Abstract
In this study, the main motivation of this work is desalination of water for irrigation arid area such as Sidri- Baba basins- south Sinai, Egypt. Also, the novelty of this work is modification of TFC surface membrane by mix of HA, DA and GO to get high performance of FO technique. Interfacial polymerization was employed to modify a thin-film composite (TFC) membrane for forward osmosis (FO) applications; moreover, graphene oxide (GO) nanosheets (GONs), a dopamine solution (DA), and naturally accessible humic acid (HA) were modified on a polyethersulfone (PES) substrate. The effects of the different quantities of GO, HA, and DA on the membrane surfaces, as well as their various cross-sectional morphologies and FO-desalination capabilities, were investigated. The integrated TFC membrane containing appropriate GO, HA, and DA blends outperformed the control membrane, obtaining high water flux, and high salt rejection. Furthermore,.
Similar content being viewed by others
Introduction
Desalination is the extraction of salts from seawater or brackish water to produce drinking water for humans and animals, as well as clean water for irrigation and industrial purposes1. Forward osmosis (FO) technology has recently piqued the interests of scholars and industries. This technology exploits the difference in the osmotic pressures of a low-concentration feed solution (FS) and a high-concentration draw solution (DS) to transport water molecules between semipermeable membrane2. FO technology exhibits several advantages, such as reduced operating energy input3, more efficient contaminant rejection4,5, and reduced likelihood of membrane fouling, over reverse osmosis (RO)6,7.
Consequently, increased efforts have been invested in recent years to optimize and improve the permeability–selectivity of membranes. Increased water permeability at constant salt rejection or decreased salt permeability at constant water permeability could increase the permeability selectivity8. A previous study demonstrated that a substrate with a large pore size benefited the synthesis of thinner polyamide (PA) layers. Thus, many hydrophilic materials, such as sulfonated polyphenylene sulfone, have been developed9, and graphene oxide (GO)10,11, carbon nanotubes12,13, titanium dioxide nanoparticles, etc., have been added to the substrate. By incorporating hydrophilic elements into the substrate and enhancing the porous structure, the water resistance of such a substrate can be enhanced. To improve the water flux of the membrane14, added GO into the formulation of the ultrafiltration polyethersulfone (PES) membrane.
These GO membranes increased the thermal stability and mechanical qualities of the PES membranes. For the interfacial polymerization of PA15, studied a PES–GO-based substrate. Thin-film-composite (TFC) FO membranes were layered. Moreover16, confirmed that the incorporation of GO can improve water permeability. Furthermore17, synthesized FO membranes by adding only 76 ppm GO to the aqueous phase during interfacial polymerization (IP)18. proved that the GO-incorporated TFC membranes exhibited improved surface hydrophilicity and smoothness, as well as thin PA layer thickness, because of the affected diffusion rate of MPD monomers and increased aqueous phase solutions viscosity with the incorporation of GO nano-sheets. Compared with the control membrane, the desalination performance of the optimal membrane was significantly improved whereas only 0.0100 wt% GO nano-sheets was applied in this method. When 2.0 M NaCl was used as the draw solution, the water flux of GO-3 membrane increased 56.97% in AL-FS mode and 42.48% in AL-DS mode, at the same time, the SRSF of the membrane decreased indicated the selectivity of the membrane improved, which was due to the charge effect and two-dimensional capillary effect of GO nano-sheets made it easier for water molecules transfer and more effective for salt ions rejection19. they present novel semi-permeable graphene-based membranes.
Composite filters were designed and fabricated on polysulfone porous scaffolding using combinations of polycrystalline large-area High Strength Metallurgical Graphene (HSMG), graphene oxide, hydrazine and an in-situ interfacial polymerized polyamide. The prepared composites were proved to be semi-permeable membranes with great ions blocking efficiency (over 95%) and water flux only one order of magnitude lower than the commercial reverse osmosis membranes. The experiments’ results demonstrated that the solutions proposed in this work indicate that graphene-based membranes can be used in water treatment technology20. suggested that surface hydrophilicity, pore size of membranes and oil–water separation performances was greatly affected by membrane shape. Owing to many advantages of HF membranes, this type of membrane has great potential for commercial applications.
Humic compounds contain polymeric carbon polymers with oxygen functional groups, e.g., hydroxyl and carboxylic acids, ketone, and quinone groups that are naturally available21,22. Moreover, humic acid (HA), which is a macromolecular material, exhibits a high dispersion capacity. Additionally, HA is an environmentally friendly material23,24 revealed that HA can improve the pore size distribution of PES membranes, thereby increasing their permeability to pure water. Moreover, studies have demonstrated that the introduction of HA into GO enhanced the molecular diffusivity of water and the permeability of the GO membrane25.
Humic substances, natural and easily accessed polymeric carbon materials, are composed by a skeleton of aromatic units, which are cross-linked by oxygen-containing functional groups such as hydroxyl, carboxylic, ketone and quinone groups26,27. Compared with other carbon materials like graphene oxide or carbon nanotube, humic acid (HA) is prevalent in environments and much cheaper. Besides, HA, as a macromolecular substance, exhibits great dispersion capacity with polyethersulfone in the casting solution. In addition, HA itself is an environmentally friendly material28. In our previous work, we surprisingly found that HA could alter pore size distribution and then substantially improve pure water permeability of polyvinylidene fluoride membrane after doping it into the substrate29. Moreover, it has been proven that the introduction of HA into graphene oxide enhanced the water molecular diffusivity and permeability of graphene oxide membrane23. Inspired by these findings, we hypothesized that HA would be a candidate in improving support layer performance and further strengthening the permeability of FO membrane.
Also, a recently developed technique to impart a hydrophilic character onto microfiltration, ultrafiltration, and forward osmosis membrane selective layers for enhanced fouling resistance to oil/water emulsions and protein mixtures was reported by30,31,32,33 using polydopamine (PDA). PDA is a polymer with a chemistry similar to the adhesive secretions of mussels34,35,36 It is formed from the spontaneous polymerization of dopamine in an alkaline aqueous solution. A subsequent study by Arena examined the first use of PDA modified membranes for osmotically driven membrane process. This was done through the application of PDA to TFC membrane support layer(s). Significant improvements in the water flux of PDA modified TFC FO membranes were observed37. Others, such as12. adopted this technique prior to synthesis of the membrane.
Furthermore, the chemistry of dopamine (DA) or polydopamine (PDA) offers a new path toward producing high-performance membranes38. designed a novel method for performing surface modification with DA and polyethylene (PE) to significantly enhance the permeability of the membranes. Employing the IP technique, single DA in an aqueous solution successfully increased the structural and chemical stabilities of the membrane39. Recently40, fabricated three types of PDA-modified substrates (coated on top, bottom, and dual-surface substrates) before synthesizing the TFC membranes41. prepared TFC-FO membranes on the top surface of PDA-modified PES substrates, and the thickness of the PA layer decreased while the salt rejection increased on the membrane substrates at a short PDA coating time.
Lu et al.42 reported that the constructed novel membrane exhibited improved water flux, following the addition of DA into a 1,3-metaphenylenediamine (MPD) solution, although it exhibited increased reverse salt flux and PA layer thickness. The thickness of the layer increased compared with that of the control membrane. Thus, the impact of the different DA concentrations of the aqueous phase that was paired with MPD on the properties and performance of the FO membrane must be investigated.
In this study, different concentrations of GO and HA were added to a PES substrate, and different concentrations of DA were added into MPD to improve the permeability–selectivity properties of a TFC-FO membrane. Further, the best concentrations of GO, HA, and DA were compared. Thereafter, membranes were modified by combining GO, HA, and DA. Thus, this study revealed an effective and ecologically acceptable substance, which could be doped into the TFC-FO membrane substrate, to alter the physicochemical properties of the membrane and improve its permeability–selectivity characteristics.
Experimental
Materials
PES was supplied by BASF Co., Germany. Polyvinylpyrrolidone (PVP), hexane, and 1,3,5-benzenetricarbonyl trichloride (TMC) with a purity of > 98% dispersed in hexane (98%; Aldrich, China) were supplied by Aladdin Co., China. N, N-dimethylacetamide (DMA) and 1,3-phenylenediamine were obtained from Sigma Co., China (MPD; 99%). HA was purchased from (Sigma-Aldrich Co., USA), and DA hydrochloride was obtained from Sigma-Aldrich Co., USA (International Laboratory, USA; 99%). Researchers have employed graphite (Sigma-Aldrich, USA), NaNO3 (Beijing Chemical Factory, China), H2O2, H2SO4 (Beijing Chemical Factory, China), and KMnO4 (Sigma-Aldrich, USA) to produce GO nanosheets (GONs), after which sodium hypochlorite was employed to assess the chlorine resistance of the membrane (Sigma Co., Egypt). For the FO experiment, sodium chloride (NaCl) was dissolved in deionized water (DI), which was prepared by a Milli-Q system (ACS reagent, Beijing Chemical Factory, China) (Millipore, USA). Sodium alginate, (KH2PO4), magnesium chloride (MgCl2), sodium hydrogen carbonate (NaHCO3), calcium chloride (CaCl2), and ammonium chloride (NH4Cl) (Chemical Supply Pty Ltd, China) were employed for the membrane fouling tests.
Methods
Preparation of GONs
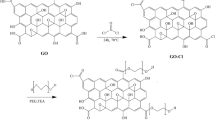
GONs were synthesized from graphite powders via the classical Hummer method43,44,45. Briefly, 1.0 g of graphite and 0.5 g of NaNO3 were added into a flask containing 23.0 mL of a concentrated H2SO4 solution and stirred in a cold water bath. Afterward, 3.0 g of KMnO4 was added gradually for 2.0 h before the mixture was transferred into a 35.0 °C water bath and stirred for 0.5 h. Thereafter, 46.0 mL of DI water was added gradually, and the reactant was kept for 0.5 h at a temperature of 98.0 °C. Finally, 140.0 mL of the DI water was added to the mixture and stirred. Also, the resulting mixture was heated for 1 h at 95 °C without allowing it to boil. Elsewhere, the heater was switched off, and the mixture was allowed to cool by adding 500 mL of DI water with stirring for 1 h. Next, 40.0 mL of an H2O2 solution was added to the mixture to terminate the reaction. To obtain the final product, the mixture was filtered and rinsed three times with DI water, after which the as-prepared GONs were washed three times with DI and methanol, successively, to remove the residues. Finally, the powders were baked for 12 h at 60 °C.
Preparation of the PES substrate
To prepare the casting solution from PES, a mixed solvent system containing PVP (5 g) and DMA (75 g) was prepared by ultrasonication for 10 min, and different concentrations of HA (0.1, 0.3, 0.5, 0.8, and 1 wt.%) and GO (0.1, 0.25, 0.5, 1, and 1.5 wt.%) were added. Employing ultrasonication, each compound was added to the solution for 30 min at room temperature. Thereafter, the solutions were added with stirring for 30 min. Next, 25 g of PES was added into the solution with stirring for 24 h and placed on a stand where it was kept overnight. Afterward, the solution was placed on a glass plate and distributed onto the films. After 30 min, the membranes were stored in a DI water bath at 4 °C.
Preparation of the TFC membranes
The TFC membranes were prepared by mixing 2.0 wt. % MPD and different concentrations of DA (0.0, 0.01, 0.05, 0.3, 0.5, and 0.8 wt. %). The PES substrate was immersed in the prepared solution for 30 min to ensure the complete soaking and adequate self-polymerization of DA (PDA). Thereafter, the substrate was dipped in a TMC solution (0.15 wt.% in hexane) for 1 min . Next, the substrate was removed from the hexane natural section and set vertically for 2 min to evaporate the residual solution. Afterward, the as-prepared substrate was vacuum dried for 5 min at 70 °C. Finally, the obtained composite membranes were washed and stored in DI water (4 °C) for the subsequent experiments.
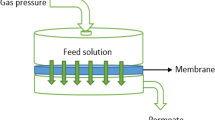
FO performance tests
The FO membrane comprising a strong membrane (42 cm2) was utilized. The temperatures of the feed and draw solutions were set at 25 °C. The membranes were examined in the FO mode (i.e., the active layer (AL) was passed through the FS). To compare the overall FO performance of the thin-film membranes, 2 M NaCl and DI water were utilized as the draw and feed solutions, respectively. The concentration of the extracted salt in DS was measured. Computer software was employed to record the mass of the permeated water (m) into the DS. FO was allowed to proceed for ~ 2 h to ensure precise measurements. The obtained values were averaged from the calculated data that were collected after 10 min of operation and replicated Three times. The FO flux (Jw, Lm−2 h−1) was calculated employing the following equation, wherein Q is the amount of permeate accumulated in “L,” A is the high area of the surface of the membrane in “m2,” and ∆t is the sampling time.
The salt rejection that was retained in the membrane (R, %) was calculated, as follows:
where Cd is the salt concentration of the DS after a given time, as obtained via the widespread curve technique employing a conductivity meter and Vd is the volume of the DS. Cf and Vf are the initial concentration and volume of the FS, respectively.
Antiorganic fouling performance evaluation
The anti-fouling performance of the FO membrane was evaluated by adopting sodium alginate as module pollutant. The feed solution was composed of 250 mg L − 1 sodium alginate, 0.45 mM KH2PO4, 9.2 mM NaCl, 0.61 mM MgCl2 Because it is binary salts., 0.5 mM NaHCO3, 0.5 mM CaCl2 and 0.93 mM NH4Cl46. 2 M NaCl was selected as the draw solution. The cumulative volume of draw solution was recorded every 2 min by a digital balance connected to a computer via a Hyperterminal Software. The entire anti-fouling evaluation process lasted 600 min.
Characterizations of the synthesized membranes
The membranes were characterized by Fourier-transform infrared (FTIR), scanning electron microscopy (SEM), and contact angle measurements. A Genesis Unicam spectrophotometer was employed for the FTIR test. The contact angle was measured by a Cam-Micro contact angle meter (Tantec Inc.) to estimate the hydrophilicity of the membrane. A water droplet (DI water) was dropped onto the surface of the air-dried membrane at 25 °C employing a numerical micro-syringe. The water contact corresponding to a median of five measurements was acquired for every membrane pattern at selected points.
FTIR analysis
Attenuated total reflectance–FTIR (ATR–FTIR) spectroscopy was performed to determine the performance of the membrane. The ATR–FTIR results of the PES substrate and TFC membranes are shown in Fig. 1. The absorption bands of PA at 1577 and 1650 cm−1, which corresponded to the aromatic ring relaxation, namely the amide I and II bands, respectively were detected in all the TFC membranes, indicating the successful fabrication of the thin-film layer via IP47,48,49. Moreover, the intensities of the signal of the hydroxyl groups at 2100–3600 cm−1 were further observed in all of the modified TFC membranes owing to the stretching vibrations of O–H and N–H in PDA, which resulted in the generation of additional hydrophilic membrane surfaces.
Ramírez et al.50 Reported that the HA-changed PES substrate exhibited a new peak at 1486 cm−1, and this could be ascribed to the deformation of O–H and C = O from alcoholic and phenolic –OH or –COO uneven stretching of HA51. Moreover, compared with the substrate, AL exhibited a high peak, which could be ascribed to the C–N stretching in amide II, at 1577 cm−1, indicating the formation of an amide. A new peak at 1610 cm−1 originated from the relaxation of the aromatic ring (PA)52. According to53, the maxima of GONs at 3400 and 1322 cm−1 were due to O–H stretching and deformation, respectively. The C = O stretching vibrations within the carboxyl group of GO were evident around 1653 cm−1, while the peak at 1011 cm−1 was caused by the C–C stretching of the epoxy and alkoxy groups. The peaks around 1669 and 1486 cm−1 were attributed to the C = O stretching of carbonyls and the deformation of the C–H bond, respectively. The peaks at 1322 and 1242 cm−1 corresponded to the stretching vibration of the C–H bond. The strongest band around 1072 cm−1 indicated the ether C–O–C functional group; concurrently, the bands at 872 and 1105 cm−1 indicated the modes of saccharide54. Moreover, the band revealed a large spectrum at 1577 cm−1 that was associated with the carbonyl (C = O) stretching vibration of the molecules in the membrane structure55. The peak corresponded to hydroxyl group was detected in HA, GO and DA samples in Fig. 1 but less visible in HA + GO + DA sample this is due to accumulation between HA, GO and DA.
Contact angle of the FO membranes
The contact angles of the control, HA/TFC; GO/TFC; DA/TFC; and HA, GO, DA/TFC-modified membranes are shown in Fig. 2. The contact angle is the most significant component for determining the hydrophilicity or hydrophobicity of membrane surfaces. Generally, a small contact angle (0° < θ < 90°) corresponds to the high hydrophilicity of the membrane, while a higher contact angle (90° < θ < 180°) corresponds to its high hydrophobicity. Figure 2 shows that the average contact angles of the control, HA/TFC, GO/TFC, DA/TFC, and HA, GO, DA/TFC-modified membranes were 69° ± 2°, 53.6° ± 1.2°, 57° ± 1.2°, 64.3° ± 1.6°, and 35.9° ± 6°, respectively, demonstrating that the membranes exhibited hydrophilic surfaces. The presence of the OH and COOH groups was due to the high hydrophilicity of the membrane. The DA/TFC-modified membrane exhibited a lower contact angle around 64° at 25 °C, signifying that its hydrophilic surfaces improved with the incorporation of DA into the polymer casting mixture. The increased hydrophilicity of the DA-modified TFC membrane could be attributed to the stronger attraction of the H2O molecules in DA.
Following the addition of DA, the contact angle was reduced, thereby increasing the surface energy of the membrane. This can allow the easy movement of H2O over the surface of the membrane to enhance the capacity of the hydrophilic pores to absorb H2O through their capillary properties. When 0.25 wt. GO/TFC membrane was added, the contact angle was reduced to 57°, increasing the hydrophilicity of the membrane by incorporating GO mainly as a pore-generating agent. Moreover, the contact angle was further reduced to 53°, following the incorporation of the TFC membrane with HA. These findings corroborated previous research on improving the hydrophilicity of PES and TFC films via the incorporation of GO and HA56. The incorporation of hydrophilic GO and HA slightly increased the hydrophilization of the membrane, as observed by a decrease in the contact angle. Further, the incorporation of the hydroxyl, carboxylate, and epoxy moieties would enhance the hydrophilicity of the HA, GO, and DA/TFC-modified membrane.
Analysis of the morphology of the membranes
SEM analysis was performed to examine the forms and dispersions of HA, DA, and GONs within the composite membranes. Figures 3a–f show the cross-sectional SEM images of the PES, control, HA/TFC, DA/TFC, GO/TFC, and HA + DA + GO/TFC-modified membranes, respectively.The prepared membranes exhibited an asymmetric structure comprising a porous support/sublayer with cellular morphologies and a thin dense skin layer. The membranes exhibited straight finger-like microvoids, which expanded and were bent toward the center and further elongated until they reached the bottom of the membrane, upon GO, HA, and DA incorporations. Further, the cellular pores were broadened, and the thin walls interacted. The microvoid structures of the composite GO, HA, and DA membranes expanded and the pores increased, following the addition of the hydrophilic materials owing to the instantaneous demixing of the membrane material in the solvent. Further, the thin layer of the composite PES membranes became thinner than that of the control PES membrane. The transportation of the water molecules across the membrane via narrow interspaces enhanced it significantly. Moreover, besides improving the permeation flux, a very hydrophilic membrane could also minimize fouling on the membrane surface57.
Results and discussions
Membrane desalination performance
Optimum conditions of the TFC control
Figure 4 shows the water fluxes of the FO membranes when different doses of MPD (0.5, 1, 2, and 3 g/100 mL) and TMC (0.05, 0.1, 0.15, and 0.2 g/100 mL) were utilized. Additionally, different times for preparing MPD (1, 2, and 3 min) and TMC (0.5, 1, and 2 min), as well as different membrane thicknesses (30, 35, and 40 µ) were exploited to obtain the optimum condition for preparing the control membrane. The DS and FS modes employed 2.0 M NaCl and DI water as DS and FS, respectively. The water flows of the optimum TFC membrane were recorded at 2 g/100 mL MPD; the TMC concentration was 0.15 g/100 mL, and the MPD and TMC preparation times were 2 and 1 min, respectively.
Water fluxes of the TFC membranes in the AL-FS mode: (a) different concentrations of MPD (0.5, 1, 2, and 3 g/100 mL), (b) different preparation times of MPD (1, 2, and 3 min), (c) different concentration of TMC (0.05, 0.1, 0.15, and 0.2 g/100 mL), (d) different preparation times of TMC (0.5, 1, and 2 min) and (e) different thicknesses of the membrane (30, 35, and 40 µ).
Effect of the different concentration of HA
The pure water permeability of the TFC membrane is shown in Fig. 5. Compared with the control membrane substrate, the HA-modified membrane substrate exhibited essentially pure water flux. It was observed that employing 0.3 wt% HA achieved the best result. The water flux increased to ~ 27.26 L/m2.h, while the salt rejection reached 82%. This result is consistent with the findings that large macrovoids58, which rendered the membrane more hydrophilic, were formed after HA doping (Fig. 6). Based on these observations, we believed that the addition of HA to the mixture reduced the hydrophilic resistance and improved the permeability of the substrate. Moreover, additional COOH functional groups were added to the PA layer to achieve a more hydrophilic surface .
Effect of the different concentrations of GONs
Figure 6 depicts the water flows of the FO membranes that were doped with different concentrations of GONs employing 2.0 M NaCl as DS. It was observed that as the concentration of GONs increased, the water fluxes increased initially before eventually reaching an ideal value (0.25 wt%). Thus, the ideal concentration of GONs was set at 0.25 wt%. The water flux increased by ~ 16.43 L/m2.h, while the salt rejection reached 89% probably because of the improvement of the hydrophilic characteristics of GO-incorporated membranes (the higher the hydrophilic characteristics of the membranes, the easier it is for water molecules to move through them)59,60,61. Furthermore, as the thickness of the PA AL of the GO-incorporated membrane reduced, the FO water flux increased. When the concentration of GONs increased dramatically, the membrane water flux decreased.
Effects of the different concentrations of DA:
Figure 7 shows the different self-polymerizations of DA in the FO mode using DI water and 2 M NaCl as FS and DS, respectively. It was observed that 0.3 wt% is the ideal concentration for the reaction. The water flux increased to ~ 13.21 L/m2.h, while the salt rejection reached 86%. Since a thin PA layer enhanced the hydrophilicity of the surface of the membrane, the water flux increased at a low degree of DA self-polymerization in the aqueous solution62.
Effect of a mix of the GO, HA, and DA membrane
Membrane desalination performance
Figure 8 shows the water fluxes of the FO membranes that were doped with a mix of the optimum concentrations of GONs, HA, and DA. Comparing the water fluxes of the HA, GO, DA, and mixed membranes, it was discovered that the mixed membrane achieved the best results. The water flux increased to ~ 33.21 L/m2.h, while the salt rejection reached 98%. Improved water flux via mixed HA,DA,GO membrane may be owing to enhancing the hydrophilicity and resaons negatively charged reactive group on to the membrane surfaces63. After ceratine time , the mixed membrane content is aggregated which subsequently leads to some pores of the membrane surfaces that could be plugged possessing to the mixed membrane aggregation resulting in water flux decay64. It is concluded that membrane structure and morphology are greatest possibly diverse where the higher hydrophilic CA membrane may cause a further swollen and flexible skin layer, thus the membrane permeability is high.Thus, the mixed membrane was the best because of its high porosity and hydrophilicity.
Evaluation of the antiorganic fouling performance of the membrane
The membrane with mix of HA, DA and GO exhibited superiority in permeability-selectivity trade-off, as analyzed above. On the other hand, the anti-fouling ability of FO is another crucial aspect for its practical application. Thus, SA was selected as a model polysaccharide to examine the flux variations under 2 M NaCl draw solution in FO mode. As shown in Figs. 9, the flux exhibited a sharp decline from 0 to 200 min and a relative stable flux profile. Similar phenomenon was reported in previous studies3,38,65. The sharp decrease in flux in the starting time could be ascribed to the bridging and gel forming of sodium alginate (SA) by Ca2+ ions. Besides, the initial high permeation drag force also contributed to the sharp decline of flux, which promoted substantial hydraulic resistance and thus a severe flux decline. After 200 min, slow flux decline was observed, suggesting that further accumulation of SA became insignificant. This result could be explained by the classical “critical flux concept”, which suggests that the fouling will become negligible once reaching critical flux58. At the end of fouling experiments, the water flux of mix HA, DA and GO-doped membrane exhibited a slower water flux decline ratio than that of the others membranes, indicating that the mix HA, DA and GO -modified membrane exhibited a higher anti-organic fouling performance. Previous studies have demonstrated a more permeable support layer would transport more water across the membrane surface and induce a membrane surface with a less fouling propensity66,67. The mix HA, DA and GO -modified support layer exhibited higher water permeability and smoother surface, leading to a higher resistance to organic foulant. Therefore, a higher anti-fouling performance could be realized after modifying FO membrane with mix HA, DA and GO membrane.
Conclusion
In this work, we demonstrate that, after blending mix of GO, HA, and DA into the PES substrate of FO membrane, the porous structure and surface chemistry of membrane were altered, and then the pure water flux of substrate was further improved. The pore structure of the membrane with mix of GO, HA, and DA doped had a higher porosity than that of the others membranes. More OH functional groups were introduced onto the polyamide layer by embedding with mix of GO, HA, and DA, leading to a more hydrophilic surface. These results endowed the membrane a better permeability-selectivity property. In addition, the anti-fouling performance for the mix of GO, HA, and DA -modified membrane was superior over the others membranes. Furthermore, the performance of the mix of GO, HA, and DA doped membrane in this work was comparable to those commercial membranes in terms of permeability and selectivity. Thereby, our results suggest that mix of GO, HA, and DA could act as an efficient and cost-effective additive and would be used for membrane fabrication.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Change history
28 February 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-023-30298-4
References
Ali, M. E. A., Wang, L., Wang, X. & Feng, X. Thin film composite membranes embedded with graphene oxide for water desalination. Desalination 386, 67–76. https://doi.org/10.1016/j.desal.02.034 (2016).
Amir, S., Hafidi, M., Merlina, G., Hamdi, H. & Revel, J. C. Elemental analysis, FTIR and 13C-NMR of humic acids from sewage sludge composting. Agronomie 24, 13–18. https://doi.org/10.1051/agro:2003054 (2004).
Ang, W. S., Tiraferri, A., Chen, K. L. & Elimelech, M. Fouling and cleaning of RO membranes fouled by mixtures of organic foulants simulating wastewater effluent. J. Membr. Sci. 376, 196–206. https://doi.org/10.1016/j.memsci.2011.04.020 (2011).
Cath, T. Y., Childress, A. E. & Elimelech, M. Forward osmosis: Principles, applications, and recent developments: Review. J. Membr. Sci. 281, 70–87. https://doi.org/10.1016/j.memsci.2006.05.048 (2006).
Chang, X. et al. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly(vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl. Surf. Sci. 316, 537–548. https://doi.org/10.1016/j.apsusc.2014.07.202 (2014).
Emadzadeh, D. W. et al. Synthesis, modification and optimization of titanate nanotubespolyamide thin film nanocomposite (TFN) membrane for forward osmosis (FO) application. Chem. Eng. J. 281, 243–251. https://doi.org/10.1016/j.cej.2015.06.035 (2015).
Emadzadeh, D., Lau, W. J., Matsuura, T., Ismail, A. F. & Rahbari-Sisakht, M. Synthesis and characterization of thin film nanocomposite forward osmosis membrane with hydrophilic nanocomposite support to reduce internal concentration polarization. J. Membr. Sci. 449, 74–85. https://doi.org/10.1016/j.memsci.2013.08.014 (2014).
FAO, In J. Bruinsma (Ed.), World Agriculture, Towards 2015/2030—An FAO Perspective, Earthscan Publications Ltd., (2003) London.
Ghaseminezhad, S. M., Barikani, M. & Salehirad, M. Development of graphene oxide-cellulose acetate nanocomposite reverse osmosis membrane for seawater desalination. Compos. B Eng. 161, 320–327. https://doi.org/10.1016/j.compositesb.2018.10.079 (2019).
Ghosh, A. K. & Hoek, E. V. M. Impacts of support membrane structure and chemistry on polyamide–polysulfone interfacial composite membranes. J. Membr. Sci. 336, 140–148. https://doi.org/10.1016/J.memesci.2009.03.024 (2009).
Guan, Y. F., Huang, B. C. C., Wang, L. F. & Yu, H. Q. Improved PVDF membrane performance by doping extracellular polymeric substances of activated sludge. Water Res. 113, 89–96. https://doi.org/10.1016/j.watres.2017.01.057 (2017).
Han, G., Zhang, S., Li, X., Widjojo, N. & Chung, T. Thin film composite forward osmosis membranes based on polydopamine modified polysulfone substrates with enhancements in both water flux and salt rejection. Chem. Eng. Sci. 80, 219–231. https://doi.org/10.1016/j.ces.2012.05.033 (2012).
He, L. et al. Promoted water transport across graphene oxide–poly(amide) thin film composite membranes and their antibacterial activity. Desalination 365, 126–135. https://doi.org/10.1016/j.desal.02.032 (2015).
Hirata, M., Gotou, T., Horiuchi, S., Fujiwara, M. & Ohba, M. Thin-film particles of graphite oxide. Carbon 42, 2929–2937. https://doi.org/10.1016/j.carbon.2004.07.003 (2004).
Huang, G. et al. One-step green hydrothermal synthesis of few-layer graphene oxide from humic acid. Nanomaterials 8, 215 (2018).
Huang, Y., Jin, H., Li, H., Yu, P. & Luo, Y. Synthesis and characterization of a polyamide thin film composite membrane based on a polydopamine coated support layer for forward osmosis. RSC Adv. 5, 106113–106121. https://doi.org/10.1039/C5RA20499B (2015).
Huang, Y. H. J., Yu, H., Li, P. & Luo, Y. Synthesis and characterization of a polyamide thin film composite membrane based on a polydopamine coated support layer for forward osmosis. RSC Adv. 5, 106113–106121. https://doi.org/10.1039/C5RA20499B (2015).
Zhanguo, L. et al. Graphene oxide incorporated forward osmosis membranes with enhanced desalination performance and chlorine resistance. Front. Chem. https://doi.org/10.3389/fchem.2019.00877 (2020).
Grzegorz, R., Konrad, D., Agata, J., Piotr, K. & Tomasz, K. Synthesis and characterization of semi-permeable graphene/graphene oxide membranes for water desalination. J. Mater. Sci. 55, 9775–9786 (2020).
Nurul, F. D. et al. Fabrication and characterization of graphene oxide–polyethersulfone (GO–PES) composite flat sheet and hollow fiber membranes for oil–water separation. J. Chem. Technol. Biotechnol. 95, 1308–1320 (2020).
Hummers, W. S. J. & Offeman, R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 13–39. https://doi.org/10.1021/ja01539a017 (1958).
Jing, H. Y. et al. Efficient adsorption photodegradation of organic pollutants from aqueous systems using Cu2O nanocrystals as a novel integrated photocatalytic adsorbent. J. Mater. Chem. 2, 14563–14570. https://doi.org/10.1039/C4TA02459A (2014).
Konch, T. J. et al. Nanofluidic transport through humic acid modified graphene oxide nanochannels. Mater. Chem. Front. 2, 1647–1654. https://doi.org/10.1039/C8QM00272J (2018).
Kong, F., Yang, H., Wang, X. & Xie, Y. F. Rejection of nine haloacetic acids and coupled reverse draw solute permeation in forward osmosis. Desalination 341, 1–9. https://doi.org/10.1016/j.desal.2014.02.019 (2014).
Kyong, H. S., Myoung, J. P., Sherub, P., Tao, H., Grace, M. N., Leonard, D. T., XueMei, L., Gang, C. and Wook, J. C. Graphene oxide incorporated polysulfone substrate for the fabrication of flat-sheet thin-film composite forward osmosis membranes.
Huang, G. et al. One-step green hydrothermal synthesis of few-layer graphene oxide from humic acid. Nanomaterials 8, 215 (2018).
Zhou, T. et al. Efficient separation of watersoluble humic acid using (3-aminopropyl) triethoxysilane (APTES) for carbon resource recovery from wastewater. ACS Sustain. Chem. Eng. 6, 5981–5989 (2018).
Jing-G, G., Xiao-L, G., Wei-W, W., Xin, Z. & Wu-Li, K. An ultrafast water transport forward osmosis membrane: Porous graphene. J. Mater. Chem. 2, 4023–4028 (2014).
Guan, Y. F., Huang, B. C., Qian, C., Wang, L. F. & Yu, H. Q. Improved PVDF membrane performance by doping extracellular polymeric substances of activated sludge. Water Res. 113, 89–96 (2017).
McCloskey, B. D. et al. (2010) Influence of polydopamine deposition conditions on purewater flux and foulant adhesion resistance of reverse osmosis, ultrafiltration, andmicrofiltration membranes. Polymer 51, 3472–3485 (2010).
McCloskey, B. D. et al. A bioinspired fouling-resistant surface modification for water purification membranes. J. Membr. Sci. 413–414, 82–90 (2012).
Miller, D. J. et al. Short-term adhesion and long-term biofouling testing of polydopamine and poly (ethylene glycol) surface modifications of membranes and feed spacers for biofouling control. Water Res. 46, 3737–3753 (2012).
Kasemset, S., Lee, A., Miller, D. J., Freeman, B. D. & Sharma, M. M. Effect of polydopamine deposition conditions on fouling resistance, physical properties, and permeation properties of reverse osmosis membranes in oil/water separation. J. Membr. Sci. 425–426, 208–216 (2013).
Lee, H., Dellatore, S. M., Miller, W. M. & Messersmith, P. B. Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430 (2007).
Lee, H. et al. Substrate-independent layer-by-layer assembly by using mussel-adhesive-inspired polymers. Adv. Mater. 20, 1619–1623 (2008).
Lee, H., Scherer, N. F. & Messersmith, P. B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. U. S. A. 103, 12999–13003 (2006).
Arena, J. T., McCloskey, B. D., Freeman, B. D. & McCutcheon, J. R. Surface modification of thin film composite membrane support layers with polydopamine: enabling use of reverse osmosis membranes in pressure retarded osmosis. J. Membr. Sci. 375, 55–62 (2011).
Lee, S. & Elimelech, M. Relating organic fouling of reverse osmosis membranes to intermolecular adhesion forces. Environ. Sci. Technol. 40, 980–987. https://doi.org/10.1021/es051825h (2006).
Yu, L. et al. Preparation and characterization of HPEI-GO/PES ultrafiltration membrane with antifouling and antibacterial properties. J. Membr. Sci. 447, 452–462. https://doi.org/10.1016/j.memsci.2013.07.042 (2013).
Liu, X., Wu, J., Hou, L. A. & Wang, J. Performance and deterioration of forward osmosis membrane exposed to various dose of gamma-ray irradiation. Ann. Nucl. Energy 135, 106950. https://doi.org/10.1016/j.anucene.2019.106950 (2020).
Lu, P. et al. Layered double hydroxide nanoparticle modified forward osmosis membranes via polydopamine immobilization with significantly enhanced chlorine and fouling resistance. Desalination 421, 99–109. https://doi.org/10.1016/j.desal.2017.04.030 (2017).
Lu, X., Romero-Vargas, C. S., Shaffer, D. L., Ma, J. & Elimelech, M. In situ surface chemical modification of thin-film composite forward osmosis membranes for enhanced organic fouling resistance. Environ. Sci. Technol. 47, 12219–12228. https://doi.org/10.1021/es403179m (2013).
Ma, N., Wei, J., Liao, R. & Tang, C. Y. Zeolite-polyamide thin film nanocomposite membranes: Towards enhanced performance for forward osmosis. J. Membr. Sci. 405–406, 149–157. https://doi.org/10.1016/j.memsci.2012.03.002 (2012).
Marcovecchio, M. G., Mussati, S. F., Aguirre, P. A. & Nicolás, J. Optimization of hybrid desalination processes including multi stage flash and reverse osmosis systems. Desalination 182, 111–122. https://doi.org/10.1016/j.desal.2005.03.011 (2005).
Ma, D., Peh, S. B., Han, G. & Chen, S. B. Thin-film nanocomposite (TFN) membranes incorporated with super-hydrophilic metal-organic framework (MOF) UiO-66: Toward enhancement of water flux and salt rejection. ACS Appl. Mater. Interfaces 9, 7523–7534. https://doi.org/10.1021/acsami.6b14223 (2017).
Mazlan, N. M., Peshev, D. & Livingston, A. G. Energy consumption for desalination a comparison of forward osmosis with reverse osmosis, and the potential for perfect membranes. Desalination 377, 138–151. https://doi.org/10.1016/j.desal.08.011 (2016).
Park, M. J. et al. Graphene oxide incorporated polysulfone, substrate for the fabrication of flat-sheet thinfilm composite forward osmosis membranes. J. Memb. Sci. 493, 496–507. https://doi.org/10.1016/j.memsci.2015.06.053 (2015).
Park, S. & Ruoff, R. S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 4, 217–224. https://doi.org/10.1038/nnano.2009.58 (2009).
Qing, L., Jingguo, L., Zhengzhong, Z., Jianping, X. & Jim, Y. L. Hydrophilic mineral coating of membrane substrate for reducing internal concentration polarization (ICP) in forward osmosis. Sci. Rep. 6, 19593. https://doi.org/10.1038/srep19593 (2016).
Ramírez, J. F., Rubio, E., Rodríguez, L. V. & Castaño, V. Purification of polluted waters by funtionalized membranes. Rev. Adv. Mater. Sci. 21, 211–216 (2009).
Roy, A. The role of fertilizers in food production. In: R. Lal, B.A. Stewart (Eds.) Food Security and Soil Quality, CRC Press. (2010).
Salehi, H., Rastgar, M. & Shakeri, A. Anti-fouling and high water permeable forward osmosis membrane fabricated via layer by layer assembly of chitosan/graphene oxide. Appl. Surf. Sci. 413, 99–108. https://doi.org/10.1016/j.apsusc.2017.03.271 (2017).
Shaffer, D., Werber, J. R., Jaramillo, H., Lin, S. & Elimelech, M. Forward osmosis: Where are we now?. Desalination 356, 271–284. https://doi.org/10.1016/j.desal.2014.10.031 (2015).
She, Q., Wong, Y. K. W., Zhao, S. & Tang, C. Y. Organic fouling in pressure retarded osmosis: experiments, mechanisms and implications. J. Memb. Sci. 428, 181–189. https://doi.org/10.1016/j.memsci.2012.10.045 (2013).
Shi, M. et al. A novel pathway for high performance RO membrane: Preparing active layer with decreased thickness and enhanced compactness by incorporating tannic acid into the support. J. Memb. Sci. 555, 157–168. https://doi.org/10.1016/j.memsci.2018.03.025 (2018).
Singh, P. S. et al. Probing the structural variations of thin film composite RO membranes obtained by coating polyamide over polysulfone membranes of different pore dimensions. J. Membr. Sci. 278, 19–25 (2006).
Song, X., Wang, L., Mao, L. & Wang, Z. Nanocomposite membrane with different carbon nanotubes location for nanofiltration and forward osmosis applications. ACS Sustain. Chem. Eng. 4, 2990–2997. https://doi.org/10.1021/acssuschemeng.5b01575 (2016).
Tang, C. Y., She, Q., Lay, W. C. L., Wang, R. & Fane, A. G. Coupled effects of internal concentration polarization and fouling on flux behavior of forward osmosis membranes during humic acid filtration. J. Membr. Sci. 354, 123–133 (2010).
Wang, Y. et al. Dopamine incorporated forward osmosis membranes with high structural stability and chlorine resistance. Processes 6, 1–12 (2018).
Wang, Y. et al. Dopamine incorporating forward osmosis membranes with enhanced selectivity and antifouling properties. Water industry and environment engineering technology research centre, 401311, Chongqing, China. RSC Adv. 8, 22469–22481. https://doi.org/10.1039/C8RA03166E (2018).
Wang, Y. X., Cheng, C., He, Y., Pan, J. & Xu, T. Second interfacial polymerization on polyamide surface using aliphatic diamine with improved performance of TFC FO membranes. J. Membr. Sci. 498, 30–38. https://doi.org/10.1016/j.memsci.2015.09.067 (2016).
Werber, J. R., Deshmukh, A. & Elimelech, M. The critical need for increased selectivity not increased water permeability, for desalination membranes. Environ. Sci. Technol. Lett. 3, 112–120. https://doi.org/10.1021/acs.estlett.6b00050 (2016).
Xi, Z. Y., Xu, Y. Y., Zhu, L.-P., Wang, Y. & Zhu, B. K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly (DOPA) and poly (dopamine). J. Membr. Sci. 327, 244–253. https://doi.org/10.1016/j.memsci.2008.11.037 (2009).
Xu, W., Chen, Q. & Ge, Q. Recent advances in forward osmosis (FO) membrane: Chemical modifications on membranes for FO processes. Desalination 419, 101–116. https://doi.org/10.1016/J.DESAL.2017.06.007 (2017).
Xie, Z., Nagaraja, N., Skillman, L., Li, D. & Ho, G. Comparison of polysaccharide fouling in forward osmosis and reverse osmosis separations. Desalination 402, 174–184 (2017).
Ramon, G. Z. & Hoek, E. M. Transport through composite membranes, part 2: Impacts of roughness. J. mater. Chem. A5, 12183–12192 (2013).
Lu, X., Ariaschavez, L. H., Romero-Vargas castrillon, S., Ma, J. & Elimelech, M. Influence of active layer and support layer surface structures on organic fouling propensity of thin film composite forward osmosis membranes. Environ. Sci. Technol. 49, 1436–1444 (2015).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
F.M.E.-S. (collection data and writting research). M.E.A.A. (Experimental). H.I. (figures). M.M.A.A. (result writing), M.M.S.A.E.-F. (desscution of results).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41598-023-30298-4
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sayed, F.M., E.A.Ali, M., Isawi, H. et al. RETRACTED ARTICLE: Surface modification of thin film composite forward osmosis membrane using graphene nanosheets for water desalination. Sci Rep 12, 21234 (2022). https://doi.org/10.1038/s41598-022-25700-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25700-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.