Abstract
Phosphoric acid is the core material for the fertilizer industry; however, it is incredibly corrosive to manufacturing plants’ structures, mainly steel. Corrosion is one of the most severe problems encountered during phosphate fertilizer manufacturing. Recently, plant extracts have been commonly used as corrosion inhibitors because they are cheap and environmentally friendly. Steel corrosion in a 20% aqueous phosphoric acid solution in the absence and presence of fenugreek seed (Fen) or cape gooseberry leaf (CgL) extracts was investigated using the electrochemical impedance spectroscopy technique, potentiodynamic polarization measurement, scanning electron microscope, and quantum chemical calculations. Fourier Transform Infrared, FTIR, was used to identify the functional groups in Fen and CgL extracts. The inhibition efficiency for steel in 20% aqueous phosphoric acid was roughly equal to 80% for 0.4 g/L CgL and 1.2 g/L Fen extracts. A scanning electron microscope showed that the chemical constituents of extracts block the surface roughness of steel, decreasing the corrosion rate. The activation parameters indicated the effectiveness of the extracts at a higher temperature. Measurements of the potential of zero charges showed that the steel surface is positively charged in the phosphoric acid solution. Quantum chemical computations were also employed to examine the corrosion inhibition mechanisms of the natural extracts.
Similar content being viewed by others
Introduction
Mild steel is significant in many industries since it is used in various equipment. Its widespread use may be due to favorable qualities such as strength, hardness, and low cost. Mild steel can be exposed to corrosive environmental conditions, making it susceptible to corrosion and thereby deteriorating its properties.
Phosphate rock (PR) and phosphoric acid (PA) are the core materials for the fertilizer industry; however, they are incredibly corrosive to manufacturing plants’ structures, mainly steel. Corrosion products and rust can contaminate fertilizers and cause severe mechanical damage and degradation to plant machinery and facilities1. Phosphoric acid is highly corrosive to steel. The 20% phosphoric acid is one of the concentrations utilized in manufacturing fertilizers. Steel materials used in H3PO4 solutions must be adequately protected. Corrosion inhibitors play a critical role in fertilizer production, storage, and handling, ensuring the long-term viability of equipment and industrial structures. Organic compounds and plant extracts are the most commonly used steel inhibitors in HCl and H2SO42,3,4,5,6,7,8,9,10.
The inhibitory effects of Zanthoxylum alatum plant extract on mild steel corrosion in 20, 50, and 88% aqueous orthophosphoric acid were evaluated utilizing weight loss and electrochemical impedance spectroscopy. In 88% phosphoric acid, Zanthoxylum alatum extract suppresses steel corrosion more efficiently than 20% phosphoric acid11. 800 ppm of an alcoholic extract of Pisidium guajava (guava) leaves reduced steel corrosion by 89% in a 1.0 M phosphoric acid solution12. The Apricot juice has a maximum inhibition efficiency of 75%13. Guar gum was discovered to be a promising carbon steel inhibitor in 2.0 M H3PO4. Maximum inhibition values of 95.9% were obtained at 1.0 g/L Guar gum concentration14. Eucalyptus leaf extract was shown to be a more effective corrosion inhibitor in 0.5 M H2SO4 solutions than in 0.5 M H3PO4 solutions15. The effect of fenugreek leaf and aqueous seed extracts on the corrosion of mild steel in HCl and H2SO4 solutions has been investigated. Both fenugreek leaf and seed extracts had higher inhibitory activity in the HCl solution than in the H2SO4 solution16. The inhibitory action of lemon peel and fenugreek leaf extracts on mild steel corrosion in 1 M HCl has been examined. The results reveal that both extracts have good inhibition characteristics17. The corrosion inhibition efficiency of acid extract of dried Indian gooseberry leaves for mild steel in 1 N HCl medium was tested. The results demonstrated that gooseberry leave is a good mixed-type corrosion inhibitor with an efficiency of 87.9% at 2% v/v inhibitor concentration18. The inhibition efficiency of Chinese gooseberry fruit shell extract as a green and cheap corrosion inhibitor for mild steel in an acidic solution was evaluated by weight loss experiments at different temperatures, and the results disclosed that during a 5-h immersion at 25 °C, the efficiency increased to 94% for a 1000 ppm sample19.
The use of toxic chemicals as corrosion inhibitors for steel in phosphoric acid is limited due to environmental issues; many countries launched major green chemistry innovations in response to the Paris climate change agreements. Few studies were performed to investigate using natural product extracts as corrosion inhibitors for steel in phosphoric acid3,4,5,6,7. Furthermore, the use of Fenugreek seed (Fen) and Cape gooseberry leaf (CgL) extracts as corrosion inhibitors for steel in phosphoric acid solutions has not been reported. As a result, the inhibitory effects of the Fen and CgL on steel corrosion were investigated in a solution simulating that used in the fertilizer industry (20% phosphoric acid at 30 °C). Various techniques such as electrochemical impedance spectroscopy, potentiodynamic polarization measurements, Fourier Transform Infrared, scanning electron microscopy, and density functional theory were used. Thermodynamics and adsorption considerations were also studied.
Experimental studies
Solution preparation
Dilutions of analytical reagent-grade 85% phosphoric acid, Scharlau chemical industry, were used to make 20% phosphoric acid solutions. Fenugreek seed (Fen) and Cape gooseberry leaf (CgL) solution were extracted, as reported by Abdel-Gaber et al.20. The solubility of Fen and CgL in 20% phosphoric acid solution is 8.4 g/L and 6.3 g/L at 30 °C respectively. Before each experiment, an appropriate volume of extract solution and a definite volume of 85% phosphoric acid is added to double distilled water in a 100 mL measuring flask and diluted to obtain a solution of 20% phosphoric acid and the required concentration of extract. Plant materials were collected in spring (March) in accordance with institutional, national, and international guidelines and legislation.
Electrochemical studies
The frequency response analyzer (FRA)/potentiostat, ACM instrument UK) was utilized to measure electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization curves. The cell setup and experimental conditions were similar to those previously mentioned21. The working electrode was made of mild steel with the following chemical composition (wt%) (P: 0.005, S: 0.001, Si: 0.260, Mn: 0.710, C: 0.164, and Fe: 98.86). A steel plate of cylindrical shape was encapsulated in Teflon, with only one surface exposed to the test solution. The exposed area (0.36 cm2) was mechanically abraded with a series of emery sheets of varying degrees, beginning with coarse (120 grade) and progressing to fine (800 grade). The data were obtained in a three-electrode mode electrochemical cell using a platinum rod (0.36 cm2) and silver-silver chloride as counter and reference electrodes. The frequency range for EIS measurements was 0.1 to 3 × 104 Hz with an applied potential signal amplitude of 10 mV around the rest potential. Polarization curve measurements were obtained at a scan rate of 30 mV/min using a potential range of ± 300 mV around the equilibrium potential. Experiments were triple checked to ensure that the measurements were accurate. The standard deviation, SD, of the inhibition efficiency of the 3 measurements was determined and reported.
Fourier transform infrared (FTIR) analysis
FTIR analysis of the Fen and CgL extracts was achieved by FTIR 8400S Shimadzu in the spectral region between 4000 and 400 cm–1.
Scanning electron microscopy (SEM)
In the absence and presence of Fen and CgL extract, abraded steel samples were immersed in a 20%t H3PO4 acid solution for 1 h. After that, the steel samples were dried at room temperature. The SEM (SEM; model: JSM-200 IT, JEOL) was used to capture micrographs of the steel surfaces.
Theoretical studies
Optimized structure of the major active ingredients of Fen and CgL extracts obtained at the B3LYP methods using quantum calculation-based density function theory (DFT). GaussView 6.0.16 was used to calculate the optimized structure's quantum chemical parameters using DFT with the B3LYP/6-311G(+) basis set, as this basis set is recognized as one of the basis sets that provides more precise results in resolving the geometries and electronic properties for many organic compounds. All the DFT calculations are performed under an aqueous phase.
Results and discussion
FTIR measurements
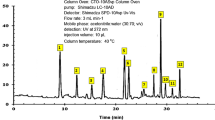
The FTIR measurements are utilized to characterize the functional groups in Fen and CgL extracts. Table 1 and Fig. 1 show the band assignments and FTIR spectrum bands for Fen and CgL extracts. Figure 1a depicts the FTIR spectra of Fen seed extract, which show an absorption peak at 3299 cm−1 due to the indole ring’s N–H stretching vibrations, absorption peaks at 2927 and 1744 cm−1 because of the aliphatic C–H bond, and the carboxylic acid group's C=O group, respectively22. These bands correspond to l-tryptophan bands, one of the chemicals extracted from Fen seed23,24,25.
The FTIR spectrum of the CgL leaf extract, Fig. 1b, shows the absorption bands for OH of the phenolic compound at 3413 cm−126. At 1732 cm−1, the stretching vibration of C=O of the carboxyl group occurs, the aromatic ring's C–H stretching vibration at 2925 cm−1, C=C vibration at 1627 cm−1, aromatic C=C bond at 1430 cm−1, unsymmetric stretching of the C–O–C bridge bond and C–O–C cyclic ether at 1154 and 1102 cm−1, respectively27. These bands are similar to those of ferulic acid, isolated from CgL leaf extract28,29. Figure 2 shows the major chemical constituents of Fen and CgL extracts.
Potentiodynamic polarization measurements
Figure 3a indicates that Fen extract influences the anodic and cathodic parts of polarization curves, suggesting that it behaves as a mixed-type inhibitor for steel in the phosphoric acid solution. According to Fig. 3b, CgL extract acts as a cathodic-type inhibitor, retarding only the hydrogen evolution process. It is obvious that both the anodic and cathodic polarization curves are linear, which means that the corrosion process is under activation control3. In air-saturated solutions with pH less than about 3.0, the hydrogen evolution reaction is generally the major cathodic reaction of steel15.
Self-catalytic processes have been proposed to explain the anodic dissolution of steel in acidic solutions16.
In water, H3PO4 is known to be partly dissociated into H+ and a series of anion ions (H2PO4−, HPO42, PO43−). As a result, iron dissolution in phosphoric acid is followed by forming a stable black iron phosphate film on the metal surface7.
Table 2 shows the electrochemical polarization parameters. The electrochemical polarization kinetics parameters were obtained by applying the Tafel extrapolation method to both anodic and cathodic polarization lines. The polarization resistances were calculated from the corrosion current density, icorr, and the anodic and cathodic Tafel slopes (βa and βc) using Eq. (6).
The inhibition efficiency, η, of the extracts was calculated from icorr utilizing Eq. (7).
In this equation, io and icorr are the corrosion current densities in the absence and presence of the extract, respectively.
The tabulated data shows that the corrosion potential values, Ecorr, vary slightly as the concentrations of both extracts increase. As a result, these extracts have the potential to be employed as pickling inhibitors. Furthermore, in the presence of the extracts, the corrosion current density (icorr) decreases while the polarization resistance (Rp) and inhibition efficiency increase confirming their inhibitive action. The decrease in icorr values can be due to the adsorption of the extracted molecules on the steel surface. CgL extract inhibits steel corrosion in 20% phosphoric acid solution more effectively than Fen extract at low extract concentrations of 0.4 g/L. Increased Fen extract concentrations above 0.4 g/L increase inhibition efficiency. The maximum inhibition efficiency for both extracts is about 80%. The significant change of βa and βc with Fen extract concentration indicates that Fen extract affects both anodic metal dissolution and hydrogen evolution reactions. The value of βc of CgL extract is around 120 mV, indicating that the proton discharge is the rate-determining step of the hydrogen evolution reaction.
EIS measurements
Figure 4 depicts the Nyquist and Bode diagrams for Fen and CgL extracts. The Nyquist plots, Fig. 4a,b, are characterized by capacitive depressed semicircles signifying that steel corrosion is due to the charge transfer process7. The depressing characteristics may be attributed to the inhomogeneities and the surface roughness of the steel30,31,32,33. As the concentration of extracts increases, the diameter of the semicircles increases, so the corrosion rate decreases.
The Bode plots for steel in 20% phosphoric acid are depicted in Fig. 4c,d. The Figure shows that increasing the extract concentration leads to increasing the impedance magnitudes at the low frequency, confirming that the extract constituents form an adsorption film at the steel surface, decreasing the corrosion rate of steel8. The phase angle plots, Fig. 4e,f, show a one-time constant, which is attributed to the electrical double layer34,35,36. With increasing the concentrations of the extracts, the phase angle at the middle frequency regions was shifted to a higher value. This may be attributed to forming of a protective layer of the extract constituents at the steel surface, so the electrode interfacial structure was changed2.
The impedance parameters were accomplished by fitting the experimental data to the equivalent circuit shown in Fig. 5 using the Zsimpwin program. Table 3 displays the obtained impedance parameters.
The solution resistances, Rs, represent the combined resistance of electrolyte and electrode material, which are nearly constant in the absence and presence of the extracts. The difference between the values of charge transfer resistance, Rct, and Rp determined by impedance and polarization measurements explains a complex reaction. The charge transfer resistance in a complex reaction is the point at which the mid-frequency loop intersects the real axis. Rp is the point on the real axis where the low-frequency loop intersects. The inhibition efficiency, η, is calculated from the charge transfer resistance, Rct, using Eq. (8)37.
where, Rcto and Rcti are the charge transfer resistances in the absence and presence of the extract, respectively. The double layer capacitance, Cdl, could be calculated from the constant phase element (CPE) value of Qdl and n using Eq. (9)38.
where n represents the deviation parameter. The n value varies between − 1 to 1; if n = − 1, it is revealed that the CPE is an inductor, while if n = 0, it implies that CPE is a pure resistor, as n = 0.5 this denotes the CPE is a Warburg impedance, and if n = 1 signifies the CPE is a pure capacitor8. The data in Table 3 shows that the value of n is lower than 1 by a small value indicating a small deviation of the CPE from being a pure capacitor. Increasing the Rct in the presence of the extracts demonstrates the inhibitive action of the extracts takes place by the adsorption mechanism. The CgL and Fen extracts have maximum inhibition efficiencies of 87.89 and 78.72 at 0.4 g/L and 1.2 g/L, respectively. The decrease in Cdl indicates that the double layer thickness is increased, which leads to a decrease in dielectric constant because the extract's constituents displace the adsorbed water molecules at the steel surface4,39,40. The results of impedance measurements are nearly similar to those of polarization measurements.
Potential of zero charge
In electrostatic processes, the potential of zero charges (PZC) is an essential parameter. Figure 6 shows the variation of the double-layer capacitances calculated from the equivalent circuit previously described in Fig. 5 as a function of applied voltage. The PZC of a material in solution is the point at which minimum capacitance is obtained. The open circuit potential with respect to the PZC determines the metal's surface charge27. The open circuit potential (Eocp) and EPZC values for steel in 20% phosphoric acid solution are − 440 mV and − 449 mV, respectively. Steel's surface charge (Er) is evaluated utilizing the equation Er = Eocp-Epzc, where Er is Antropov's rational corrosion potential41. The results show that the steel surface is positively charged (+ 9 mV) in a 20% phosphoric acid solution.
Adsorption considerations
Adsorption isotherms were applied to demonstrate the interaction between Fen and CgL extracts and mild steel surface in 20% H3PO4. Theoretical fitting of the Kinetic-thermodynamic model42 Langmuir isotherm, Flory–Huggins, and Temkin models43 are depicted in Fig. 7. The degree of surface coverage (θ = η × 100) values of the extract in 20% H3PO4 were obtained from EIS measurements.
Table 4 depicts the fitting parameters derived by fitting the experimental data to the examined isotherms. The ideal behavior of adsorption Fen and CgL extract at the steel surface is explained by the fitting of the experimental data to Langmuir adsorption isotherm and confirmed by the values of 1/y that indicates that a single inhibitor molecule occupies one active site. The ideal behavior of adsorption Fen and CgL extract at the steel surface was also confirmed by the size parameter (x) values that explain the number of water molecules displaced by one inhibitor molecule; its value equals the values of 1/y. These results confirm that the size of inhibitor molecules equals that of a water molecule. The Temkin isotherm shows that the positive value of the adsorption parameter (f) suggests mutual repulsion of molecules44. Moreover, as reported, the efficiency of a corrosion inhibitor is proportional to the magnitude of its binding constant K, i.e., large values of K mean better and strong metal-inhibitor interactions45. Thus, according to the numerical values obtained from Langmuir, kinetic–thermodynamic model, and Temkin isotherms, the binding constant K value for CgL extract is greater than that of Fen extract in H3PO4 solutions, which confirms the experimental results.
Effect of temperature
Many studies have focused on the influence of temperature on steel corrosion in the phosphoric acid solution46. In the present work, we investigate the corrosion of steel in 20% phosphoric acid in the presence of 0.8 g/L Fen and 0.4 g/L CgL extract at 30 °C, 40 °C, 50 °C, and 60 °C.
The polarization curves in Fig. 8 show that increasing temperature enhances the hydrogen evolution reaction in the presence of both extracts. Furthermore, the temperature influences metal dissolution in the presence of Fen extract. The obtained electrochemical polarization parameters at different temperatures are shown in Table 5.
Figure 9 shows Nyquist diagrams of steel in 20% phosphoric acid in the presence of 0.8 g/L Fen and 0.4 g/L CgL extract at 30 °C, 40 °C, 50 °C, and 60 °C. As seen in the Figure, the Nyquist diagrams show a capacitive depressed semicircle signifying that the corrosion of steel is due to the charge transfer process; the size of the depressed capacitive semicircle diminishes as the temperature rises indicating an increased corrosion rate. The obtained electrochemical impedance parameters at different temperatures are depicted in Table 6. The values of Rct decrease, and those of Cdl increase with increasing temperature. These are consistent with the physical adsorption of Fen and CgL extract molecules to the metal surface, with the adsorption–desorption equilibrium shifted toward desorption with increasing temperature.
The reciprocal of the absolute temperature 1/T has a linear relationship with the logarithm of the corrosion rate (k), according to many authors (Arrhenius equation)47:
where Ea is the apparent effective activation energy, T is the absolute temperature, R is the universal gas constant, and A is Arrhenius pre-exponential factor.
The transition state equation is an alternate formulation of the Arrhenius equation:
where N is Avogadro's number, h is Plank's constant, ∆H* is the activation enthalpy, and ∆S* is the activation entropy. The corrosion rates were taken as the corrosion current density. Figure 10 shows the plots used to determine the activation parameter of steel in 20% phosphoric acid in the absence and presence of 0.8 g/L Fen and 0.4 g/L CgL extract.
Table 7 displays the activation parameter values that were obtained. The variation in the activation energy values may be attributed to the alteration of the mechanism of the corrosion process in the presence of extract constituents48. The higher values of Ea and ∆H* in the presence of Fen extract indicate the extract's effectiveness at higher temperatures. The active complex denotes an association rather than a dissociation step, as shown by the negative value of ∆S*. As you progress from reactants to active complexes, you'll see a decrease in disordering. The entropy of activation changes signs from negative to positive due to the addition of the efficient CgL extract. Because the discharge of hydrogen ions to create adsorbed hydrogen atoms on the steel surface is the rate-determining step, the presence of efficient molecules will almost completely cover the surface, preventing the discharge process49.
SEM examination
Figure 11 shows a SEM micrograph of steel in the presence and absence of 0.8 g/L Fen and 0.4 g/L CgL extracts. In the absence of extracts, Fig. 11a shows a rough surface with general corrosion and a big shallow pit, suggesting the phosphoric acid's vigorous attack on the steel surface. However, in the presence of Fen extract, Fig. 11b, the surface morphology becomes smooth, which may be related to the adsorption of chemical constituents of Fen extracts, blocking the surface roughness and shallow pits. Figure 11c also clarifies the formation of the smooth film due to the adsorption of chemical constituents of CgL extracts (Ferulic acid) over the steel surface. This behavior corroborated the results of polarization and impedance tests.
Quantum calculation
The use of quantum calculations in corrosion inhibitor research has received a lot of interest in the recent decade. Because of its theoretical background, quantum calculation-based density function theory (DFT) is widely accepted as a "green corrosion inhibition technology". The higher occupied molecular orbital and lower unoccupied molecular orbital energy, EHOMO and ELUMO, of Ferulic acid and l-Tryptophan which are the major chemical constituents in CgL and Fen extracts, respectively, are shown in Fig. 12. Table 8 depicts the calculated quantum chemical descriptors for the optimized structure of Ferulic acid, and l-Tryptophan obtained using DFT with the B3LYP/6-311G(+) basis set in the aqueous phase50.
According to Table 8, ferulic acid has less negative HOMO energy and a smaller energy gap (ΔE = EHOMO − ELUMO), implying a stronger adsorption bond51,52.
The ionization potential (I) can be calculated using DFT-Koopmans' theorem as the negative of the highest occupied molecular orbital energy (I = − EHOMO).
Decreased ionization potential greatly affects Ferulic acid's adsorption and inhibitory characteristics. X signifies the absolute electronegativity of the inhibitor molecule (Χ = − ½[EHOMO + ELUMO]), and η denotes the absolute hardness of the inhibitor molecule (η = ½ ΔE). The ability of atoms in molecules to attract electrons is measured using electronegativity. The greater its value, the easier it is to attract electrons, indicating a stronger inhibition effect.
The softness parameter, S, is the inverse of the hardness parameter that measures the softness of the inhibitor and, thus, its reactivity. The most effective metal corrosion inhibitors are those with the highest softness51. This is because a soft molecule (inhibitor) is more reactive toward a metal surface than a hard molecule (inhibitor)53. Thus, ferulic acid with higher χ and S but lower I and η is a better corrosion inhibitor than l-Tryptophan. These observations are consistent with the results of the experimental measurements for CgL and Fen extracts.
Inhibition mechanism
To discuss the mechanism of inhibition of the major active ingredient of the extracts, it is crucial to calculate the Extended Huckel charge, Table 9, for the 3-dimensional structure of l-Tryptophane and Ferulic acid, which are the major chemical constituents in Fen and CgL extracts respectively, Fig. 13.
l-Tryptophan zwitterion is generated by the internal transfer of a hydrogen ion from the –COOH group to the –NH2 group, resulting in an ion with both a negative and positive charge. The inhibition of steel corrosion by tryptophan zwitterion could be attributed to the adsorption of (O14) of the carboxylate group over the positively charged iron phosphate film. In comparison, the inhibition of steel corrosion by Ferulic acid could be attributed to the adsorption of (O13) of the carboxylate group over the positively charged iron phosphate film.
Conclusions
-
1.
Fenugreek seed extract (Fen) and Cape gooseberry leaf extract (CgL) act as eco-friendly corrosion inhibitors for mild steel in phosphate fertilizer manufacturing. The inhibition efficiency increases with the extract's concentrations reaching an inhibition efficiency of roughly 80% for 0.4 g/L CgL and 1.2 g/L Fen extracts.
-
2.
The binding constant, obtained from the tested isotherms, indicated that CgL extract is a better and strong metal inhibitor than Fen extract. Increasing temperature increases the corrosion rate in the presence of both extracts suggesting a physisorption mechanism.
-
3.
SEM micrograph of steel in the presence and absence of Fen and CgL extracts clarifies the formation of smooth film that can be due to the adsorption of chemical constituents of extracts.
-
4.
The calculated quantum chemical descriptors for the optimized structure of Ferulic acid and l-Tryptophan indicated that ferulic acid is a better corrosion inhibitor than l-Tryptophan. These results confirm the potentiodynamic polarization and EIS results.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Prabhu-Gaunkar, G. V. & Raman, A. Corrosion inhibitors in fertilizer production and handling. In NACE-Int. Corros. Conf. Ser. 1996-March (1996).
Galai, M. et al. Chemically functionalized of 8-hydroxyquinoline derivatives as efficient corrosion inhibition for steel in 1.0 M HCl solution: Experimental and theoretical studies. Surf. Interfaces 21, 100695 (2020).
Abousalem, A. & Rbaa, M. Experimental, DFT calculations and MC simulations concept of novel quinazolinone derivatives as corrosion inhibitor for mild steel in 1.0 M HCl medium Nordine. J. Mol. Liq. 312, 113413 (2020).
Kadiri, L. et al. Coriandrum sativum L. seeds extract as a novel green corrosion inhibitor for mild steel in 1.0 M hydrochloric and 0.5 M sulfuric solutions. Anal. Bioanal. Electrochem. 10, 249–268 (2018).
Alaoui, K. et al. Poly (1-phenylethene): As a novel corrosion inhibitor for carbon steel/hydrochloric acid interface. Anal. Bioanal. Electrochem. 8, 830–847 (2016).
Alaoui, K. et al. Anti-corrosive properties of polyvinyl-alcohol for carbon steel in hydrochloric acid media: Electrochemical and thermodynamic investigation. J. Mater. Environ. Sci. 7, 2389–2403 (2016).
El Faydy, M. et al. Experimental and theoretical studies for steel XC38 corrosion inhibition in 1 M HCl by N-(8-hydroxyquinolin-5-yl)-methyl)-N-phenylacetamide. J. Mater. Environ. Sci. 7, 1406–1416 (2016).
Yan, T. et al. Investigation of imidazole derivatives as corrosion inhibitors of copper in sulfuric acid: Combination of experimental and theoretical researches. J. Taiwan Inst. Chem. Eng. 106, 118–129 (2020).
Abd-El-nabey, B. A., Abd-El-khalek, D. E., El-Housseiny, S. & Mohamed, M. E. Plant extracts as corrosion and scale inhibitors: A review. Int. J. Corros. Scale Inhib. 9, 1287–1328 (2020).
Abd-El-Nabey, B. A., El-Housseiny, S., Eldissouky, A. & Mohamed, M. E. Trizma as an efficient inhibitor for the corrosion of aluminium in acid solutions containing chloride ions. Russ. J. Electrochem. 57, 765–773 (2021).
Gunasekaran, G. & Chauhan, L. R. Eco friendly inhibitor for corrosion inhibition of mild steel in phosphoric acid medium. Electrochim. Acta 49, 4387–4395 (2004).
Noyel Victoria, S., Prasad, R. & Manivannan, R. Psidium guajava leaf extract as green corrosion inhibitor for mild steel in phosphoric acid. Int. J. Electrochem. Sci. 10, 2220–2238 (2015).
Yaro, A. S., Khadom, A. A. & Wael, R. K. Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alexandria Eng. J. 52, 129–135 (2013).
Messali, M. et al. Guar gum as efficient non-toxic inhibitor of carbon steel corrosion in phosphoric acid medium: Electrochemical, surface, DFT and MD simulations studies. J. Mol. Struct. 1145, 43–54 (2017).
Abdel-Gaber, A. M., Rahal, H. T. & Beqai, F. T. Eucalyptus leaf extract as a eco-friendly corrosion inhibitor for mild steel in sulfuric and phosphoric acid solutions. Int. J. Ind. Chem. 11, 123–132 (2020).
Noor, E. Comparative study on the corrosion inhibition of mild steel by aqueous extract of fenugreek seeds and leaves in acidic solution. J. Eng. Appl. Sci. 2008(3), 23–30 (2008).
Agarwal, K. Fenugreek leaves and lemon peel as green corrosion inhibitor for mild steel in 1M HCl medium. J. Mater. Sci. Surf. Eng. 1, 44–48 (2014).
Saratha, R. & Vasudha, V. G. Emblica officinalis (Indian gooseberry) leaves extract as corrosion inhibitor for mild steel in 1N HCL medium. E-J. Chem. 7, 677–684 (2010).
Dehghani, A., Bahlakeh, G. & Ramezanzadeh, B. A detailed electrochemical/theoretical exploration of the aqueous Chinese gooseberry fruit shell extract as a green and cheap corrosion inhibitor for mild steel in acidic solution. J. Mol. Liq. 282, 366–384 (2019).
Abdel-Gaber, A. M., Abdel-Nabey, B. A. & Saadawy, M. The co-operative effect of chloride ions and some natural extracts in retarding corrosion of steel in neutral media. Mater. Corros. 63, 161–167 (2012).
Abdel-Gaber, A. M., Hijazi, K. M., Younes, G. O. & Nsouli, B. Comparative study of the inhibitive action between the bitter orange leaf extract and its chemical constituent linalool on the mild steel corrosion in HCl solution. Quim. Nov. 40, 395–401 (2017).
Mohamed, M. E. & Abd El Nabey, B. A. Fabrication of a biological metal–organic framework based superhydrophobic textile fabric for efficient oil/water separation. Sci. Rep. https://doi.org/10.1038/s41598-022-19816-y (2022).
El-Bahy, G. M. S. FTIR and Raman spectroscopic study of fenugreek (Trigonella foenum graecum L.) seeds. J. Appl. Spectrosc. 72, 111–116 (2005).
Bukhari, S. B., Bhanger, M. I. & Memon, S. Antioxidative activity of extracts from fenugreek. Pak. J. Anal. Environ. Chem. 9, 78–83 (2008).
Wani, S. A. & Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 17, 97–106 (2018).
Mohamed, M. E., Mahgoub, F. M., Ragheb, D. M. & Abdel-Gaber, A. M. Novel and facile method for fabrication of robust superhydrophobic film on copper surface and its chemical, mechanical, and corrosion performance. Surf. Eng. 37, 1255–1265 (2021).
Ragheb, D. M., Gaber, A. M. A., Mahgoub, F. M. & Mohamed, M. E. Eco-friendly method for construction of superhydrophobic graphene-based coating on copper substrate and its corrosion resistance performance. Sci. Rep. https://doi.org/10.1038/s41598-022-22915-5 (2022).
Ertürk, Ö., ÇolAyvaz, M., Can, Z., Karaman, Ü. & Korkmaz, K. Antioxidant, antimicrobial activities and phenolic and chemical contents of Physalis peruviana L. from Trabzon, Turkey. Indian J. Pharm. Educ. Res. 51, S213–S216 (2017).
Popova, V. T. et al. The leaves and stems of Cape gooseberry (Physalis peruviana L.) as an alternative source of bioactive substances. In IOP Conf. Ser. Mater. Sci. Eng. vol. 1031, (2021).
Mohamed, M. E., Ezzat, A. & Gaber, A. M. A. Fabrication of eco-friendly graphene-based superhydrophobic coating on steel substrate and its corrosion resistance, chemical and mechanical stability. Sci. Rep. 12, 1–15 (2022).
Kong, H. Different forms of corrosion classified on the basis of appearence. Corros. Protect. https://doi.org/10.1007/978-1-85233-845-9_7 (2007).
Aramaki, K., Hagiwara, M. & Nishihara, H. Impedance study on inhibition and stimulation of iron corrosion in acid solution by various inorganic anions and tetra-alkylammonium cation. J. Electrochem. Soc 0, 1364–1369 (1988).
El, B., Baddouh, A., Oukhrib, R., El, S. & Hafidi, Z. Electrochemical and in silico investigations into the corrosion inhibition of cyclic amino acids on tin metal in the saline environment. Surf. Interfaces 23, 100966 (2021).
Nady, H., El-Rabiei, M. M. & Samy, M. Corrosion behavior and electrochemical properties of carbon steel, commercial pure titanium, copper and copper–aluminum–nickel alloy in 3.5% sodium chloride containing sulfide ions. Egypt. J. Pet. 26, 79–94 (2017).
Mahdavian, M. & Naderi, R. Corrosion inhibition of mild steel in sodium chloride solution by some zinc complexes. Corros. Sci. 53, 1194–1200 (2011).
Berrani, A. et al. Analytical & bioanalytical electrochemistry. Anal. Bioanal. Electrochem. 10, 1299–1316 (2018).
Mohamed, M. E. & Abd-El-Nabey, B. A. Corrosion performance of a steel surface modified by a robust graphene-based superhydrophobic film with hierarchical roughness. J. Mater. Sci. https://doi.org/10.1007/s10853-022-07325-2 (2022).
Hsu, C. H. & Mansfeld, F. Concernng the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 57, 747–748 (2001).
Galai, M. et al. α-Brass and (α+β) brass degradation processes in Azrou soil medium used in plumbing devices. J. Bio-Tribo-Corros. 3, 1–15 (2017).
Dahmani, K. et al. Quantum chemical and molecular dynamic simulation studies for the identification of the extracted cinnamon essential oil constituent responsible for copper corrosion inhibition in acidified 3.0 wt% NaCl medium. Inorg. Chem. Commun. 124, 108409 (2021).
Popova, A., Sokolova, E., Raicheva, S. & Christov, M. AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros. Sci. 45, 33–58 (2003).
El-Awady, A. A., Abd-El-Nabey, B. A. & Aziz, S. G. Kinetic-thermodynamic and adsorption isotherms analyses for the inhibition of the acid corrosion of steel by cyclic and open-chain amines. J. Electrochem. Soc. 139, 2149–2154 (1992).
Ayawei, N., Ebelegi, A. N. & Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. vol. 2017 (2017).
Rahal, H. T., Abdel-Gaber, A. M. & Younes, G. O. Inhibition of steel corrosion in nitric acid by sulfur containing compounds. Chem. Eng. Commun. 203, 435–445 (2016).
Al-Moghrabi, R. S., Abdel-Gaber, A. M. & Rahal, H. T. A comparative study on the inhibitive effect of Crataegus oxyacantha and Prunus avium plant leaf extracts on the corrosion of mild steel in hydrochloric acid solution. Int. J. Ind. Chem. 9, 255–263 (2018).
Benabdellah, M. & Hammouti, B. Corrosion behaviour of steel in concentrated phosphoric acid solutions. Appl. Surf. Sci. 252, 1657–1661 (2005).
Abdel-Gaber, A. M. Effect of immersion time and temperature on the inhibition of the acid corrosion of zinc by fenugreek seeds extract. Int. J. Appl. Chem. 3, 161–174 (2007).
Oukhrib, R. et al. Ziziphus lotus as green inhibitor of copper corrosion in natural sea water. Port. Electrochim. ACTA 35, 187–200 (2017).
Ameer, M. A., Khamis, E. & Al-Senani, G. Effect of temperature on stability of adsorbed inhibitors on steel in phosphoric acid solution. J. Appl. Electrochem. 32, 149–156 (2002).
Manahan, M. P., MacDonald, D. D. & Peterson, A. J. Determination of the fate of the current in the stress corrosion cracking of sensitized type 304SS in high temperature aqueous systems. Corros. Sci. 37, 189–208 (1995).
Khaled, K. F. & Al-Qahtani, M. M. The inhibitive effect of some tetrazole derivatives towards Al corrosion in acid solution: Chemical, electrochemical and theoretical studies. Mater. Chem. Phys. 113, 150–158 (2009).
Finley, H. F. & Hackerman, N. The effect of adsorption of polar organic compounds on the reactivity of steel. Preprints 3, 1–5 (1958).
Pearson, R. G. Hard and soft acids and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.E.M.: writing—review and editing, methodology, supervision, data curation, and writing—original draft. A.E.: methodology, data curation, and writing—review and editing. A.M.A.: writing—review and editing, supervision, discussion and validation. At the end, this manuscript has resulted by the collaboration of all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Gaber, A.M., Ezzat, A. & Mohamed, M.E. Fenugreek seed and cape gooseberry leaf extracts as green corrosion inhibitors for steel in the phosphoric acid industry. Sci Rep 12, 22251 (2022). https://doi.org/10.1038/s41598-022-26757-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26757-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.