Abstract
Cardiovascular function is related to age, sex, and state of consciousness. We hypothesized that cardiovagal baroreflex sensitivity (BRS) demonstrates different patterns in both sexes before and after 50 years of age and that these patterns are associated with patterned changes during the sleep–wake cycle. We recruited 67 healthy participants (aged 20–79 years; 41 women) and divided them into four age groups: 20–29, 30–49, 50–69, and 70–79 years. All the participants underwent polysomnography and blood pressure measurements. For each participant, we used the average of the arterial pressure variability, heart rate variability (HRV), and BRS parameters during the sleep–wake stages. BRS and HRV parameters were significantly negatively correlated with age. The BRS indexes were significantly lower in the participants aged ≥ 50 years than in those aged < 50 years, and these age-related declines were more apparent during non-rapid eye movement sleep than during wakefulness. Only BRS demonstrated a significantly negative correlation with age in participants ≥ 50 years old. Women exhibited a stronger association than men between BRS and age and an earlier decline in BRS. Changes in BRS varied with age, sex, and consciousness state, each demonstrating a specific pattern. The age of 50 years appeared to be a crucial turning point for sexual dimorphism in BRS. Baroreflex modulation of the cardiovascular system during sleep sensitively delineated the age- and sex-dependent BRS patterns, highlighting the clinical importance of our results. Our findings may aid in screening for neurocardiac abnormalities in apparently healthy individuals.
Similar content being viewed by others
Introduction
The cardiovascular system is controlled by the baroreflex and central autonomic brain regions1. Baroreflex sensitivity (BRS) is considered an index of cardiac autonomic regulation in humans; it comprises cardiovagal and sympathetic components, which are responsible for both short- and long-term blood pressure regulation2. In general, BRS and arterial pressure variability (APV) are crucial mechanisms underlying the neural regulation of the cardiovascular system3. Typically, daily activities are related to the state dependency of the patterned responses of cardiovascular function. For instance, the sleep–wake cycle is related to stable arterial pressure maintenance and pattern differences in the cardiovascular system4. We previously reported that differences in cardiovascular variabilities between Wistar–Kyoto rats (WKYs) and spontaneously hypertensive rats (SHRs) were more evident during sleep; BRS tended to be significantly higher in the WKYs during sleep but was nonsignificant in the SHRs5,6. These results are similar to those of a human study indicating that BRS is higher during non-rapid eye movement sleep (nREM) than during wakefulness7. However, due to the lack of relevant comprehensive studies, the effects of basic physiological factors on BRS remain unclear.
Cardiovascular events may be related to both circadian variations and the sleep–wake cycle8. The sympathetic BRS acts as a buffer that dampens surges in sympathetic activation by rapidly changing cardiac vagal circuits throughout the overnight sleep period9. Therefore, BRS is an essential factor in humans during sleep. Recently, a population-based study confirmed that age and sex were important factors associated with BRS10; however, research on BRS in both the sexes of different ages during different consciousness states remains scant. Nevertheless, the CNS-ANS coupling is different among the conscious states11. Only analyzing the awake state cannot fully reflect the impact of biological variables on BRS. In our previous animal study, SHRs demonstrated increased sympathetic modulation during sleep; however, this sympathetic modulation was less evident in SHRs who were awake12. Measuring HRV and BRS indexes during sleep can have considerable implications in research on neurocardiac events. However, few studies have investigated the effects of physiological factors on neural cardiovascular regulation during sleep, particularly using spontaneous BRS methods.
In our previous studies, we observed age-dependent differences in HRV; for instance, postmenopausal women had lower high-frequency of HRV (HF) but higher low-frequency of HRV (LF) percentage and LF/HF ratio than did young premenopausal women13. Moreover, normal aging led to declines in both cardiac parasympathetic nerve activity14 and sympathetic BRS15. Autonomic activity prominently declined before the age of 50 years16. However, the effects of age and sex on low-frequency power of APV (BLF) and BRS have not been reported thus far. In particular, age-related changes in BRS remain unclear, possibly because studies conducted thus far have included ethnically different populations, a small sample size, or only a few age groups.
We previously investigated the effects of sex on the sympathetic and parasympathetic control of HR. The results revealed that sex-related differences in parasympathetic regulation were not observed after the age of 50 years, whereas the dominance of sympathetic regulation diminished later in men than in women17. Notably, estrogen is a major factor controlling sex-related autonomic differences13. The rate of the BRS decline throughout the entire lifespan was similar for both sexes18. In addition, menopause was noted to be a crucial time point, where BRS impairment is observed19. Moreover, the age of 50 years appeared to be a crucial turning point for physiological and psychological functioning in both sexes16,17. However, few studies have investigated sex-related differences in BRS among all age groups under different consciousness states.
On the basis of the aforementioned findings, we hypothesized that in both sexes, cardiovagal BRS demonstrates different patterns before and after the age of 50 years. Moreover, the different patterns are associated with patterned changes during the sleep–wake cycle. Therefore, the current study investigated the effects of age and sex on blood pressure (BP), APV, and BRS during the states of wakefulness and sleep and compared age and sex-related pattern differences in BP, APV and BRS between wakefulness and sleep.
Methods
Participants
In total, 67 healthy volunteers aged 20 to 79 years (41 women) were recruited through an online advertisement. We believe that special consideration should be given to the menopausal and andropausal interval in the age variable in order to more accurately detect the impact of sex on BRS, so we use 50 years as the cutpoint for subject grouping20,21,22. All of them had a regular sleep–wake pattern (i.e., night sleep) and did not consume any medication, alcohol, caffeine, or nicotine. Body mass index (BMI) of all participants were in the normal range of 18.5–25 kg/m2. None of the participants had a reported medical history of psychiatric, neurological, and cardiovascular illnesses. Moreover, they did not demonstrate any signs of substance abuse or sedative or hypnotic drug use.
Experimental procedures and data recording
Initially, all the participants were divided into 4 age groups: 20–29, 30–49, 50–69, and 70–79 years. All the participants underwent laboratory polysomnography (PSG) at noon and BP recording for 1 h. Here, we used standard PSG measurements (Embla SX, Natus Medical Incorporated, USA) to assess sleep cycle. Brain waves were recorded at four reference points (C3-A2, C4-A1, O1-A2, and O2-A1) by electroencephalogram (EEG) while eye movements were recorded through electrooculography. Chin muscle tone was measured through electromyography (chin-EMG), and cardiac activity was recorded by electrocardiogram (ECG) at the V5 site on the chest. Continuous BP measurements were obtained using Finometer PRO (Finapres Medical Systems, Amsterdam, Netherlands). For finger blood calibration, a 2-min baseline recording was performed using an upper arm cuff before continuous recording was implemented during sleep. Real-time BP signals were synchronized using the PSG software program Somnologica (Embla, Inc., Denver, CO).
Sleep stages, namely wakefulness, rapid eye movement sleep (REM), and nREM, were scored in accordance with standard criteria described elsewhere16,23.
Signal processing and data analysis
Power spectral analysis was used for the HRV frequency domain measurement. The stationary R-R interval (RR) signal was curtailed into successive 64-s (4096 point) time segments (i.e., windows or epochs) with 50% overlap for analysis. Analytical procedures used here for HRV analysis have been detailed elsewhere12,24,25. Through Fourier transformation, the spectral information of HRV was classified into total power (TP), HF (0.15–0.4 Hz), LF (0.04–0.15 Hz), LF/HF ratio, and normalized LF (LF%) of the RR spectrogram were enumerated for each time segment. RR and TP are related to both sympathetic and parasympathetic nervous systems17. HF can reflect vagal modulation, and LF% and LF/HF are considered to be sympathetic modulation markers or represent the sympathovagal balance26.
For sleep stage analysis, we performed computerized sleep analysis in line with the criteria defined by Rechtschaffen and Kales and the American Academy of Sleep Medicine16,27. All the obtained results were verified by a qualified sleep technician. All PSG data were manually scored in 30-s epochs for each consciousness state including wakefulness and nREM.
We designed a special program in the Pascal language (Borland Pascal 7.0, Borland, USA) for analyzing our EEG and ECG data; ECG signals were preprocessed in accordance with recommended procedures28 as indicated in our previous studies17,29. In brief, both ventricular premature complexes and artifacts were identified and the QRS complex was acquired using a computer algorithm. For the continuity in the time domain, resampling and linear interpolation were applied at a rate of 64 Hz to RR. Moreover, the sampling rate was adjusted to 64 Hz for all EEG signals. Power spectral analysis was performed to measure EEG, APV, and HRV amplitudes. EEG, arterial pressure, and RR signals were truncated into successive 64-s (4096 points) time segments (windows or epochs) with 50% overlap. In addition, the Hamming window was applied24. Subsequently, the power density of the spectral components was estimated on the basis of fast Fourier transformation, and attenuation originating from sampling was corrected; the Hamming window was also implemented here17.
For APV and HRV analysis, we used a previously reported methodology5,12,17,25,26. In brief, the mean AP (MAP) and mean RR were estimated from the digitized AP and ECG signals, respectively. In addition, resampling and interpolation were performed to provide continuity in the time domain. Fast Fourier transformation and the Hamming window were used for these sequences24. Either HF (0.15–0.4 Hz) or LF% (0.04–0.15 Hz) was quantified from the RR spectrogram. BLF (0.04–0.15 Hz), a marker of sympathetic vasomotor control, and the high-frequency power of APV (BHF) was quantified from the AP spectrogram from each time segment25,26.
Spontaneous BRS was derived from the AP–RR transfer function and AP–RR linear regression25,26. In brief, the transfer magnitude at HF (BrrHF) and LF (BrrLF) ranges were estimated using transfer function analysis. For linear regression analysis, the ascending and descending slopes of AP and RR pairs were defined as BrrA and BrrD, respectively. Finally, APV, HRV, and BRS during the sleep–wake state were averaged for each participant.
Statistical analysis
Continuous data (HF, LF%, LF/HF, BP, BLF, BHF, BrrLF, BrrHF, BrrA, and BrrD) are presented as medians (min–max), whereas categorical data (sex and age groups) are presented as numbers (percentages).
We used the Kruskal–Wallis test followed by the Mann–Whitney U test with Bonferroni adjustment to determine between-group differences. The relationship between parameters was analyzed using Spearman correlation analysis. We next performed linear regression analysis to confirm the association among BP, APV, BRS, and age. All statistical analyses were performed using SPSS (version 17; SPSS, Chicago, IL, USA). In general, p < 0.05 was assumed to indicate statistical significance. However, in post hoc analysis, p < 0.008 was assumed to indicate significance.
Ethical approval
All the participants provided written informed consent for participation after the experimental procedures had been described to them. The procedures used in this study were approved by the Institutional Review Board of Taipei Veterans General Hospital (approval number: 1000057). All research was performed in accordance with relevant guidelines.
Our research is classified in observational studies which don't test potential treatments; instead we observe participants and track health outcomes over time. We followed the ICMJE (INTERNATIONAL COMMITTEE of MEDICAL JOURNAL EDITORS) definition of clinical trial: Purely observational studies (those in which the assignment of the medical intervention is not at the discretion of the investigator) will not require registration.
Results
Basic characteristics and HRV and BRS indexes among different age group
Table 1 summarizes the demographic characteristics of the included 67 patients. No significant differences in BMI, systolic BP, and diastolic BP were noted among the different age groups (Table 1).
The analysis of HRV indexes among the different age groups (Table S1) revealed the correlation of autonomic function with age and consciousness states; this finding is consistent with that of our previous study16. In general, BRS indexes were more strongly and inversely correlated with age compared with HRV indexes (Table S2).
BP/APV profiles and correlation coefficients between BP/APV and age in all participants under different consciousness states
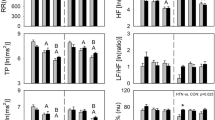
During wakefulness, BP and BHF tended to increase with age. During nREM, compared with the individuals aged 20–29 years, BP, BLF, and BHF were significantly elevated in the individuals aged 50–69 years (p = 0.004, 0.001, and 0.006, respectively), whereas only BHF was significantly elevated in the individuals aged 70–79 years (p = 0.004). In all the participants in this study (aged 20–79 years), the results of simple linear regression indicated that BP was positively correlated with age in both the AW and nREM stages. However, this correlation was stronger during nREM. In contrast to BP, BLF and BHF significantly differed among different consciousness states in the individuals aged 50–69 years. Similarly, age demonstrated a moderately positive correlation with BLF (r = 0.47, p < 0.05) and BHF (r = 0.52, p < 0.05) during nREM but not during wakefulness. Notably, the correlation between BP and age was stronger during nREM (r = 0.39, p < 0.05). Furthermore, changes in sympathetic activity with normal aging were noted in the individuals aged 50–69 years only during nREM. We noted that BLF, BHF, and HF were moderately correlated with age (Fig. 2; Table S2); specifically, the BLF–age and BHF–age correlation was positive, but the HF–age correlation was negative (Fig. 2; Table S2). These findings indicated the importance of sympathetic activity and BP during sleep (Fig. 1).
Blood pressure (BP)/arterial pressure variability (APV) parameters during wakefulness (AW) and non-rapid eye movement sleep (nREM) in the different age groups, and the comparisons of BP/APV between the AW and nREM for the different age groups in all participants (A). *p < 0.05 vs. 20–29. †p < 0.05 vs. the awake stage. The values are presented as means ± SEMs. Two-dimensional scattergrams for the BP/APV–age relationship during AW and nREM in all participants (B). *p < 0.05. r1: correlation coefficient in the 20–49-year age group. r2: correlation coefficient of 50–79-year age group. r3: correlation coefficient of 20–79-year age group. Abbreviations: ln, natural logarithm; BLF, LF of arterial pressure variability.
BRS profiles and correlation coefficients between BRS and age in all participants under different consciousness states
Before the age of 50 years, BRS was higher during nREM than during wakefulness. During wakefulness, significantly lower BRS indexes (i.e., BrrLF, BrrHF, and BrrA) were observed in the individuals aged 30–49 years (p = 0.006, 0.007, and 0.002, respectively) compared with the individuals aged 20–29 years. Moreover, compared with the individuals aged 20–29 years, those aged 50–69 and 70–79 years demonstrated significantly lower BrrLF (p = 0.008 and 0.006, respectively). Among sleep-related BRS measurements, BrrHF, BrrA, and BrrD were significantly lower in the individuals aged 50–69 years (all p < 0.001) and 70–79 years (p = 0.001, 0.003, and 0.003, respectively) than in those aged 20–29 years. Moreover, BrrHF and BrrD were significantly lower in the individuals aged 70–79 years (p = 0.006 and 0.008, respectively) than in those aged 30–49 years. BrrA was significantly lower in the individuals aged 50–69 and 70–79 years than those aged 20–29 years (p = 0.001 and 0.003, respectively), and it was significantly lower in the those aged 50–69 years than in those aged 30–49 years (p = 0.007); moreover, BrrLF was significantly lower in the individuals aged 70–79 years than in those aged 20–29 years (p = 0.005). Compared with BLF (r = 0.47, p < 0.001), the BRS parameters (i.e., BrrLF, BrrHF, BrrA, and BrrD) demonstrated a negative correlation with age (r = − 0.53, p < 0.001; r = − 0.65, p < 0.001; r = − 0.66, p < 0.001; r = − 0.64, p < 0.001) during nREM. Significant negative correlations were observed between BrrLF and age among all the age groups (r = − 0.53, p < 0.001) and in the 50–79-year age group (r = − 0.50, p = 0.020); however, this correlation was not observed in the 20–49-year age group. Moreover, BrrA (r = − 0.38, p = 0.023) and BrrD (r = − 0.37, p = 0.028) demonstrated a more considerable decline in the earlier life stages than did BrrLF. Moreover, the age-related decline in BRS (i.e., BrrLF, BrrHF, BrrA, and BrrD) was more apparent during nREM (r = − 0.53, − 0.65, − 0.66, and r = − 0.64, respectively; all p < 0.001) than during AW (r = − 0.59, p < 0.001; r = − 0.50, p < 0.001; r = − 0.55, p < 0.001; and r = − 0.46, p = 0.001, respectively). Therefore, BRS indexes during sleep are a vital indicator of the effect of age on BRS (Fig. 2).
Baroreflex sensitivity (BRS) parameters during wakefulness (AW) and non-rapid eye movement sleep (nREM) in the different age groups in all participants and their comparison for different consciousness states (A). *p < 0.05 vs. 20–29-year age group. †p < 0.05 vs. 30–49-year age group. The values are presented as means ± SEMs. Two-dimensional scattergrams for the BRS–age relationship during AW and nREM in all participants (B). *p < 0.05. r1: correlation coefficient in the 20–49-year age group. r2: correlation coefficient of 50–79-year age group. r3: correlation coefficient of 20–79-year age group. Abbreviations: BrrHF/BrrLF, magnitude of the MAP–R-R interval transfer function; BrrA/BrrD, slopes of MAP–R-R interval linear regression.
Comparison of BP/APV between sexes during different consciousness states across different age groups
Changes in BP with aging were more notable during nREM than during wakefulness. A significant correlation was noted between BP and age both during wakefulness and nREM in women (r = 0.50, p = 0.006 and r = 0.41, p = 0.018, respectively); however, this correlation was noted only during nREM in men (r = 0.48, p = 0.020). If we examine all the participants aged 20–79 years, BP during nREM was observed to have a moderate to strong relationship with age both in the men and women (r = 0.48, p = 0.020 and r = 0.41, p = 0.018, respectively). In addition, the men aged 20–49 years were found to have significantly higher BP than did the women during wakefulness (p = 0.041) and nREM (p = 0.005). During nREM, BLF was significantly and positively correlated with age in the women aged 20–79 years and those aged 50–79 years (r = 0.62, p < 0.001 and r = 0.75, p = 0.002, respectively); however, this result was not observed in the men. During wakefulness, significant sex differences were noted. Among the individuals aged 20–29 years, BLF was higher in the men than in the women (p = 0.037). During nREM, BHF was positively correlated with age among the women (r = 0.55, p = 0.001) and men (r = 0.55, p = 0.007) of all ages and in the women aged 50–79 years (r = 0.66, p = 0.011). Finally, significant differences were observed in BHF between the men and women in the 50–69-year group (p = 0.036) only during nREM (Fig. 3).
Comparison of blood pressure (BP)/arterial pressure variability (APV) between wakefulness (AW) and non-rapid eye movement sleep (nREM) for the different age groups in men and women (A). * p < 0.05 vs. 20–29-year age group. †p < 0.05 vs. men. The values are presented as means ± SEMs. Two-dimensional scattergrams for the BP/APV–age relationship during AW and nREM in men and women (B). *p < 0.05. r1: correlation coefficient in the 20–49-year age group. r2: correlation coefficient of 50–79-year age group. r3: correlation coefficient of 20–79-year age group. Abbreviations: ln, natural logarithm; BLF, LF of arterial pressure variability.
Comparison of BRS and age between sex under different consciousness states across different age groups
The BRS pattern along with aging was similar during wakefulness to that during nREM. Our simple linear regression analysis results indicated that aging was associated with a decline in BRS in both the men and women. Moreover, a relatively significant decline was noted in BrrA and BrrD among the 20–49-year-old women during wakefulness (r = − 0.52, p = 0.023 and r = − 0.54, p = 0.017, respectively) and nREM (r = − 0.60, p = 0.007 and r = − 0.62, p = 0.005, respectively). During nREM, the tendency for the attenuation of BrrHF, BrrA, and BrrD demonstrated a highly negative correlation in both the men (r = − 0.68, p < 0.001; r = − 0.64, p = 0.001; and r = − 0.62, p = 0.002, respectively) and women (r = − 0.74, p < 0.001; r = − 0.70, p < 0.001; r = − 0.72, p < 0.001, respectively). The correlation coefficient was higher in the women than in the men. Moreover, during nREM, compared with the men, the women demonstrated a significantly higher BrrHF in the 20–29-year age group (p = 0.007) and significantly higher BrrHF, BrrA, and BrrD in the 50–69-year age group (p = 0.041, 0.027, and 0.020, respectively; Fig. 4).
Comparison of baroreflex sensitivity (BRS) between wakefulness (AW) and non-rapid eye movement sleep (nREM) for the different age groups in men and women (A). *p < 0.05 vs. 20–29-year-old men. †p < 0.05 vs. 20–29-year-old women. ‡ p < 0.05 vs. men. The values are presented as means ± SEMs. Two-dimensional scattergrams for the BRS–age relationship during AW and nREM in men and women (B). *p < 0.05. r1: correlation coefficient in the 20–49-year age group. r2: correlation coefficient of 50–79-year age group. r3: correlation coefficient of 20–79-year age group. Abbreviations: ln, natural logarithm; BrrHF, magnitude of MAP–R-R interval transfer function; BrrA/BrrD, slope of MAP–R-R interval linear regressions.
Discussion
In this study, we determined various HRV and APV parameters during different consciousness states in the 20–79-year-old individuals to evaluate the spontaneous effects of sex, age, and consciousness states on cardiac sympathetic and parasympathetic activity and BRS. By performing AP–RR transfer function and AP-RR linear regression analyses, we quantified changes in spontaneous BRS to investigate relationships between the activities of autonomic nervous system (ANS) and central nervous system (CNS), which control the heart and blood vessels. The major finding of this study is that the central effect on BRS is different before and after the age of 50 years in both sexes; here, the age of 50 years appeared to be the cutoff point. Our findings indicated a stronger correlation between circulation system function and aging in the women than in the men. In addition, degeneration in BRS with age was observed more clearly in the nREM stage than during wakefulness.
Each consciousness state demonstrates unique behavioral and physical patterns owing to specific underlying neurophysiologic mechanisms30. Of these, sleep exerts a larger effect on physiological regulation; specifically, physiological changes related to motor control, BP, HR, and respiratory activity occur during sleep, through an antagonistic effect between sympathetic and parasympathetic cardiovascular regulation31. The central neural pathways related to autonomic commands underlie the features of BRS during nREM; in other words, the baroreflex resetting toward the lower values of heart rate (HR), BP, and sympathetic nerve activity leads to higher BRS during nREM than during wakefulness. These phenomena possibly illustrate the mechanism underlying CNS–ANS coupling during sleep11. In a previous study, WKYs demonstrated significant BRS enhancement during sleep, whereas SHRs exhibited enhanced sympathetic vasomotor activity but attenuated BRS; however, these phenomena were not observed under wakefulness5,6. An age-related decline in autonomic functioning was observed in individuals aged > 29 years16.
The circadian rhythmicity of the functional regulation of the cardiovascular system is unequivocal32. Nocturnal cardiovascular events tend to have a bimodal distribution, leading to their more frequent occurrence at the beginning and end of the night33,34. In susceptible patients, the risk of numerous adverse cardiac events, such as arrhythmia, acute myocardial infarction, and sudden cardiac death, are higher during REM, whereas that of ischemic events is higher during nREM32,35. We previously observed that changes in sympathetic vasomotor activity and BRS were associated with alterations in the sleep–wake cycle5. These findings are corroborated by the current findings regarding sleep-related changes in BP, BLF, BHF and BRS (Figs. 1 and 2). Notably, recordings obtained during nREM highlighted the patterns of BP, APV and BRS, all of which were affected by aging. These changes before and after the age of 50 years were found to be different. As such, cardiovascular vulnerability during sleep is a major factor in susceptible patients.
Similar to the different degeneration pattern of autonomic functioning, which declined rapidly before the age of 50 years and relatively slow after it16, our current findings revealed the turning point of BLF and BHF to be approximately the age of 50 years; BLF and BHF demonstrated a considerable increase after 50 years of age (Fig. 1). This increase in sympathetic activity may be related to cardiovascular problems during middle age—the age range in which the apparent attenuation of BRS occurs36.
Previous research pointed out normotensive premenopausal women show a vagal predominance of cardiac autonomic modulation, whereas age-matched men show a predominance of sympathetic modulation37. Another related research divided the subjects into three age groups (< 30 yrs, 30–60 yrs, and > 60 yrs), and found that there were significant statistical differences in BRS among the three groups38. The average age of menopause is around 50 in the Asian and the United States20,21. Besides, andropause should also be taken into consideration22. Thus we believe that special consideration should be given to the menopausal interval in the age variable in order to more accurately detect the impact of sex on BRS, so we use 50 years as the cutpoint.
Our findings regarding the age-dependent changes were more obvious in BRS than in HRV and APV in the 20–49-year age group, particularly during sleep (Table S2). These findings indicated that the significant degeneration in the ANS may account for the apparent decline in BRS before the age of 50 years, whereas the increase in cardiac sympathetic modulation and sympathetic-driven peripheral vasomotor responses occurred only after the age of 50 years.
Before the age of 50 years, BP and BLF were significantly lower in the women than in the men (Fig. 3), possibly because of the strengthening of β-adrenergic receptor–mediated dilatation and circulating female sex hormones in younger women39. Our results are consistent with those reported by Matsukawa et al.: at the age of < 50 years, muscle sympathetic nerve activity was lower in women than in men, but these levels became similar between the sexes after the age of 50 years40. In this study, although BP was higher in the men than in the women before the age of 50 years, APV increased in women after the age of 50 years. According to our previous findings, cardiac sympathetic regulation could be evaluated based on respiratory-related APV41,42. Moreover, in anaesthetized and positive pressure–ventilated rats, graded hemorrhage was noted to accompany an increase in respiratory-related APV in correlation with autonomic function, particularly β-adrenoceptors43. Across all age groups (20–79 years), we found that BLF was positively correlated with age only in the women. Moreover, BLF and BHF were positively correlated with age only in the women, with a sharp increase in their values after the age of 50 years. This observation was noted only during sleep (Fig. 3). The current findings are in line with the observation that the incidence of hypertension is considerably higher around the age of menopause in women and that these this incidence may be identical to or even higher than that in men of the same age group44.
Recently, a population-based study confirmed that age and sex were important factors associated with BRS10. However, no studies have been found to discuss the impact of biological variables on BRS for sleep–wake cycle. Nevertheless, the CNS-ANS coupling is different in the awake and sleep stages11, only the single awake state cannot fully reflect the impact of biological variables on BRS.
Sexual dimorphism has been noted in BRS function. Considerable variabilities, particularly those related to sex differences, lead to beat-to-beat vascular transduction45. Studies have reported inconsistent results related to sex differences in BRS among young individuals46,47,48. Nonetheless, around middle age, women have been noted to have higher arterial stiffness than men do18. Animal studies have confirmed the negative consequences of the oscillation and deprivation of ovarian hormones on BRS, which is involved in cardiovascular autonomic control, as indicated by changes in arterial pressure49. In addition, estrogen treatment significantly increased BRS in ovariectomized rats; however, the β-adrenergic receptor was found to not be involved in this effect50. A prospective clinical study reported that a reduction in estradiol levels leads to decreased carotid-vasomotor BRS51. In an animal study examining whether testosterone can facilitate baroreflex responsiveness, baroreflex was measured in sham-operated rats and castrated rats treated with phenylephrine and sodium nitroprusside, respectively. The results indicated that decreased plasma testosterone levels attenuated reflex bradycardia in the castrated rates compared with the sham-operated rats52. Another study demonstrated obvious cardiovascular changes following the administration of testosterone alone in adolescent rats; these changes included increased arterial pressure, decreased HR, and exacerbated tachycardiac baroreflex response53. In the current study, the men demonstrated significantly lower BRS at the age of 50–69 years than did the women during sleep (Fig. 4); this result is in contrast to that of a previous study, which reported that BRS was attenuated in middle-aged women but not in middle-aged men54. The possible reason for this inconsistency is as follows: the previous study used the Valsalva method to detect BRS, whereas we measured BRS spontaneously. Moreover, we divided our patients into different age groups and detected their parameters at different consciousness states. Notably, our findings revealed that the women exhibited attenuation in BRS before the age of 50 years; however, the women aged 20–69 years had higher BRS than did the men aged 20–69 years (Fig. 4). This phenomenon was associated with parasympathetic reduction in cardiac parasympathetic activity; however, BRS weakened with age at a relatively slow rate in the men. In addition, all the men demonstrated a significant negative correlation between age and BRS during both nREM and wakefulness, but they did not demonstrate significant differences before and after the age of 50 years (Fig. 4). Autonomic status, BRS, and hormone status have been demonstrated to be correlated, indicating that the lower levels of circulating androgens and estrogens accompany lower HRV and decreased BRS55. Thus, both the men and women demonstrate a physiological turning point around middle age; however, this reduction in hormone production appears to be slower in men than in women. The above results are consistent with our hypothesis that the trend of BRS changes with age for men and women before and after the age of 50 is different.
Perspective and significance
In summary, the aforementioned results supported the hypothesis that when considering sex differences, the patterns of APV and BRS are different across an individual’s lifespan. Around middle age, individual differences in APV and BRS demonstrated considerable variations among the women (Figs. 3 and 4). These results may be related to menopause: sympathetic activity was determined to be significantly higher in perimenopausal women56. Moreover, estrogen may play a crucial role in sex-related autonomic differences13. Similarly, in the men, andropause was a likely reason for the marked decrease in BRS in the 50–69-year age group than in the 20–29-year age group (Fig. 4). Evidence suggests a positive interaction between testosterone and cardiomotor vagal activity52. Testosterone may be involved, at least partly, in the augmentation of cardiac vagal efferent activity, which may have contributed to the results in our study. The aforementioned results supported the hypothesis that the 50 years of age is a critical turning point in human life. Moreover, ANS activity varies with age, with a turning point around the age of 50 years16. Therefore, not only neural and hemodynamic factors but also hormones possibly contribute to age- and sex-related differences in BRS.
Limitations
This study has few limitations. First, although we excluded individuals diagnosed as having hypertension, sporadic BP fluctuations were still noted in our data. In addition, because we did not perform 24-h BP monitoring in our participants, we could not exclude individuals with altered circadian BP rhythms, such as nondipping and inverted dipping. Second, although we controlled for BMI in each age group, the sex ratio in each group was less controlled for and the number of participants in each subgroup was unequal. Finally, because we did not perform any invasive vascular and hormone measurements, the collected indirect data may have had some deviation from the actual data.
Conclusions
In the present study, changes in BRS were found to vary with age, sex, and consciousness state, with each exhibiting a specific pattern. The age of 50 years seems to be a crucial turning point for sexual dimorphism in BRS. Baroreflex modulation of the cardiovascular system during sleep can delineate the age and sex–dependent BRS pattern more sensitively, indicating the clinical importance of this result. Age-related cardiovascular function impairment demonstrated sex differences. The measurements used in this study may aid in screening for neurocardiac abnormalities in apparently healthy individuals. Additional studies focused on generating a normative database of a healthy population according to age, sex, and consciousness state are warranted.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
DiFrancesco, M. W. et al. Age-related changes in baroreflex sensitivity and cardiac autonomic tone in children mirrored by regional brain gray matter volume trajectories. Pediatr. Res. 83, 498–505. https://doi.org/10.1038/pr.2017.273 (2018).
Head, G. A., Saigusa, T. & Mayorov, D. N. Angiotensin and baroreflex control of the circulation. Braz. J. Med. Biol. Res. 35, 1047–1059. https://doi.org/10.1590/s0100-879x2002000900005 (2002).
Adoor, M. et al. Influence of age and gender on blood pressure variability and baroreflex sensitivity in a healthy population in the Indian sub-continent. J. Basic Clin. Physiol. Pharmacol. 29, 329–337. https://doi.org/10.1515/jbcpp-2017-0125 (2018).
Miki, K. & Yoshimoto, M. Sympathetic nerve activity during sleep, exercise, and mental stress. Auton. Neurosci. Basic Clin. 174, 15–20. https://doi.org/10.1016/j.autneu.2012.12.007 (2013).
Kuo, T. B. J. & Yang, C. C. H. Sleep-related changes in cardiovascular neural regulation in spontaneously hypertensive rats. Circulation 112, 849–854. https://doi.org/10.1161/circulationaha.104.503920 (2005).
Legramante, J. M. & Galante, A. Sleep and hypertension: A challenge for the autonomic regulation of the cardiovascular system. Circulation 112, 786–788. https://doi.org/10.1161/circulationaha.105.555714 (2005).
Smyth, H. S., Sleight, P. & Pickering, G. W. Reflex regulation of arterial pressure during sleep in man. A quantitative method of assessing baroreflex sensitivity. Circ. Res. 24, 109–121. https://doi.org/10.1161/01.res.24.1.109 (1969).
Peter-Derex, L. & Derex, L. Wake-up stroke: From pathophysiology to management. Sleep Med. Rev. 48, 101212. https://doi.org/10.1016/j.smrv.2019.101212 (2019).
Iellamo, F. et al. Baroreflex buffering of sympathetic activation during sleep: Evidence from autonomic assessment of sleep macroarchitecture and microarchitecture. Hypertension 43, 814–819. https://doi.org/10.1161/01.HYP.0000121364.74439.6a (2004).
Man, T. et al. Spontaneous baroreflex sensitivity and its association with age, sex, obesity indices and hypertension: A population study. Am. J. Hypertens. 34, 1276–1283. https://doi.org/10.1093/ajh/hpab122 (2021).
de Zambotti, M., Trinder, J., Silvani, A., Colrain, I. M. & Baker, F. C. Dynamic coupling between the central and autonomic nervous systems during sleep: A review. Neurosci. Biobehav. Rev. 90, 84–103. https://doi.org/10.1016/j.neubiorev.2018.03.027 (2018).
Kuo, T. B. J., Lai, C. J., Shaw, F. Z., Lai, C. W. & Yang, C. C. H. Sleep-related sympathovagal imbalance in SHR. Am. J. Physiol. Heart Circ. Physiol. 286, H1170-1176. https://doi.org/10.1152/ajpheart.00418.2003 (2004).
Liu, C. C., Kuo, T. B. J. & Yang, C. C. H. Effects of estrogen on gender-related autonomic differences in humans. Am. J. Physiol. Heart Circ. Physiol. 285, H2188-2193. https://doi.org/10.1152/ajpheart.00256.2003 (2003).
Stratton, J. R. et al. Effects of aging on cardiovascular responses to parasympathetic withdrawal. J. Am. Coll. Cardiol. 41, 2077–2083. https://doi.org/10.1016/s0735-1097(03)00418-2 (2003).
Ebert, T. J., Morgan, B. J., Barney, J. A., Denahan, T. & Smith, J. J. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am. J. Physiol. 263, H798-803. https://doi.org/10.1152/ajpheart.1992.263.3.H798 (1992).
Kuo, T. B. J., Li, J. Y., Kuo, H. K., Chern, C. M. & Yang, C. C. H. Differential changes and interactions of autonomic functioning and sleep architecture before and after 50 years of age. Age 38, 5. https://doi.org/10.1007/s11357-015-9863-0 (2016).
Kuo, T. B. J. et al. Effect of aging on gender differences in neural control of heart rate. Am. J. Physiol. 277, H2233-2239. https://doi.org/10.1152/ajpheart.1999.277.6.H2233 (1999).
Fu, Q. & Ogoh, S. Sex differences in baroreflex function in health and disease. J. Physiol. Sci. JPS 69, 851–859. https://doi.org/10.1007/s12576-019-00727-z (2019).
Vongpatanasin, W. Autonomic regulation of blood pressure in menopause. Semin. Reprod. Med. 27, 338–345. https://doi.org/10.1055/s-0029-1225262 (2009).
Shi, J. et al. Age at menarche and age at natural menopause in East Asian women: A genome-wide association study. Age (Dordr.) 38, 513–523. https://doi.org/10.1007/s11357-016-9939-5 (2016).
Minkin, M. J. Menopause: Hormones, lifestyle, and optimizing aging. Obstet. Gynecol. Clin. North Am. 46, 501–514. https://doi.org/10.1016/j.ogc.2019.04.008 (2019).
Wu, C. Y., Yu, T. J. & Chen, M. J. Age related testosterone level changes and male andropause syndrome. Chang Gung Med. J. 23, 348–353 (2000).
Iber, C. The AASM manual for the scoring of sleep and associated events: Rules. Terminology and Technical Specification (2007).
Kuo, T. B. J. & Chan, S. H. Continuous, on-line, real-time spectral analysis of systemic arterial pressure signals. Am. J. Physiol. 264, H2208-2213. https://doi.org/10.1152/ajpheart.1993.264.6.H2208 (1993).
Kuo, T. B. J. et al. Diminished vasomotor component of systemic arterial pressure signals and baroreflex in brain death. Am. J. Physiol. 273, H1291-1298. https://doi.org/10.1152/ajpheart.1997.273.3.H1291 (1997).
Yang, C. C. H., Kuo, T. B. J. & Chan, S. H. Auto- and cross-spectral analysis of cardiovascular fluctuations during pentobarbital anesthesia in the rat. Am. J. Physiol. 270, H575-582. https://doi.org/10.1152/ajpheart.1996.270.2.H575 (1996).
Hori, T. et al. Proposed supplements and amendments to “A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects”, the Rechtschaffen & Kales (1968) standard. Psychiatry Clin. Neurosci. 55, 305–310. https://doi.org/10.1046/j.1440-1819.2001.00810.x (2001).
Camm, A. J., Malik, M., Bigger, J. T., Breithardt, G., Cerutti, S., Cohen, R. J., ... & Singer, D. H. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task force of the European society of cardiology and the North American society of pacing and electrophysiology. Eur. Heart J. 17, 354–381 (1996).
Yang, C. C. H., Lai, C. W., Lai, H. Y. & Kuo, T. B. J. Relationship between electroencephalogram slow-wave magnitude and heart rate variability during sleep in humans. Neurosci. Lett. 329, 213–216. https://doi.org/10.1016/s0304-3940(02)00661-4 (2002).
Harris, C. D. Neurophysiology of sleep and wakefulness. Respir. Care Clin. N. Am. 11, 567–586. https://doi.org/10.1016/j.rcc.2005.08.001 (2005).
Silvani, A. et al. Sleep-dependent changes in the coupling between heart period and blood pressure in human subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1686-1692. https://doi.org/10.1152/ajpregu.00756.2007 (2008).
Portaluppi, F. et al. Circadian rhythms and cardiovascular health. Sleep Med. Rev. 16, 151–166. https://doi.org/10.1016/j.smrv.2011.04.003 (2012).
Boudreau, P., Yeh, W. H., Dumont, G. A. & Boivin, D. B. Circadian variation of heart rate variability across sleep stages. Sleep 36, 1919–1928. https://doi.org/10.5665/sleep.3230 (2013).
Lavery, C. E., Mittleman, M. A., Cohen, M. C., Muller, J. E. & Verrier, R. L. Nonuniform nighttime distribution of acute cardiac events: A possible effect of sleep states. Circulation 96, 3321–3327. https://doi.org/10.1161/01.cir.96.10.3321 (1997).
Holty, J. E. & Guilleminault, C. REM-related bradyarrhythmia syndrome. Sleep Med. Rev. 15, 143–151. https://doi.org/10.1016/j.smrv.2010.09.001 (2011).
Monahan, K. D. Effect of aging on baroreflex function in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R3–R12. https://doi.org/10.1152/ajpregu.00031.2007 (2007).
Philbois, S. V. et al. Important differences between hypertensive middle-aged women and men in cardiovascular autonomic control-a critical appraisal. Biol. Sex Differ. 12, 11. https://doi.org/10.1186/s13293-020-00355-y (2021).
Boettger, M. K. et al. Influence of age on linear and nonlinear measures of autonomic cardiovascular modulation. Ann. Noninvasive Electrocardiol. 15, 165–174. https://doi.org/10.1111/j.1542-474X.2010.00358.x (2010).
Hart, E. C., Joyner, M. J., Wallin, B. G. & Charkoudian, N. Sex, ageing and resting blood pressure: Gaining insights from the integrated balance of neural and haemodynamic factors. J. Physiol. 590, 2069–2079. https://doi.org/10.1113/jphysiol.2011.224642 (2012).
Matsukawa, T., Sugiyama, Y., Watanabe, T., Kobayashi, F. & Mano, T. Baroreflex control of muscle sympathetic nerve activity is attenuated in the elderly. J. Auton. Nerv. Syst. 73, 182–185. https://doi.org/10.1016/s0165-1838(98)00128-3 (1998).
Yang, C. C. H. & Kuo, T. B. J. Assessment of cardiac sympathetic regulation by respiratory-related arterial pressure variability in the rat. J. Physiol. 515(Pt 3), 887–896. https://doi.org/10.1111/j.1469-7793.1999.887ab.x (1999).
Yang, C. C. H. & Kuo, T. B. J. Impact of pulse pressure on the respiratory-related arterial pressure variability and its autonomic control in the rat. Pflugers Arch. 439, 772–780. https://doi.org/10.1007/s004249900230 (2000).
Lai, H. Y. et al. Respiratory-related arterial pressure variability as an indicator of graded blood loss: Involvement of the autonomic nervous system. Clin. Sci. 105, 491–497. https://doi.org/10.1042/cs20030080 (2003).
Kearney, P. M. et al. Global burden of hypertension: Analysis of worldwide data. Lancet (London, England) 365, 217–223. https://doi.org/10.1016/s0140-6736(05)17741-1 (2005).
Robinson, A. T. et al. Relation between resting sympathetic outflow and vasoconstrictor responses to sympathetic nerve bursts: Sex differences in healthy young adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 316, R463-r471. https://doi.org/10.1152/ajpregu.00305.2018 (2019).
Beske, S. D., Alvarez, G. E., Ballard, T. P. & Davy, K. P. Gender difference in cardiovagal baroreflex gain in humans. J. Appl. Physiol. 91, 2088–2092. https://doi.org/10.1152/jappl.2001.91.5.2088 (2001).
Tank, J., Diedrich, A., Szczech, E., Luft, F. C. & Jordan, J. Baroreflex regulation of heart rate and sympathetic vasomotor tone in women and men. Hypertension 45, 1159–1164. https://doi.org/10.1161/01.HYP.0000165695.98915.9a (2005).
Klassen, S. A., Chirico, D., Dempster, K. S., Shoemaker, J. K. & O’Leary, D. D. Role of aortic arch vascular mechanics in cardiovagal baroreflex sensitivity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R24-32. https://doi.org/10.1152/ajpregu.00491.2015 (2016).
Ferreira, M. J. et al. Ovarian status modulates cardiovascular autonomic control and oxidative stress in target organs. Biol. Sex Differ. 11, 15. https://doi.org/10.1186/s13293-020-00290-y (2020).
Pourshanazari, A. A., Mohagheghi, O., Pilavarian, A. A., Enayatfard, L. & Shafei, M. N. Involvement of β-adrenergic receptor of nucleus tractus solitarius in changing of baroreflex sensitivity by estrogen in female rats. Adv. Biomed. Res. 3, 83. https://doi.org/10.4103/2277-9175.127996 (2014).
Brunt, V. E. et al. Short-term administration of progesterone and estradiol independently alter carotid-vasomotor, but not carotid-cardiac, baroreflex function in young women. Am. J. Physiol. Heart Circ. Physiol. 305, H1041-1049. https://doi.org/10.1152/ajpheart.00194.2013 (2013).
El-Mas, M. M., Afify, E. A., Mohy El-Din, M. M., Omar, A. G. & Sharabi, F. M. Testosterone facilitates the baroreceptor control of reflex bradycardia: Role of cardiac sympathetic and parasympathetic components. J. Cardiovasc. Pharmacol. 38, 754–763. https://doi.org/10.1097/00005344-200111000-00012 (2001).
Engi, S. A. et al. Cardiovascular complications following chronic treatment with cocaine and testosterone in adolescent rats. PLoS ONE 9, e105172. https://doi.org/10.1371/journal.pone.0105172 (2014).
Huikuri, H. V. et al. Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation 94, 122–125. https://doi.org/10.1161/01.cir.94.2.122 (1996).
Rydlewska, A. et al. Circulating testosterone and estradiol, autonomic balance and baroreflex sensitivity in middle-aged and elderly men with heart failure. Aging Male 16, 58–66. https://doi.org/10.3109/13685538.2013.768979 (2013).
Kishan, A., Marakur, N., Moodithaya, S. & Mirajkar, A. M. Electrodermal response to auditory stimuli in relation to menopausal transition period. J. Basic Clin. Physiol. Pharmacol. 29, 123–129. https://doi.org/10.1515/jbcpp-2017-0057 (2018).
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Funding
This study was supported by grants (11101-62-026 and 11101-62-044) from the Taipei City Hospital; a grant (111W32505) from Brain Research Center, National Yang Ming Chiao Tung University; a grant (111-2622-E-A49-019) from National Science and Technology Council; and from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education, Taiwan.
Author information
Authors and Affiliations
Contributions
T.B.J.K.: Software, Methodology, Supervision. C.C.H.Y.: Writing—Reviewing and Editing. K.-L.K.: Investigation. H.-Y.H.: Formal analysis, Visualization. C.-M.C.: Investigation. J-Y.L.: Conceptualization, Writing- Reviewing and Editing. C.-H.Y: Conceptualization, Writing—Original draft preparation, Writing—Reviewing and Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yeh, CH., Kuo, T.B.J., Li, JY. et al. Effects of age and sex on vasomotor activity and baroreflex sensitivity during the sleep–wake cycle. Sci Rep 12, 22424 (2022). https://doi.org/10.1038/s41598-022-26440-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26440-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.