Luminescence 32, 177–181 (2017).
Ragab, M. A. A. & El-Kimary, E. I. Investigation of the spectrofluorimetric behavior of azelastine and nepafenac: Determination in ophthalmic dosage forms. Spectrochim. Acta A. 204, 260–266 (2018).
Shekhar, S. & Bali, A. Spectrofluorimetric method for the determination of azelastine hydrochloride in bulk and nasal formulations. J. Appl. Spectrosc. 88, 674–680 (2021).
Gouda, A. A., El Sheikh, R. & El Saied, H. Extractive spectrophotometric determination of azelastine hydrochloride in pure form and pharmaceutical formulations. Can. Chem. Trans. 3, 29–41 (2015).
Hassouna, M. E., Abdelrahman, M. M. & Mohamed, M. A. Determination of azelastine hydrochloride and benzalkonium chloride in their ophthalmic solution by different spectrophotometric methods. World J. Appl. Chem. 2, 48–56 (2017).
El-Masry, A. A., Hammouda, M. E. A., El-Wasseef, D. R. & El-Ashry, S. M. Validated spectroscopic methods for determination of anti-histaminic drug azelastine in pure form: Analytical application for quality control of its pharmaceutical preparations. Spectrochim. Acta A. 191, 413–420 (2018).
Castillo, M., Scott, N. W., Mustafa, M. Z., Mustafa, M. S. & Azuara‐Blanco, A. Toxpical antihistamines and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis. Cochrane Database Syst. Re. 6. (2015).
Rozet, E., Lebrun, P., Hubert, P., Debrus, B. & Boulanger, B. Design Spaces for analytical methods. TrAC Trends Anal. Chem. 42, 157–167 (2013).
Pietrowska, K. et al. Analysis of pharmaceuticals and small molecules in aqueous humor. J. Pharm. Biomed. Anal. 159, 23–36 (2018).
Nikolin, B., Imamović, B., Medanhodzić-Vuk, S. & Sober, M. High perfomance liquid chromatography in pharmaceutical analyses. Bosn. J. Basic Med. Sci. 4, 5–9 (2004).
Macri, A. et al. An artificial aqueous humor as a standard matrix to assess drug concentration in the anterior chamber by high performance liquid chromatography methods. Clin. Lab. 61, 47–52 (2015).
Moffat, A. C., Osselton, M. D., Widdop, B. & Watts, J. Clarke’s analysis of drugs and poisons (Pharmaceutical Press, 2011).
Mendez, A., Bosch, E., Roses, M. & Neue, U. D. Comparison of the acidity of residual silanol groups in several liquid chromatography columns. J. Chromatogr. A. 986, 33–44 (2003).
Guideline, I. H. T. Validation of analytical procedures: Text and methodology. Q2 (R1) 1(20), 05 (2005).
Miller, J. N. & Miller, J. C. Statistics and chemometrics for aanalytical chemistry (Pearson Eductaion, 2008).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
W.N.A. carried out the lab work and statistical analysis. F.A.A., Z.A.S., and S.S. supervised the work and made the critical revision for the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd-AlGhafar, W.N., Aly, F.A., Sheribah, Z.A. et al. Factorial design-assisted reverse phase HPLC–UV approach for the concurrent estimation of cetirizine and azelastine in aqueous humor. Sci Rep 12, 22435 (2022). https://doi.org/10.1038/s41598-022-26774-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26774-y
This article is cited by
-
Factorial design-assisted spectrophotometric methods for the determination of total catechins in green tea extract via reaction with MBTH. Application to commercial tablet
Monatshefte für Chemie - Chemical Monthly (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.