Abstract
This study describes a molecular dynamics computational modelling informed bioengineering of nano-scaled 2-D hydronium jarosite. More specifically, a phyto-engineering approach using green nano-chemistry and agro-waste in the form of avocado seed natural extract was utilized as a green, economic, and eco-friendly approach to synthesize this unique mineral at the nanoscale via the reduction of iron (II) sulphate heptahydrate. The nanoproduct which was found to exhibit a quasi-2D structure was characterized using a multi-technique approach to describe its morphological, optical, electrochemical, and magnetic properties. Radial distribution function and electrostatic potential maps revealed that flavone, a phenolic compound within the avocado seed natural extract, has a higher affinity of interaction with the nanoparticle's surface, whilst vanillic acid has a higher wetting tendency and thus a lower affinity for interacting with the hydronium jarosite nanoparticle surface compared to other phytoactive compounds. XRD and HRTEM results indicated that the nanoscale product was representative of crystalline rhombohedral hydronium jarosite in the form of quasi-triangular nanosheets decorated on the edges with nanoparticles of approximately 5.4 nm diameter that exhibited significant electrochemical and electroconductive behaviours. Magnetic studies further showed a diamagnetic behaviour based on the relationship of the inverse susceptibility of the nanomaterial with temperature sweep.
Similar content being viewed by others
Introduction
In 2004 the Opportunity rover reported widespread existence of jarosite at Meridiani Planum 21, Since then, the mineral has been frequently identified on Mars2,3,4 and has been interpreted as an evidence for the occurrence of liquid water5 as on Earth, jarosite forms as the result of acidic oxidative low-temperature weathering of iron-bearing minerals in water-limited settings6. Likewise, jarosite crystals adhering on residual silica-rich particles have been identified in the Talos Dome ice core (East Antarctica) and interpreted as products of weathering involving aeolian dust and acidic atmospheric aerosols7,8.
Hydronium jarosite is a relatively rare mineral belonging to the jarosite series of secondary iron sulfate minerals synthesized via the oxidation of sulfide minerals, in particular, pyrite9. As examplified by Fig. 1a, Jarosites are layered systems characterized by the formula A1−x(H3O)xFe3 + 3-y(SO4)2(OH)6-3y (H2O)3y, where the A sites are occupied by a monovalent (K, Na, H3O+, NH4, Ag and Tl) or divalent (Pb) cation10, with hydronium jarosite [H3OFe3 (SO4)2(OH)6] containing an elusive H3O+ group that was previously characterized in more detail by Majzlan et al.11. Jarosite-type minerals generally occur in fluvial environments contaminated by acid rock or acid mine drainage sediments or in mine tailings of sulfide ore deposits. They are of economic importance, and show potential for utilization in multifunctional applications12. For example, hydronium jarosite serves as an iron scavenger in the hydro-metallurgic industry as a raw material in the production of pigments or construction materials13,14,15,16,17,18, as a heterogeneous Fenton catalyst in the degradation of azo dye methyl orange (MO)19, and for the photocatalytic reduction of specific carcinogenic and mutagenic organic as well as inorganic pollutants such as Cr(VI)20 released by leather tanning, metal plating, pigment manufacturing, and chromate production industrial activities among others.
Since hydronium jarosite minerals harbour diverse catalytic applications within the chemical industry and as an effective photocatalytic reducing agent with potential applications in environmental remediation, researchers have tried to synthesize such jarosite-type minerals using both chemical and biological oxidation of Fe approaches21,22. In this regard, Jarosite minerals have been synthesized using different methods including chemical synthesis23. Even though some jarosite minerals only require Fe2 (SO4)3 and water, some also require the use of high temperatures and necessitate longer synthesis times19. Chemical methods employ a number of toxic chemicals24, whilst the biological oxidation of Fe during hydronium jarosite formation is significantly affected by acidity and temperature25,26. Only very recently, the synthesis and characterization of rod-shaped jarosite nanoparticles using a microwave-assisted hydrothermal method have also been reported25. As nanomaterials have been shown to have increased reactivity for application across multidisciplinary research fields due to their unique physico-chemical properties and larger surface to volume ratio, the catalytic potential of hydronium jarosite may be further developed by tuning its physico-chemical properties to the nanoscale. Yet, there is no literature available on the engineering of hydronium jarosite at the nanoscale.
Phyto-engineering of nanomaterials has increased in popularity over recent years due to the fact that this approach is environmentally safe and possible at standard conditions of temperature and pressure26. More specifically, phyto-engineering involves the use of plant extracts to produce safe and biocompatible nanomaterials27. Plants contain phytochemicals including phenolic compounds that have been shown to serve as excellent reducing and chelating agents for synthesizing nanomaterials27,28. Since the interaction between different types of plant phytochemical compounds and nanoparticles plays a critical role in phyto-engineering an end product with unique physico-chemical properties27, delineating this interaction at the molecular level is important. Yet, studies exploring the molecular interaction between specific plant phytochemical compounds and nanoparticles using methods such as computational simulation remain to our knowledge largely limited. In effect, molecular dynamics computational simulation—a technique for exploring conformational space using various sized molecules, including proteins, active compounds, and nanoparticles29,30,31—may present as a powerful computational approach to explore the interaction between plant phytochemical compounds serving as nanoparticle stabilizing agents during phyto-engineering of nanomaterials that may exhibit unique physico-chemical properties for diverse applications.
This study reports for the first time on the synthesis of nano-scaled 2-D Hydronium jarosite. More precisely, this study combines computational simulation for informing the phyto-engineering of hydronium jarosite nanoparticles utilizing an agro-waste product rich in phytochemicals—Persea americana (avocado) seeds32—as reducing and chelating agents for the reduction of iron (II) sulphate hepta-hydrate (FeSO4·7H2O) using green nano-chemistry approach. More specifically, the interactions between hydronium jarosite nanoparticles, water, and eight phyto-active, including phenolic compounds, found in avocado seeds (caffeic acid, chlorogenic acid, epicatechin, ferulic acid, flavone, neochlorogenic acid, procyanidin, and vanillic acid) were investigated using a molecular dynamics computational simulation. In addition, full physicochemical characterization of the hydronium jarosite phyto-engineered at the nanoscale were studied using a multi-technique approach. Although hydronium jarosite minerals have a unique chemical formulation that may endow it with prominent optical, electrical, electrochemical, and magnetic features not yet explored, this study also investigated these parameters in phyto-engineered hydronium jarosite nanoparticles.
Materials and methods
Chemicals used for nanoparticle synthesis
Analytical grade iron (II) sulphate hepta-hydrate (FeSO4·7H2O) and Alconox were purchased from Sigma-Aldrich. The glassware used in all experiments was washed with Alconox and rinsed thrice with DI H2O. All aqueous solutions were prepared with double distilled deionized (DI) H2O.
Preparation of avocado seed aqueous extract and hydronium jarosite nanoparticles
The preparation of avocado seed aqueous extract and phyto-engineering of hydronium jarosite nanoparticles were completed by following a green chemistry approach and the method of Bhattacharjee et al.33. The mixture constituted of FeSO4·7H2O dissolved in DI water and combined with avocado seed extract was stirred at 70 °C for 3 h. The hydronium jarosite nanoparticles were separated and isolated from the solution using centrifugation at 4000 rpm for 20 min. The pellet obtained after centrifugation was washed with DI H2O and dried in a Memmert hot air oven at 50 °C for 2 h.
Morphological, elemental, optical, electrochemical and magnetic characterization
Sample purity and elemental composition was determined with Energy Dispersive X-rays Spectroscopy (EDS) using a Nova Nano-SEM equipped with an Oxford X-Max detector (20 mm2) at 20 kV and a working distance of 6 mm and Oxford INCA software. HRTEM and SAED images were captured using a Tecnai F20 FEG-TEM at 200 keV in bright field mode. The Direct Electron DE16 camera detection system was used to capture images, whilst ImageJ open source software was used to evaluate particle size distribution.
Phase identification and sample crystallography were performed using X-Rays Diffraction (XRD) measured with a Bruker Advanced AXS D8 diffractometer with a monochromatic CuK-1 radiation (λ = 1.54060 Å) operating in the Bragg–Brentano geometry and a step size of 0.2° with 0.2 s per step for angles between 5° and 85°. To characterize chemical bonds and the molecular structure of functional groups originating from organic phyto-constituent molecules responsible for the reduction and stabilization of the Fe ions, Fourier Transform Infrared Spectroscopy (FTIR) using potassium bromide pellet technology and a PerkinElmer 100 Spectrometer in the wave number 400–4000 cm−1 range was used.
For the optical characterization, UV–VIS absorbance and photoluminescence investigations were carried out. A Cary5000 UV–VIS–NIR spectrophotometer with double beam and an integrating sphere was used to measure UV–VIS absorbance at room temperature within the spectral range of 200–800 nm for the avocado seed and colloidal nanoparticle solution and hence the bio-reduction and formation of the nanoparticles. A fibre-optics linked ocean optics system consisting of a high-powered UV Light emitting diode source (240 nm) coupled to a high sensitivity QE ProFL spectrometer was used for the photoluminescence measurements.
For the magnetic characterization, a cryogenics vibrating stage magnetometer was used to study the magnetic properties of the sample. The sample was mounted on a sample holder and centred between two pick up coils, while the sample stage vibrated at a frequency of 50 Hz and at an approximate amplitude range of 3 mm. The zero field cooling (ZFC) and field cooling (FC) measurements were conducted from 3 to 300 K at a set field of 1 T.
For the electrochemical investigations, electrochemical properties of the material were conducted on a CH Instruments Autolab Potentiostat electrochemical workstation using two techniques including cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The instrument is a three-electrode system in which a glassy carbon electrode is used as a working electrode, a platinum wire as a counter electrode, and Ag/AgCl as reference electrodes combined with a 3 M NaCl salt bridge solution. CV experiments were performed on the potential window from − 0.6 to 0.6 V with a scanning rate of 50 mV/s and various scans from 20 to 100 mV/s. EIS measurements were conducted at a perturbation amplitude of 10 mV within the frequency range of 0.1–100 Hz. All experimental measurements were conducted using a 0.1 M KOH solution as an electrolyte. High purity argon gas was used to de-oxygenate and blanket the experimental solution during all measurements at room temperature. The working electrode (GCE/hydronium jarosite) was prepared by adding 2 µl of a 5% Nafion solution in a small amount of hydronium jarosite material dissolved in ethanol. The mixture was ultra-sonicated in a warm water bath for 15 min to make a colloidal solution. For the analysis, a small amount of the solution was drop-coated on the surface area of the glassy carbon electrode that was cleaned before coating using alumina powder (1.0, 3.0, 0.05) followed by ultra-sonication in ethanol and subsequently water, for 5 min. The modified glassy carbon electrode was dried for 20 min in an oven at 35 °C before being immersed into 0.1 M KOH for the experimental measurements.
Computational and modelling: molecular dynamics computational simulation
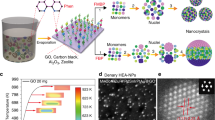
To perform the molecular dynamics simulations, a sphere of hydronium jarosite nanoparticles produced by Atomsk34 with a diameter of 2.5 nm and containing 981 atoms were placed in the centre of a simulation cubic box. Ten molecules of each phyto-active compound were subsequently randomly inserted into the box, with a minimum distance of 1 nm from the simulation box's edge sides (Fig. 1). GROMACS 2019 software35 was used to perform all molecular dynamics simulations under periodic boundary conditions, using the CHARMM36 force field36 and SPC water model37. The TIP3P model was used to solvate the water molecules in the complex. The systems were optimized in terms of energy using the steepest descent algorithm for all atoms38. Each system was equilibrated using the NVT ensemble [constant number of particles (N), volume (V), and temperature (T)] coupled to the V-rescale thermal bath at 300 K for 200 ps, and the NPT ensemble (constant number of N, P, and T) coupled to the Berendsen pressure bath at 1 atm for 300 ps. Each system was then conducted at a 50 ns molecular dynamics simulation with a time step of 2 fs under constant conditions of 1 atm, and 300 K. The LINCS algorithm39 was used to constrain the lengths of H-bonds. The particle mesh Ewald40 approach was used to apply long-range electrostatics. GROMACS utilities were used to analyze the trajectory data and VMD 1.9.341 was used to produce molecular graphics and visualizations. The force fields of hydronium jarosite nanoparticles and compounds were determined using CHARMM CGenFF42. The electronic structures of eight phytoactive chemicals (caffeic acid, chlorogenic acid, epicatechin, ferulic acid, flavone, neochlorogenic acid, procyanidin, and vanillic acid) were done at the density functional theory (DFT) level using the Gaussian program, version 0943. The geometry optimization of the molecules was carried out at the B3LYP/6-311++g(d,p) level of theory.
Ethical statement
In the study, avocado seeds were used and this requires no compliance with any institutional, national, and international guidelines and legislation since the avocado seeds presented as household waste in the form of food waste.
Results and discussion
Morphology and size distribution studies
The shape and the size of the green synthesized hydronium jarosite particles were characterized by high resolution transmission electron microscopy (HRTEM). Figure 2 displays a typical HRTEM and SAED images and size distribution histogram of the hydronium jarosite nanoparticles. The HRTEM image of Fig. 2a indicates that the bio-engineered material exhibits a crystal clear shape anisotropy. More precisely, they seem forming triangular shaped 2-D sheets grafted with nanoparticles with a significant nano-particles population both on the basal surface and at the edge of the 2-D triangular sheets as schematically summarized in Fig. 2e. The measured average length of the triangular shaped sheets shown in is ~ 193.03 nm. The agglomeration of the particles at the edges of the sheets may be due to the electrostatic field on the edges as their chemical environments are different from the atoms on the basal plane due to the termination of these atoms44. The average diameter of the decorating particles is ~ 5.4 nm. The histogram diagram of their particle size distribution is displayed in Fig. 2b.
Yet it is early to suggest the growth model of the obtained triangular 2-D nanosheets, it is likely that the process of growth follows the summarized model of Fig. 2c. If one borrows from the thin films epitaxial growth governed by the 3 major models; Volmer–Weber, Stranski–Krastanov and Frank–van der Merwe45,46, it is likely the last one that fits with the current observations. If so, within this model, at room temperature, through the Brownian motion, their kinetic energy can be approximated as 3/2kBT ~ 4.23 10–2 eV, the so called on plane-crystallites diffuse (equivalent of seed atoms/clusters) to coalesce so to form the 2-D sheets controlling the thickness of the formed sheets. The edge crystallites are likely to diffuse reaching the edge of the 2-D sheets contributing to their spatial extension. If so, the 2-D sheets growth would be similar to that of thin films governed by Frank–van der Merwe model47. If so, the current jarosite growth would be similar to that observed on graphene by Wang et al.48. Also, this growth is common to to the formation mechanism in a series of antiprismatic jarosite-type compounds as validated by Xin Yang et al.49, Fig. 2c and its zoom Fig. 2d display parallel lattice fringes with an inter-reticular d- spacing of to 0.48 nm as measured by Image-J suggesting that the 2-D sheets present a certain degree of crystallinity at least locally. The Selected Area Electron Diffraction (SAED) (Fig. 2g) on a thicker jarosite 2-D sheet of Fig. 2f suggests that the prepared Jarosite 2-D sheets are crystalline in nature with a significant textured orientation (highly intense/bright spots) with a likely hexagonal crystallographic symmetry which is an intrinsic crystallographic orientation of hydronium jarosites50.
Crystallographic properties
In relation to the crystallographic properties of the bio-engineered sample, Fig. 3 reports the room temperature X-Rays Diffraction pattern (XRD) of the bio-engineered Hydronium jarosite nanoparticles. The spectrum displays a rich set of Bragg diffraction peaks located at 14.8°, 15.6°,17.3°, 24.1°,25.1°, 28.4°, 28.8°, 29.9°, 31.5°, 35.1°, 38.8°, 39.6°, 45.5°, 46.9°, 48.0° and 49.5° corresponding to the (JCP2_31-0650) Bragg peaks of (101), (003), (012), (110), (104), (021), (113), (202), (006), (024), (122), (303), (027), (009) and (220), respectively. Following a Maud and a Rietveld treatment, such an XRD pattern is in full agreement with the rhombohedral hydronium jarosite (JCP2_31-0650)51,52. It is worth noting that the XRD patterns showed no impurities or other competitive jarosite phases. These patterns are comparable with the reported hydronium jarosites obtained through bio-mineralization53 and hydrothermal synthesis51. The XRD peaks further confirm the crystallinity of the nanoparticles, in support of the previous SAED data.
Vibrational properties
In relation to the atomic vibrational properties of the bio-engineered sample, Fig. 4 displays the FTIR spectrum of the bio-engineered Hydronium jarosite sample. The spectrum has a broad stretching band in the region from 3000 to 3600 cm−1 ascribed to the ν(O‒H) vibration from the hydronium ions at 3390 cm−1. The vibrations at 1439 and 1392 cm−1 are attributed to ν(O‒H) whereas the doublets in the region 1000–1200 cm−1 at 1092 cm−1 and 1018 cm−1 are assigned to SO4 vibrations. The spectrum also shows metal coordination bands around 600 to 400 cm−1 which are assigned to the FeO6 coordination19,54. The band at around 1600 which, according to Plasil et al.10, is due to the bending modes of H2O and H3O+ that overlap, which is mainly a result of the OH and H3O interaction forming H2O. In a summary, the FTIR spectrum of the bio-engineered Hydronium jarosite sample exhibits the same stretching bands and vibrations as the various reported jarosite analogues55. This confirms that bio-engineered 2-D nanosheets are as a Hydronium jarosite analogue.
UV–VIS, photoluminescence
In relation to the optical properties of the bio-engineered 2-D hydronium jarosite sample, Fig. 5 reports the UV–VIS absorbance spectrum (a) and its corresponding Tauc plot (b) as well as the fluorescence spectrum (c), of the bio-engineered 2-D hydronium jarosite sample. The UV–VIS absorbance spectrum (Fig. 5a) displays a relatively broad absorption maxima in the UV region, with two shoulders centered at the vicinity of 242 and 287 nm which might be attributed to the charge transfer between valence and conduction bands56. Such an elevated UV-Bleu optical absorbance could be exploited for photocatalytic activity in waste water treatment. The absorbance data was used to estimate the optical band gap by employing the Tauc’s approximation57. The derived direct band gap energy is about ~ 3.4 eV. The Photoluminescence data displays several emissions with the maximum centered at the vicinity of 500 nm. While this latter is likely to be caused by surface oxygen deficiencies, the others in the near Infrared region (~ 750 nm, ~ 850 nm, ~ 770 nm, ~ 950 nm) could be ascribed to volume O or Fe defects.
Elemental chemical properties
Figure 6 displays a typical Energy Dispersive X-rays Spectroscopy spectrum of the bio-engineered 2-D hydronium jarosite sample. In addition to the Jarosite major elements of Fe, K and O, one can distinguish Phosphorus at energy channels centered at the vicinity of 2.1 keV. The presence of this contaminant is likely to originate from the avocado seed extract used as a chelating agent. Its small ionic radius is likely to favour their diffusion within the crystallographic open channels offered by the bio-engineered 2-D hydronium jarosite nanoparticles.
Magnetism properties
As established, the jarosite family provides an ideal model for Kagome antiferromagnets as schematically illustrated in Fig. 7a. The Fe3+ ions sit at the vertices of well-separated Kagome layers and behave as an S = 5/2 Heisenberg spins coupled through strong nearest-neighbour antiferromagnetic exchange (Curie–Weiss constants are typically ~ 700 K). Most members of the jarosite family show long-range magnetic order with the q = 0 spin configuration below 55 K as validated by Towsend et al.58,59. Likewise, it is to be noted that some jarosites show long-range magnetic order implies that further-neighbour interactions may be significant in these materials, stabilising a particular spin configuration relative to the others60. Elucidation of the response of hydronium jarosite to diamagnetic dilution may require a better understanding of further-neighbour exchange in the jarosite family, and of the influence this has on theoretical models. In addition, it has been reported that the jarosite phases that order magnetically at low temperatures tend to behave different from the known magnetic solids61. Grohol et al. found KGa3(SO4)2(OH)6 to be a diamagnetic analogue of KFe3(SO4)2(OH)6 jarosite62.
(a) Kagome lattice of anti-ferromagnetically coupled Heisenberg spins with the so-called q = 0 structure in Jarosites, (b) plot of zero field cool (ZFC) and field cool (FC) of the sample showing a blocking temperature of the sample, (c) inverse susceptibility plot versus temperature with the applied field at 1 T. (d) Magnetization plot of the sample at various temperatures and (e) zoomed in plots of the magnetization of the sample at various temperatures.
In relation to the magnetic properties of the bio-engineered 2-D hydronium jarosite sample it can be seen in Fig. 7b from the ZFC and FC plots that the two plots intersect at approximately ~ 274 K, which is the region of the blocking temperature of the system. In this region, the spins are random and magnetic domains in the material are not aligned due to thermal component. Figure 7c displays the magnetization curves (from − 5 to 5 T) of the sample measured at the temperatures 20 K, 40 K, 50 K, 100 K, 200 K and 300 K. Zooming in at the axis intercepts of the plots, coercivity, remnant field, and magnetic saturation can be measured as shown in Fig. 7d, which shows that the sample exhibit higher coercivity of 0.11 T at 300 K, with a low coercivity of 0.007 T at 20 K. The remnants field is higher at 50 K. The sample show some saturation magnetization at lower temperatures (20–100 K). While these rich magnetic results are preliminary, it is intended to perform similar studies with SQUID on a single thin and thick triangular sheets of the bio-engineered 2-D hydronium jarosite.
Electrochemical properties
In relation to the electrochemical properties of the bio-engineered sample, cyclic voltammetry is used63,64. Figure 8a displays typical voltammograms for bare GCE and deposition of the hydronium jarosite 2-D nanosheets at a scan rate of 50 mV/s and various scan rates from 20 to 100 mV/s. Based on these results, there is no peak observed for the unmodified electrode (bare GCE), with peaks and higher current density observed after the modified electrode with hydronium jarosite nanoparticles. The peaks observed at 0, 22 V and 5, 9 × 10–6 represent the strong anodic peak which corresponds to the oxidation process of Fe(II) ions to Fe(III). No clear reduction peak was observed with respect to the potential range, indicating that the oxidation process is pseudo-irreversible. The CV results are an indication that the material have a good electrochemical behaviour, therefore it is considered as a promising electro catalyst for electrochemical applications. The scan rate (v) effect was studied as shown in Fig. 8b, where the chosen v was 20, 40, 60, 80 and 100 mV/s. The performance was evaluated by observing the peak current in the anodic scan. The oxidation peak current increases linearly as a function of scan rate, suggesting a fast diffusion-controlled electrolyte ion transport kinetic at the interface of nanoparticles on the electrode. The oxidation peak potentials shift slightly to more positive values, which may be attributed to the fast faradaic redox reaction because of the good interaction between the conductive GC electrode and the electroactive hydronium jarosite in the alkaline electrolyte.
EIS was used to further investigate the electric conductivity and kinetics of the hydronium jarosite behaviour using the Nyquist and bode plot analysis. Figure 8c shows the Nyquist plot (imaginary Z″ VS real Zʹ) which is a flat semi-circle region observed at the high frequency region ascribed to the low charge transfer resistance Rct or high electric conductivity of the sample. The semi-circle continued by a straight line in a low frequency region, which is related to Warburg impedance or diffusive resistance between the electrode pores and the electrolyte ions. This could be indicating that the electrochemical performance is highly related to the interfacial charge transfer process and diffusion control65,66,67. Figure 8d provides information on the impedance and phase angle as a function of frequency. The bode analysis is plotted log frequency vs log imaginary Zʺ and phase angle (degrees). The phase angle in a plot shows the maximum angle at 79°, this resultant of a higher metallic conductivity of the sample. The results reveal good electronic conductivity as well as electrochemical stability of the hydronium jarosite nanomaterial.
Molecular dynamics computational simulation
The root mean square displacements (RMSDs) of phyto-active compounds describe when the analysed systems attain their equilibrium states during simulations. Their plots demonstrated that in simulations with drastically reduced RMSD fluctuations, all systems reach their equilibrium state before the duration of 10 ns. As a result, we used the molecular dynamics trajectories extracted after 10 ns for further analysis. The charge distribution and electrostatic potential map of eight active compounds (caffeic acid, chlorogenic acid, epicatechin, ferulic acid, flavone, neochlorogenic acid, procyanidin, and vanillic acid) in Fig. 9 illustrate the active sites of these molecules in their interactions with the surface of the nanoparticle. The scale bar at the top of this figure demonstrates regions of electron excess (reddish area) to regions of electron deficiency (bluish area) The Hirshfeld point charges and electrostatic potential maps for the optimized structure were obtained using DFT calculations with water as the solvent. The results indicate that the most prominent red areas in the charge distribution maps (Fig. 9) correspond to the active sites of molecules interacting with the nanoparticle. In this case, the atom O4 in caffeic acid, ferulic acid, procyanidin, and vanillic acid; the atom O2 in flavone; the atoms O3 and O4 in chlorogenic acid; the atoms O2 and O4 in epicatechin; and the atoms O6 and O7 in neochlorogenic acid have the highest affinity for interacting with the hydronium jarosite nanoparticle surface.
Charge distribution (Hirshfeld point charges) of eight phytoactive compounds and their electrostatic potential map for (a) Caffeic acid, (b) Epicatechin, (c) Chlorogenic acid, (d) Ferulic acid, (e) Flavone, (f) Neochlorogenic acid, (g) Procyanidin, and (h) Vanillic acid. The scale bar at the top of this figure demonstrates regions of electron excess (reddish area) to regions of electron deficiency (bluish area). The geometry optimization of active compounds was carried out at the B3LYP/6-311++g(d,p) level of theory.
The Radial Distribution Function (RDF), g(r), of molecules' active sites with respect to the surface of a spherical hydronium jarosite nanoparticle with a diameter of 2.5 nm is shown in Fig. 10. As illustrated, the g(r) has a maximum peak for the atoms O4 in caffeic acid, ferulic acid, procyanidin, vanillic acid, and chlorogenic acid; O2 in epicatechin and flavone; and O7 in neochlorogenic acid, which exhibit the highest affinity in comparison to other types of oxygen and carbon atoms, which agrees with DFT calculations (Fig. 9). Water solubility is a measure of the amount of active compounds that can dissolve in water at a specific temperature. LogS is directly proportional to an active compound's water solubility and is defined as a standard solubility unit equal to the 10-based logarithm of a molecule's solubility in mol/L. It is reported that logS values greater than − 1 indicate a highly polar molecule, implying higher hydrophilicity and a greater proclivity to interact with water68. According to empirical evidence, molecules with log S values between − 1 and − 5 exhibit hydrophilicity, lipophilicity, and aqueous solubility, which enable them to interact with hydrophobic surfaces68,69. This section determines logS values for water solubility using the ALOPGPS 2.1 software70,71. Flavone has the lowest calculated value of − 4.43, followed by procyanidin, epicatechin, ferulic acid, caffeic acid, neochlorogenic acid, chlorogenic acid, and vanillic acid, which have computed values of − 3.49, − 2.65, − 2.33, − 2.05, − 2.02, − 2.01 and − 1.47, respectively (see Table 1).
RDF plots for the active sites of (a) Caffeic acid, (b) Chlorogenic acid, (c) Epicatechin, (d) Ferulic acid, (e) Flavone, (f) Neochlorogenic acid, (g) Procyanidin, and (h) Vanillic acid with respect to the surface of hydronium jarosite nanoparticles. RDF plots of eight phytoactive compounds with respect to the surface of hydronium jarosite nanoparticles (i).
The RDF graphs for each phytoactive compound with respect to the surface of the nanoparticle are shown in Fig. 10i. As shown in Fig. 10i, flavone has the highest peak for g(r) and, consequently, the highest density near the nanoparticle’s surface at approximately 3 Å. In addition, as stated earlier, flavone has the lowest value for logS (− 4.43), indicating that it is the least hydrophilic among these active compounds. Together, these two parameters indicate that flavone has a greater affinity to interact with the surface of nanoparticles. Conversely, vanillic acid with the lowest g(r) peak and the highest logS (− 1.47) value has the least affinity to interact with the surface of the nanoparticle. Additionally, the interaction energies (Van der Waals plus electrostatic potential energies) shown in Table 2 reveal that the interaction energy between the active compounds and the nanoparticle is decreasing in the following order: hydronium jarosite nanoparticles—flavone > hydronium jarosite nanoparticles—procyanidin > hydronium jarosite nanoparticles—epicatechin > hydronium jarosite nanoparticles—ferulic acid > hydronium jarosite nanoparticles—caffeic acid > hydronium jarosite nanoparticles—neochlorogenic acid > hydronium jarosite nanoparticles—chlorogenic acid > hydronium jarosite nanoparticles—vanillic acid, while the interaction energy between active compounds and water is decreasing in the following order: water—vanillic acid > water—chlorogenic acid > water—neochlorogenic acid > water—caffeic acid > water—ferulic acid > water—epicatechin > water—procyanidin > water—flavone. The interaction energies (Table 2) and the RDF graphs in Fig. 8 corroborate the discussion on logS values, which indicate that vanillic acid is considerably more hydrophilic (meaning more tendency to interact with water than nanoparticle) than chlorogenic acid, neochlorogenic acid, caffeic acid, ferulic acid, epicatechin, procyanidin, and flavone, respectively.
As a preliminary pre-conclusion, one could mention the followings: (i) while the phenolic compound flavone originating from the avocado seed extracts seems driving the formation and the growth of the hydronium jarosite early clusters, (ii) as pointed out previously in section “Morphology and size distribution studies”, the 2-D sheets formation might be driven by an equivalent Frank–van der Merwe layer by layer growth. However, a model of the formation of a very thin precursor film (PF), usually a single molecular layer propagating ahead of the nominal contact line should be explored. This sound hypothesis which deserves to be explored has been demonstrated in several cases where wetting of 2 different fluids gives rise to such a thin precursor film as per the relatively recent investigations of Yuan et al. and Li et al.72,73.
Conclusion
Within this contribution, it is reported for the first time on the bio-engineering of 2-D triangular hydronium jarosite using natural extract of avocado as an effective chelating agent. The molecular dynamics computational modelling showed that the phenolic compound flavone originating from the avocado seed extract, has the highest affinity for interacting with the nanoparticle surface during the phyto-engineering of the hydronium jarosite nanoparticles and the reduction of iron (II) sulphate heptahydrate. XRD and morphological characterization showed that the synthesized hydronium jarosite was representative of crystalline rhombohedral hydronium jarosite in the form of triangular nanosheets decorated on the edges with nanoparticles of approximately 5.4 nm diameter, with EDX elemental profiling confirming the presence of potassium and phosphorus most likely originating from the avocado seed extract. The synthesized nanomaterial further showed diamagnetic behaviour with a high coercivity at a high temperature and low coercivity at low temperatures as well as good electrochemical and electroconductive behaviour, indicating that the phytoengineered hydronium jarosite nanomaterial may find unique utility for electrochemical and diamagnetic applications. Since it has been investigated and reported that the electrochemical and magnetic behaviour of the material is greatly influenced by its morphology, particle size, shape, crystallinity and optical properties74,75, future research should focus on using different plants and experimental parameters to optimize the morphological features as well as the electrochemical and magnetic behaviour of hydronium jarosite nanoparticles.
Data availability
In line with the journal’s policy and regulations, the datasets used and/or analysed during the current study is available from the corresponding author [Nandipha Botha] on reasonable request.
References
Baccolo, G. et al. Jarosite formation in deep Antarctic ice provides a window into acidic, water-limited weathering on Mars. Nat. Commun. 12, 436 (2021).
Farrand, W. H., Glotch, T. D., Rice, J. W. Jr., Hurowitz, J. A. & Swayze, G. A. Discovery of jarosite within the Mawrth Vallis region of Mars: Implications for the geologic history of the region. Icarus 204, 478–488 (2009).
Weitz, C. M., Noe Dobrera, E. & Wray, J. J. Mixtures of clays and sulfates, within deposits in western Melas Chasma, Mars. Icarus 251, 291–314 (2015).
Rampe, E. B. et al. Mineralogy of an ancient lacustrine mudstone succession, from the Murray formation, Gale crater, Mars. Earth Planet. Sci. Lett. 471, 172–185 (2017).
Elwood Madden, M., Bodnar, M. E. & Rimstidt, J. D. Jarosite as an indicator, of water-limited chemical weathering on Mars. Nature 431, 821–823 (2004).
Papike, J. J., Karner, J. M. & Shearer, C. K. Comparative planetary mineralogy: Implications of Martian and terrestrial jarosite. A crystal chemical perspective. Geochim. Cosmochim. Acta 70, 1309–1321 (2006).
Montagnat, M. et al. Measurements and numerical simulation of fabric evolution along the Talos Dome ice core, Antarctica. Earth Planet. Sci. Lett. 357–358, 168–178 (2012).
Delmonte, B. et al. Aeolian dust in the Talos Dome ice core (East Antarctica, Pacific/Ross Sea sector): Victoria Land versus remote sources over the last two climatic cycle. J. Quatern. Sci. 25, 1327–1337 (2010).
Bayliss, P., Kolitsch, U., Nickel, E. H. & Pring, A. Alunite supergroup: Recommended nomenclature. Mineral. Mag. 74, 919–927 (2010).
Plasil, J. et al. Hydroniumjarosite, (H3O)+ Fe3(SO4)2(OH)6, from Cerros Pintados, Chile: Single-crystal X-ray diffraction and vibrational spectroscopic study. Mineral. Mag. 78, 535–547 (2014).
Majzlan, J. et al. Thermodynamic properties, low-temperature heat-capacity anomalies and single crystal X-ray refinement of hydronium jarosite, (H3O)Fe3(SO4)2(OH)6. Phys. Chem. Minerals 31, 518531 (2004).
Eftekhari, N., Mohammad Kargar, M., Zamin, F. R., Rastakhiz, N. & Manafi, Z. A review on various aspects of jarosite and its utilization potentials. Ann. Chim. Sci. Matériaux 44(1), 43–52 (2020).
Gräfe, M., Beattie, D., Smith, E., Skinner, W. & Singh, B. Copper and arsenate co-sorption at the mineral-water interfaces of goethite and jarosite. J. Colloid Interface Sci. 322, 399–413 (2008).
Asta, M., Cama, J., Martinez, M. & Giménez, J. Arsenic removal by goethite and jarosite in acidic conditions and its environmental implications. J. Hazard. Mater. 171, 965–972 (2009).
Dutrizac, J. & Chen, T. The behaviour of phosphate during jarosite precipitation. Hydrometallurgy 102, 55–65 (2010).
Katsioti, M. et al. Utilization of jarosite/alunite residue for mortars restoration production. Matér. Constr. 43, 167–177 (2010).
Vu, H., Jandova, J. & Hron, T. Recovery of pigment-quality magnetite from jarosite precipitates. Hydrometallurgy 101, 1–6 (2010).
Ahamed, A. M. et al. New pathway for utilization of jarosite, an industrial waste of zinc hydrometallurgy. Miner. Eng. 170, 107030 (2021).
Xu, Z., Liang, J. & Zhou, L. Photo-Fenton-like degradation of azo dye methyl orange using synthetic ammonium and hydronium jarosite. J. Alloys Compd. 546, 112–118 (2013).
Xu, J. et al. Impregnation synthesis of TiO2/hydroniumjarosite composite with enhanced property in photocatalytic reduction of Cr(VI). Mater. Chem. Phys. 152, 4–8 (2015).
Jerry, M. et al. Characterization of jarosites produced by chemical synthesis over a temperature gradient from 2 to 40 °C. Int. J Miner. Process. 94, 121–128 (2010).
Jones, F. S., Bigham, J. M., Gramp, J. P. & Tuovin, O. H. Synthesis and properties of ternary (K, NH4, H3O)-jarosites precipitated from Acidithiobacillus ferrooxidans cultures in simulated bioleaching solutions. Mater. Sci. Eng. C 44, 391–399 (2014).
Ray, L. F., Rachael-Anne, W., Kloprogge, J. T. & Martens, W. N. Thermal decomposition of hydronium jarosite (H3O)Fe3(SO4)2(OH)6. J. Therm. Anal. Calorim. 83(1), 213–218 (2006).
Bigham, J. M. et al. Characterization of jarosites produced by chemical synthesis over a temperature gradient from 2 to 40 °C. Int. J Miner. Process. 94, 121–128 (2010).
Labib, Sh., Abdelaal, S., Abdelhady, A. M. & Elmaghraby, E. Preparation and characterization of jarosite nanorods synthesized by microwave hydrothermal method. Mater. Chem. Phys. 256, 123654 (2020).
Bhardwaj, B., Singh, P., Kumar, A., Kumar, S. & Budhwar, V. Eco-friendly greener synthesis of nanoparticles. Adv. Pharm. Bull. 10, 566–576 (2020).
Sackey, J. et al. Industrial textile removal using date pit assisted CuO–MgO nanocomposite: Molecular dynamics and biosynthesis analysis. J. King Saud Univ. Sci. 34, 101840 (2022).
Balciunaitien, A. et al. Eucalyptus globulus and Salvia officinalis extracts mediated green synthesis of silver nanoparticles and their application as an antioxidant and antimicrobial agent. Plants 11, 1085 (2022).
Morad, R. et al. First principle simulation of coated hydroxychloroquine on Ag, Au and Pt nanoparticles. Sci. Rep. 11, 1–9 (2021).
Akbari, M., Morad, R. & Maaza, M. First principle study of silver nanoparticle interactions with antimalarial drugs extracted from Artemisia annua plant. J. Nanopart. Res. 22, 1–9 (2020).
Sackey, J. et al. Molecular dynamics and bio-synthesis of phoenix dactylifera mediated Mn3O4 nanoparticles: Electrochemical application. J. Alloys Compd. 854, 156987 (2020).
Silva, G. G. et al. Phytochemicals of avocado residues as potential acetylcholinesterase inhibitors, antioxidants, and neuroprotective agents. Molecules 27, 1892 (2022).
Bhattacharjee, S., Habib, F., Darwish, N. & Shanableh, A. Iron sulfide nanoparticles prepared using date seed extract: Green synthesis, characterization and potential application for removal of ciprofloxacin and chromium. Powder Technol. 380, 219–228 (2021).
Hirel, P. Atomsk: A tool for manipulating and converting atomic data files. Comput. Phys. Commun. 197, 212–219 (2015).
Abraham, M. J., van der Spoel, D., Lindahl, E., Hess, B. & The GROMACS development team. GROMACS User Manual version 2019. http://www.gromacs.org (2019).
Huang, J. & MacKerell, A. D. Jr. Charmm36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 34, 2135–2145 (2013).
Jorgensen, W. L., Chandrasekhar, J. & Madura, J. D. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Adcock, S. A. & McCammon, J. A. Molecular dynamics: Survey of methods for simulating the activity of proteins. Chem. Rev. 106, 1589–1615 (2006).
Hess, B., Bekker, H. & Berendsen, H. J. C. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Essmann, U., Perera, L. & Berkowitz, M. L. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Humphrey, W., Dalke, A. & Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Vanommeslaeghe, K. & MacKerell, A. D. Jr. Automation of the CHARMM general force field (CGenFF) I. Bond Percept. Atom Typing 52, 12 (2012).
Frisch, M. J., Trucks, G. W. & Schlegel, H. B. Gaussian 09, Revision A.02 (Gaussian, Inc., 2009).
Ni, B. & Wang, X. Face the edges: Catalytic active sites of nanomaterials. Adv. Sci. (Weinh.) 2(7), 1500085 (2015).
Schneemeyer, L. F. Crystal growth. In Encyclopedia of Physical Science and Technology, 3rd ed. (Academic press Inc., London, 2003).
Hideaki Adachi, H. & Wasa, K. Thin films and nanomaterials. In Handbook of Sputtering Technology, 2nd ed. (Elsevier Inc., Amsterdam, 2012)
Lorenz, M. et al. Focus Issue: Jan van der Merwe: Epitaxy and the computer age. J. Mater. Res. 32(21), 3936–3946. https://doi.org/10.1557/jmr.2017.266 (2017).
Wang, M. J. et al. Matter 4 3339–3353 (Cell Press, 2021). https://doi.org/10.1016/j.matt.2021.08.017.
Yang, X. et al. Formation mechanism of a series of trigonal antiprismatic jarosite-type compounds. J. Crystal Growth 429, 49–55 (2015).
Majzlan, J. et al. Thermodynamic properties, low-temperature heat-capacity anomalies, and single-crystal X-ray refinement of hydronium jarosite, (H3O)Fe3(SO4)2(OH)6. Phys. Chem. Miner. 31, 518–531 (2004).
Spratt, H., Rintoul, L., Avdeev, M. & Martens, W. The thermal decomposition of hydronium jarosite and ammoniojarosite. J. Therm. Anal. Calorim. 115, 101–109 (2014).
Sklute, E. C. et al. Optical constants of synthetic potassium, sodium, and hydronium jarosite. Am. Mineral. 100, 1110–1122 (2015).
Oggerin, M. et al. Specific jarosite biomineralization by Purpureocillium lilacinum, an acidophilic fungi isolated from Río Tinto. Environ. Microbiol. 15(8), 2228–2237 (2013).
Aguilar-Carrillo, J., Villalobos, M., Pi-Puig, T., Escobar-Quiroz, I. N. & Romero, F. M. Synergistic arsenic (v) and lead (ii) retention on synthetic jarosite. I. Simultaneous structural incorporation behaviour and mechanism. Environ. Sci. Process. Impacts 20, 354–369 (2018).
Forray, F. L., Navrotsky, A., Hudson-Edwards, K. & A.,. Synthesis, characterization and thermochemistry of synthetic Pb–As, Pb–Cu and Pb–Zn jarosites. Geochim. Cosmochim. Acta 127, 107–119 (2014).
Bashir, A. K. H. et al. Investigation of electrochemical performance, optical and magnetic properties of NiFe2O4 nanoparticles prepared by a green chemistry method. Physica E Low-dimens. Syst. Nanostructures 119, 114002 (2020).
Rehman, S., Mumtaz, A. & Hasanain, S. K. Size effects on the magnetic and optical properties of CuO nanoparticles. J. Nanopart. Res. 13, 2497–2507 (2011).
Townsend, M. G., Longworth, G. & Roudaut, E. Theory of finite-temperature screening in a disordered two-dimensional electron gas. Phys. Rev. B 33, 49129 (1986).
Lee, S. H. et al. Long-range order induced by diamagnetic dilution of jarosites, model Kagomé antiferromagnets. Phys. Rev. B 56, 8091 (1997).
Harris, A. B., Kallin, C. & Berlinsky, A. J. Possible Néel orderings of the Kagomé antiferromagnet. Phys. Rev. B 45, 2899 (1992).
Majzlan, J. et al. Heat capacity, entropy, and magnetic properties of jarosite-group compounds. Phys. Chem. Miner. 37, 635–651 (2010).
Grohol, D. et al. Spin chirality on a two-dimensional frustrated lattice. Nat. Mater. 4, 323–328 (2005).
Arteaga, J. F. et al. Comparison of the simple cyclic voltammetry (CV) and DPPH assays for the determination of antioxidant capacity of active principles. Molecules 17, 5126–5138 (2012).
Harnisch, F. & Freguia, S. ChemInform Abstract: A basic tutorial on cyclic voltammetry for the investigation of electroactive microbial biofilms. Chem. Asian J. 7, 466–475 (2012).
Fuku, X., Kaviyarasu, K., Matinise, N. & Maaza, M. Punicalagin green functionalized Cu/Cu2O/ZnO/CuO nanocomposite for potential electrochemical transducer and catalyst. Nanoscale Res. Lett. 11, 1–12 (2016).
Dodson, J. J., Neal, L. M. & Hagelin-Weaver, H. E. The influence of ZnO, CeO2 and ZrO2 on nanoparticle-oxide-supported palladium oxide catalysts for the oxidative coupling of 4-methylpyridine. J. Mol. Catal. A Chem. 341, 42–50 (2011).
Suo, Z., Dong, X. & Liu, H. Single-crystal-like NiO colloidal nanocrystal-aggregated. J. Solid State Chem. 206, 1–8 (2013).
Jorgensena, W. L. & Duffy, E. M. Prediction of drug solubility from structure. Adv. Drug Deliv. Rev. 54, 355–366 (2002).
Stefani, R. Computational study of natural phenolic acid solubility and their interactions with chitosan. https://doi.org/10.13140/RG.2.1.2055.9123 (2016).
Tetko, I. V. et al. Virtual computational chemistry laboratory—design and description. J. Comput. Aid. Mol. Des. 19, 453–463 (2005).
VCCLAB. Virtual Computational Chemistry Laboratory. http://www.vcclab.org (2005).
Yuan, Q. & Zhao, Y.-P. Precursor film in dynamic wetting, electrowetting, and electro-elasto-capillarity. Phys. Rev. Lett. 104, 246101 (2010).
Li, P., Xianfu, H. & Zhao, Y.-P. Active control of electro-visco-fingering in HeleShaw cells using Maxwell stress. iScience 25, 105204. https://doi.org/10.1016/j.isci.2022.105204 (2022).
Seevakana, K., Manikandan, A., Devendranc, P., Baykald, A. & Alagesan, T. Electrochemical and magneto-optical properties of cobalt molybdate nanocatalyst as high-performance supercapacitor. Ceram. Int. 44, 17735–17742 (2018).
Paulose, R., Mohan, R. & Parihar, V. Nanostructured nickel oxide and its electrochemical behaviour—A brief review. Nano-Structures Nano-Objects 11, 102–111 (2017).
Acknowledgements
The authors acknowledge the Centre for High Performance Computing, South Africa, for providing computational resources and facilities for this research. The University of South Africa and iThemba labs (NRF) Cape Town, the Abdus Salam International Centre for Theoretical Physics (Abdus Salam-ICTP), the International Organization for Women in Science (OWSD).
Funding
This study was funded by UNISA and UNESCO.
Author information
Authors and Affiliations
Contributions
For the manuscript entitled: “Computationally informed phytoengineering of nanoscale hydronium jarosite with electrocatalytic, electroconductive, and diamagnetic properties” by N.L.B., G.G.W., K.J.C., M.A., R.M., C.K., N.M., R.B., and M.M. Contributions are as follows: N.L.B. and K.J.C. performed the lab work and wrote the manuscript, M.A. and R.M. performed the computational studies, C.K. performed the magnetism studies, N.M. performed the electro chemistry investigation, R.B. and S.A. completed the XRD experiments, and M.M. supervised the work and was in charge of the acquisition of funding and resources.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Botha, N.L., Cloete, K.J., Welegergs, G.G. et al. Physical properties of computationally informed phyto-engineered 2-D nanoscaled hydronium jarosite. Sci Rep 13, 2442 (2023). https://doi.org/10.1038/s41598-022-25723-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25723-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.