Abstract
Enclosure and grazing can significantly change the turnover of nitrogen in grassland soil. Changes of soil nitrogen mineralization and ammonium-oxidizing microorganisms caused by enclosure in different grazing intensities (about 30 years of grazing history) grassland, however, has rarely been reported. We selected the grassland sites with high and medium grazing intensity (HG and MG, 4 and 2 sheep ha−1, respectively) and had them enclosed (45 × 55 m) in 2005 while outside the enclosure was continuously grazed year-round. A two factorial study was designed: grazing intensity (MG and HG sites) and enclosure (fence and non-fence). Nitrogen mineralization was detected through a laboratory incubation experiment. The abundance and community structure of soil ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) were analyzed using quantitative PCR (q-PCR), terminal-restriction fragment length polymorphism (T-RFLP), cloning, and sequencing. Results showed that compared with MG site, at HG site the AOB abundance and community structure of AOB changed significantly while the AOA abundance and community structure did not change obviously. Enclosure significantly decreased the cumulative mineralized N, N mineralization rate, the abundance of AOB and the AOB community structure at the HG site, while at MG site, enclosure did not change these parameters. Potential nitrification rate (PNR) was positively correlated with the abundance of AOA and AOB at the MG and HG sites, respectively. The abundance of AOA was significantly correlated with soil pH; however, AOB abundance was significantly correlated with soil available N, total N, C/N ratio, pH, etc. The phylogenetic analysis showed that Nitrososphaeraceae and Nitrosomonadaceae were the dominant AOA and AOB, respectively. Totally, the responses of AOB and AOA mainly were associated to changes in soil physicochemical properties caused by different intensity grazing; AOB and AOA may be the dominant functional players in ammonia oxidation processes at HG and MG site, respectively.
Similar content being viewed by others
Introduction
With important ecological functions and economic value, inner Mongolia grassland is one of the largest and well-preserved natural grasslands in the world. At present, overgrazing has altered the nutrient cycle of the grassland ecosystem, resulting in nutrient loss, grassland degradation, and lead to variations in both microbial diversity and the potential functioning of micro‑organisms1,2,3. As an important means of restoring degraded grassland, enclosures have been widely used to prevent over-exploitation4,5.
Nitrogen is widely considered as the principal growth-limiting nutrient for plant and microorganisms in soil6. The change of land use patterns significantly affects the nitrogen cycling and the availability of nitrogen in grassland7, further affecting the productivity level and function of grassland ecosystems. Soil N mineralization, as an important ecological process, determines soil nitrogen supply capacity8,9. Previous studies have detected the influences of grazing on soil mineralizable N (Nmin) and have found that grazing can stimulate the accumulation of Nmin in typical grasslands of Inner Mongolia10, and moderate grazing results in the largest accumulation of available N11. Synthesis data from multiple studies on grazed Northern Great Plains ecosystems also showed that grazing enhanced soil Nmin12; however, the influence of enclosures on Nmin is uncertain. Enclosures significantly increased soil Nmin in temperate grassland of Hulunbeir, Inner Mongolia and Kobresia alpine grassland, Tibet13,14; however; it was found that enclosures nearly had no significant effect on soil Nmin in the semi-arid steppe in central Argentina15. These differences of the findings could be attributed to dissimilar grassland types, soil characteristics, or microbial effects.
Nitrification plays an important role in the nitrogen cycle, which determines the effectiveness of nitrogen on plants16,17,18. Ammonia oxidation is the rate limiting step of nitrification process, which was considered to be performed by ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB)16. It was found that the change of grassland use pattern had different effects on the abundance and community structure of ammonia oxidizing microorganisms and then affected the nitrogen cycle19,20. In typical grasslands of Inner Mongolia, AOA varied with the change of grassland management (grazing and mowing), while abundance and community structure of AOB remained unchanged under different grassland management19. However, in the desert steppe of Inner Mongolia, grazing did not affect the abundance of AOA or community structure of AOB but significantly reduced the abundance of AOB as well as affected the community structure of AOA14. Many studies on the effects of grazing and enclosures on soil Nmin and microorganisms have been carried out and the findings vary due to the differences in environmental factors. However, there are few studies on the effects of enclosure in different grazing intensities sites (long grazing history for about 30 years) in soil Nmin and ammonia-oxidizing microorganisms in the typical grasslands of Inner Mongolia.
This study mainly focused on the influences of the enclosure in different grazing intensities (moderate grazing (MG) and heavy grazing (HG)) sites on soil Nmin and ammonia-oxidizing microorganisms. The results of study are of great significance in understanding the nitrogen turnover mechanism in grassland with enclosure management in different grazing intensity sites.
Materials and methods
Description of site
The study was conducted at the Inner Mongolia Grassland Ecosystem Research Station (IMGERS) in the Xilin River Basin (43°26′–44°29′ N, 115°32′–117°12′ E), Inner Mongolia, China. The average annual rainfall is 343 mm, and 60–80% of precipitation occurs from May to late August21. The mean annual temperature is 0.7 °C, with the highest monthly average of 19.0 °C in July, and the lowest monthly average of −21 °C in January. We selected two representative sites, which differed in stocking rate over the past 30 year (from 1970s): (a) moderately grazed (MG, 2 sheep ha−1) and (b) heavily grazed (HG, 4 sheep ha−1). In this area of Inner Mongolia, these grazing patterns are typical. Soil physicochemical properties of the two sites were shown in Table 1, and soil is calcic chernozems.
Experimental design
At the beginning of May 2005, a 45 × 55 m area was enclosed at both the MG and HG sites. Outside the enclosure, the grassland was grazed continuously all year (4 and 2 sheep ha−1, respectively). Therefore, a two factorial experiment was designed in this study: grazing patterns (MG and HG sites) and enclosure (fence and non-fence), with four replicates. At the end of August, vegetation in all enclosure plots was cut to 5 cm in height and removed from the site.
Soil sampling for N mineralisation incubation experiment
To minimize the effect on experimental plots, we only extracted undisturbed soil samples in the four enclosure plots and the four random points outside the enclosure at both the MG and HG sites. PVC cylinders (17 cm in length, 5 cm in diameter), with one side sharpened, were hammered 15 cm into the soil to extract undisturbed soil cores for the incubation experiment. Twelve soil cores (6 incubation periods × 2 water levels) were taken in each subplot. A total of 192 soil cores (2 fence levels × 2 sites × 4 replications × 6 incubation periods × 2 water levels) were taken for incubation. The PVC cores were covered with the Parafilm to reduce water loss but allow gas exchange during transport. These soil cores were incubated at a constant temperature of 25 ℃ and soil moisture of 5 and 10 g H2O/100 g in the laboratory with four replications22. Constant soil moisture was maintained by adding water based on the weighing method every 3 days. The six soil cores from the same subplot were randomly assigned to one of six different incubation periods of 3, 7, 14, 28, 56, 112 days. After incubation, the soil cores were sieved (2 mm mesh) and extracted with 0.01 M CaCl2, and then the extracts were used to analyze ammonium (NH4+-N) and nitrate (NO3– N) through Continuous Flow Analysis (Auto Analyzer 3, Nordstadt, Germany).
Soil sampling for microorganism and potential nitrification rate (PNR) measurement
Soil samples for microorganism measurement were taken from the same plots as the soil N mineralization incubation experiment (total 16 sample: 2 fence levels × 2 sites × 4 replications) in 2014. Five drills samples with diameters of 2 cm were randomly sampled from 0 to 15 cm of soil at each sampling subplots, and five drills were mixed into one sample and passed through 2 mm sieves. Stones, plant residue, and roots in soil samples were removed. The soil sample was selected using the ‘quartering method (the process of mixing the sample evenly and then dividing the sample by a ratio of 2/4 is called quartering)’, and fresh soil samples were stored at 4 °C during transportation. Each fresh soil sample was separated into three sub-samples: one sub-sample was preserved at 4 °C for determine nitrification potential within 48 h; one sub-sample was stored at −80 °C for microbial analysis within a week; the third sub-sample was air-dried to measure soil physicochemical properties within a week.
Soil physicochemical properties analysis
Air-dried soils were sieved (0.25 mm mesh) before physicochemical properties were measured. The soil pH (soil:water = 1:5) was measured using a conductivity meter (Thermo Orion, United States). The soil organic carbon (SOC) and total nitrogen (TN) were measured using an elemental analyzer (Elementar, Germany). Total phosphorus (TP) was determined colourimetrically after wet digestion with H2SO4 and HClO4. The soil physicochemical properties are showed in Table 1.
Soil potential nitrification rate (PNR)
The chlorate inhibition method23 was used for measuring PNR. For the assay, 5 g of fresh soil was added to 50 mL centrifuge tubes, which containing 20 mL of phosphate buffer solution (PBS) (g·L−1: NaCl, 8.0; KCl, 0.2; Na2HPO4, 0.2; NaH2PO4, 0.2; pH 7.4) with a final concentration of 1 mM (NH4)2SO4. KClO3 10 mM was added for inhibiting nitrite oxidation. The soil slurry was incubated at 25 °C for 24 h, and then nitrite was extracted with 5 mL of 2 M KCl, and shaken extracts were measured using a continuous flow analyzer.
DNA extraction and purification
DNA was extracted using the WelPrep DNA kit (Welgene Biotech Co., Ltd) according to the manufacturer’s instructions24. The extracted DNA was quantified using a UV–Vis Spectrophotometer (ND-1000, NanoDrop, USA).
Quantitative PCR (q-PCR) for amoA gene
The AOA and AOB amoA gene copy numbers were quantified by q-PCR using a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Primers and thermal cycling conditions used for q-PCR are listed in Table S1. Product specificity was checked through melting curve analysis and agarose gel electrophoresis. The standard curves were established using amoA gene fragments cloned into a plasmid pGEM-T Easy Vector (3015 bp, Promega Madison, USA). Positive clones were extracted using a Plasmid Mini Kit (Qiagen Nordic). The AOA and AOB amoA gene copy numbers were calculated from the concentrations of the corresponding extracted plasmid DNA with concentrations ranging from 1.732 × 101 copies µL−1 to 1.732 × 108 copies µL−1 and 2.384 × 101 copies µL−1 to 2.384 × 106 copies µL−1, respectively. The efficiency of q-PCR was 102.3% (r2 = 0.992) for AOA and 105.2% (r2 = 0.995) for AOB.
PCR amplification and T-RFLP (terminal restriction fragment length polymorphism) analysis of amoA gene
The T-RFLP method was used to evaluate the effects of grazing and enclosure on ammonia-oxidizing microbial community structure, and the PCR amplification was performed using the same primer pairs as the q-PCR assays (Table S1), with the forward primer labeled with 6-carboxyfluorescein. The 25 μl PCR reactions included 2.5 μl 10 × PCR buffer (Mg2+ plus), 2 μl 2.5 mM dNTPs, 0.25 μl Ex Taq HS polymerase (5 U μl−1, Takara Biotechnology, Dalian, China), 0.5 μl of each primer, and 2 μl diluted DNA template. PCR products were gel-purified using PCR Clean-Up System (Promega, USA). The PCR products were digested with the restriction enzyme FastDigest® Mbo I (Takara Biotechnology, China) at 37 °C for 5 min and subsequently at 65 °C for 15 min. The digested products were purified by ethanol precipitation and mixed with deionised formamide, and then determined with an ABI PRISM 3130XL Genetic Analyzer (Applied Biosystems).
Phylogenetic analysis
The amoA genes of AOA and AOB were constructed from both the HG and MG sites in the fenced treatment soil by using the same primer pairs as q-PCR. PCR was performed by mixing DNA from three duplicate soil samples in each treatment. The PCR products were purified and ligated into the pGEM-T Easy Vector (Promega, Madison, WI). Plasmids were transformed into Escherichia coli JM109 (Takara Biotechnology Company, China). 166 and 100 positive clones from each treatment were selected for amoA genes of archaeal and bacterial, respectively, and were sequenced by an ABI 3730 sequencer (Applied Biosystem, USA). The operational taxonomic unit (OTU) defined at 96% similarity was estimated using Clustal X (1.83). A representative sequence of each OTU and the related sequences obtained from the NCBI database were used for constructing the phylogenetic tree with MEGA 5.0 using the neighbour-joining method.
Calculations and statistical analysis
The cumulative mineralized soil N (Nmin) and Soil net N mineralization rates (Rmin) were calculated according to the following formulas:
where ti and ti+1 are the beginning and end dates of each incubation period, respectively. NH4+-Ni, NO3−-Ni and NH4+-Ni+1, NO3−-Ni+1 were the concentrations of soil NH4+-N and NO3–N in the initial and incubated samples, respectively25.
All statistical analyses were performed using SPSS Version 17.0 for Windows (SPSS Inc., Chicago, Illinois). The significance of treatment effects and their interactions on the observed parameters were detected through a three-way analysis of variance (ANOVA). Quantitative differences between treatments were examined using a least-significant difference (LSD) test. Figures were generated using the Origin 8.0 package (Origin Lab Corporation, USA).
Ethical approval
All authors declared that they had no known competing financial interests or personal relationships that seemed to affect the work reported in this article. All authors followed the ethical responsibilities of this journal.
Results
Soil properties
Heavy grazing significantly decreased SOC, TN, N/P, and water content but significantly increased soil pH and C/N compared with those at the MG site (Table 1). Long-term enclosure did not significantly change soil pH, SOC, TP, C/N, and water content but significantly increased soil N/P at both sites (Table 1). Long-term enclosure significantly increased soil TN at the MG site, while it significantly decreased the soil NO3- -N concentration at the HG site (Table 1).
Cumulative soil mineralized N (Nmin) and N mineralization rate (Rate_Nmin)
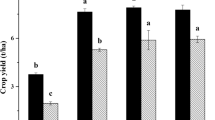
Compared with non-fenced treatment, the fenced treatment did not significantly change soil Nmin and Rate_Nmin at the MG site (Fig. 1a–d). Under the 5% soil water content level, enclosure significantly decreased the soil Nmin and Rate_Nmin on days 14 and 112 for the HG site, respectively (Fig. 1a,e); under the 10% soil water content level, the enclosure decreased the soil Nmin significantly and obviously decreased Rate_Nmin on days 14–56 for the HG site (Fig. 1b,f). The soil Nmin at the HG site was significantly higher than that at the MG site in non-fenced treatment under a 5% soil moisture level; however, the soil Nmin was not obviously different between the MG and HG sites in fenced treatments (Fig. 1a). The Nmin at the HG site was significantly higher than that at the MG site after 28 d in the non-fenced treatment; meanwhile, in the fenced treatment, the Nmin was significantly lower at the HG site than at the MG site on days 7–56 under a 10% soil moisture level (Fig. 1b).
Soil cumulative inorganic N and net N mineralization rate (mean ± SE, n = 4) as affected by sites with different grazing intensity (MG and HG) and enclosure under different soil water contents (5% and 10%). MG moderately grazed site, HG heavily grazed site, NF non-fenced, F fenced treatment. ANOVA results are available in Table S2. Enclosure effects at a given incubation time are indicated above the data as: ***p < 0.001, **p < 0.01, *p < 0.05.
Quantification of ammonia-oxidizing microorganisms
High copy numbers of the amoA gene of AOA (approximately 2.5 × 108 copies kg−1 dry soil) were detected in both the MG and HG sites, and they were two orders of magnitude greater than that of AOB in the two sites (Fig. 2a,b). For AOA, the amoA gene of AOA was not obviously different between the MG and HG sites in both fenced and non-fenced treatments (Fig. 2a). For AOB, compare with no-fence treatment, amoA gene copy numbers of AOB decreased significantly in fence treatment at the HG site but not obviously changed at MG site. The amoA gene copy numbers of AOB were significantly higher at the HG site than that at the MG site in both fenced and non-fenced treatments (Fig. 2b).
Effects of grazing intensity and enclosure on the abundance of AOA (a) and AOB (b) in soil. MG moderately grazed site, HG heavily grazed site, NF non-fenced, F fenced treatment. Different lowercase letters indicate differences between NF and F treatment at the MG and HG sites, respectively (p < 0.05, n = 4). Different capital letters indicate differences between the MG and HG sites across NF and F treatment (p < 0.05, n = 8).
Correlation analysis between the AOA and AOB abundance and soil physicochemical properties and PNR
A correlation analysis between abundance of AOA and AOB and soil physicochemical properties and PNR was used to identify the environmental variables which significantly control the abundance of AOA and AOB (Table 2). AOA was significantly and negatively correlated with pH (R = −0.52, p < 0.05). AOB was significantly and positively correlated with pH (R = 0.67, p < 0.01), NO3–N (R = 0.77, p < 0.01), NH4+-N + NO3–N (R = 0.65, p < 0.01), and C/N ratio (R = 0.52, p < 0.05) but significantly negatively correlated with TN (R = −0.53, p < 0.05) and N/P ratio (R = −0.58, p < 0.05).
PNR was positively correlated with the AOA and AOB amoA gene copy numbers at the MG and HG sites, respectively (Fig. 3a,d).
T-RFLP combined with cloning and sequencing for ammonia-oxidizing microorganisms
T-RFLP analyses of AOA showed more T-RFs (seven to eight fragments) than that of AOB (six fragments) (Fig. 4). In both fenced and non-fenced treatments, the fragments of 558 bp and 448 bp of AOA significantly increased; however, the 74 bp fragment of AOA significantly decreased at the HG site compared with those at MG site (Fig. 4a,b; Table S3). The 154 bp fragments of AOB also significantly decreased at the HG site compared with those in both non-fenced and fenced treatments at the MG site (Fig. 4c,d; Table S4). At the MG site, the T-RFLP patterns of AOA and AOB showed almost no variation between fenced and non-fenced treatment (Fig. 4a,c). At the HG site, the T-RFLP patterns of AOA showed no significant variation between fenced and non-fenced treatment (Fig. 4b), however, the fragments of 256 bp of AOB decreased significantly in fenced treatment compared with those in non-fenced treatment (Fig. 4d).
A total of 166 and 100 positive clones of amoA gene of AOA and AOB were randomly selected and sequenced, respectively (Figs. 5, 6). Based on deduced amoA amino acid sequences and reference sequences obtained from GenBank database, the neighbour-joining trees were constructed. Three clusters of the amoA gene of AOA, and AOB obtained from trees. Fifteen and ten OTUs were detected in AOA and AOB sequences, respectively. All AOA sequences in Cluster 1 had the highest similarity (95%-100%) with the amoA gene of AOA sequences obtained from uncultured crenarchaeote or thaumarchaeote (Fig. 5); Almost all the sequences in Cluster 2 belonged to Nitrososphaeraceae. There were fifteen and twenty-one sequences of the AOA amoA gene in Cluster 3, which belonged to Nitrosopumilaceae. The sequences of the amoA gene of AOB obtained in treated soil were affiliated with Nitrosospira and Nitrosomonas species, and most of the sequences were grouped into Nitrosospira species (Fig. 6).
Discussion
Effects of grazing intensity and long-term enclosure on Nmin
Our results indicated that the soil Nmin and Rate_Nmin at the HG site were higher than that at the MG site in typical grasslands of Inner Mongolia (Fig. 1, Table S2). It indicated that grazing promoted nitrogen mineralization and accelerated soil nitrogen turnover, such as alpine meadow in eastern Qinghai-Tibetan plateau in China, semi-arid grasslands on the Loess Plateau in China, and the Northern Great Plains of North America12,21,26.
Enclosures are an important measure for restoring degraded grassland because it affects soil fertility19,27. Our results indicated that after 10 years enclosure, the soil Nmin did not significantly change at the MG site; however, the Nmin and Rate_Nmin significantly decreased at the HG site (Fig. 1). According to Pan et al.1,2, ~ 70 to 90% of the N returned to the grassland soils via animal excreta. In addition, numerous studies found that the stability of soil aggregates would be improved after grazing stopped, thus increasing soil microbial immobilization of available nitrogen10,28. Therefore, reduces in animal excreta and microbial changes may explain the decrease of Nmin and Rate_Nmin at the HG site after enclosure. In contrast, studies in the Kobresia alpine grassland in Tibet and in the Hulunbeier grassland in Inner Mongolia showed that enclosure increased Nmin14,29, this may be because the enclosure induced changes in the composition of plant residue (e.g., higher cellulose, lower lignin/N ratio), which promoted decomposition and improved C release27,30, and a higher input of labile C promoted the reproduction of microorganisms after enclosure31. Therefore, the different soil physicochemical properties and microbial activity caused by enclosure measures impact soil Nmin accumulation.
Effects of grazing intensity and long-term enclosure on the abundance of ammonia-oxidizing microorganisms and drivers for ammonia-oxidizing microorganism abundance
AOA abundance was two orders of magnitude greater than that of AOB in both MG and HG sites, which coincided with other grassland ecosystem19,32,33. AOB abundance at the HG site was significantly higher than that at the MG site (Fig. 2b). Enclosure significantly reduced AOB abundance compared with that under non-fence treatment at the HG site, however, enclosure did not obviously change AOB abundance at the MG site (Fig. 2b). The positive relationships were found between PNR and the abundance of AOB and AOA at the HG and MG sites, respectively (Fig. 3, Table 1), indicating that AOB and AOA may separately be the dominant functional players in ammonia oxidation processes at HG and MG site, because PNR was directly regulated by the ammonia oxidizers community34.
Relationships between potential nitrification rate (PNR) and the amoA gene copy numbers of AOA (a,b) and AOB (c,d) indifferent treatments. MG moderately grazed site, HG heavily grazed site, NF non-fenced, F fenced treatment. Single asterisk and double asterisk denote the significance at p < 0.05 and p < 0.01, respectively.
The differences in AOB and AOA abundances and/or community compositions between HG and MG sites may be due to their differential responses to soil environmental factors35,36,37. Soil pH were 7.17–7.41 and 7.97–8.15 at the MG and HG sites in this study, respectively. This indicated that HG increased soil pH by nearly 0.8 after treatment. Correlation analysis revealed that soil pH was positively and negatively correlated with the abundance of AOB and AOA, respectively (Table 2). AOA played an important role in nitrification in the temperate grassland of southwestern Germany with acidic soil (pH 5.7–6.9) and the typical steppe of Inner Mongolia with neutral soil (pH 7.13–7.61)19,34,35. AOB played an great role in nitrification in desert steppes of Inner Mongolia and plateau steppes in Wuchuan County with alkaline soil (pH 8.12–8.27)14,36. The abundance of AOB was positively and significantly correlated with soil NO3–N (R2 = 0.77, p < 0.05) and available nitrogen (NH4+-N + NO3−-N) (R2 = 0.65, p < 0.05), but there was no significant relationships between the AOA abundance and soil nitrogen in this study (Table 2). AOB adapted better to higher N environments, including high NH4+-N and NO3–N, whereas AOA were more favored by a low N environment34,37,38,39,40. There were also significant relationships between soil NO3–N and inorganic N concentrations and AOB abundance in grassland soil in southern England41. However, N availability alone is not the crucial factor controlling AOB abundance according to other studies42. The low C:N ratio was a primary factor regulating AOB abundance in Wessen et al. study43, which agrees with the study’s results, wherein, AOB abundance was significantly and positively correlated with soil C/N ratio.
Effects of grazing intensity and long-term enclosure on the community structure of ammonia-oxidizing microorganisms
More T-RFs fragments (seven to eight) of AOA were observed compared with those (six T-RFs fragments) of AOB from T-RFLP (Fig. 4), indicating that the diversity of AOA was higher than that of AOB in our study. Grazing pattern was important in regulating the distribution of soil microorganisms and affected nitrification microbial communities by influencing soil physical and chemical properties (e. g. bulk density, NH4+-N)1,2. Enclosure of grassland from grazing affects microbial biomass and ammonia-oxidizing populations17. Compared with the MG site, the AOA and AOB community structures showed significant changes in the HG site. Enclosure conditions at the HG site significantly affected the community structure of AOB but not that of AOA compared with those under non-fence conditions (Fig. 4d). Enclosure measures increased soil physicochemical properties such as C/N ratio and NH4+ in the Haibei Alpine Meadow of Tibet, which significantly affected the AOA and AOB community structures44. The correlation analysis revealed that the T-RFs of 256 bp of AOB relative abundance was significantly positively correlated with NO3–N and NH4+-N + NO3–N at the HG site (Table S5). Therefore, the decrease of available nitrogen content entering the soil in the form of livestock excrement after enclosure at the HG site may explain the change in the AOB community structure (Table S5).
Terminal restriction fragment (T-RF) analyses targeting the functional gene amoA gene for AOA (a,b) and AOB (c,d) in soil. MG moderately grazed site, HG heavily grazed site, NF non-fenced, F fenced treatment. ANOVA results are available in Table S3 and Table S4. Correlation coefficient between soil physicochemical properties and T-RFs of AOB at HG site in Table S5.
The phylogenetic analysis showed that three and two groups of the amoA gene of AOA and AOB were obtained from two trees. The sequences of AOA in Cluster 1 were affiliated with the amoA gene of uncultured crenarchaeote and thaumarchaeote. Nearly all the sequences in Cluster 2 belonged to Nitrososphaeraceae, and that in Cluster 3 belonged to Nitrosopumilaceae (Fig. 5). This coincided with early studies19,45,46, which found that most AOA sequences were affiliated with cluster Nitrososphaera (designated as I.1b AOA lineage47). Surprisingly, a Nitrosopumilaceae (designated as marine47) origin of AOA in our study was also found (Fig. 5).
Neighbour-joining phylogenetic tree based on ammonia oxidizing archaeal amoA amino acid sequences. Clones with > 96% sequence similarity were considered as the same OTU and were named with OTU and numbers. Additional amoA sequences were obtained from the GenBank database. The scale bar represents 1% sequence divergence.
The sequences of the amoA gene of AOB obtained in treated soil were affiliated with Nitrosospira and Nitrosomonas species, and most of the sequences were grouped into Nitrosospira species (Fig. 6). This indicates that Nitrosospira species are ubiquitous in the studied soil. Nitrosospira species as predominates in the AOB community were also found in studies targeting grassland soils19,45,48, arable soil33, and acidic upland soil49,50. However, Pan et al.1,2 and Olivera et al.51 detected a high number of Nitrosococcus lineages of AOB in different grazing intensity grassland soils, which is likely correlated with soil conditions caused by specific management measures. In addition, fifteen and ten OTUs were detected from AOA and AOB sequences, respectively, indicating that the diversity of AOA was higher than that of AOB in this study. Thus, Nitrososphaeraceae and Nitrosospira were the main AOA and AOB, respectively, regulating the nitrification process in this grassland soil.
Neighbour-joining phylogenetic tree based on ammonia oxidizing bacterial amoA amino acid sequences. Clones with > 97% sequence similarity were considered as the same OUT and were named with OTU and numbers. Additional amoA sequences were obtained from the GenBank database. The scale bar represents 1% sequence divergence.
Conclusions
Enclosure significantly decreased the cumulative soil mineralized N and N mineralization rate at the HG site but not at MG site. Enclosure at the HG site significantly decreased the AOB abundance and changed the community structure of AOB. However, enclosure at the MG site did not significantly affect AOA and AOB abundance and community structures. PNR was positively correlated with the AOB and AOA abundance at the HG and MG sites, respectively. The abundance of AOA was significantly correlated with soil pH; however, AOB abundance was significantly correlated with several factors, such as soil pH, NO3–N, NH4+-N + NO3–N, C/N ratio, TN, and N/P ratio. The phylogenetic analysis showed that Nitrososphaeraceae and Nitrosospira were the main AOA and AOB, respectively, which may separately be the dominant influences on ammonia oxidation at the MG and HG sites and regulating nitrification processes in this grassland soil. In brief, enclosure measure in different grazing intensity grassland likely determined the niche special of ammonia-oxidizing microbes in the study soils through their effects on soil physicochemical properties and elements nutrient availability. The results of this study are of great significance for evaluating the ecological effects of enclosure in different grazing intensities.
Data availability
All data generated or analyzed during this study were included in this published article.
References
Pan, H. et al. Archaea and bacteria respectively dominate nitrification in lightly and heavily grazed soil in a grassland system. Biol. Fert. Soils. 54(1), 41–54 (2018).
Pan, H. et al. Understanding the relationships between grazing intensity and the distribution of nitrifying communities in grassland soils. Sci. Total Environ. 634, 1157–1164 (2018).
Dong, L., Li, J. J., Sun, J. & Yang, C. Soil degradation influences soil bacterial and fungal community diversity in overgrazed alpine meadows of the Qinghai-Tibet plateau. Sci. Rep. 11, 11538 (2021).
Oduor, C. O. et al. Enhancing soil organic carbon, particulate organic carbon and microbial biomass in semi-arid rangeland using pasture enclosures. BMC Ecol. 18, 45 (2018).
Wang, S. Z., Fan, J. W., Li, Y. Z. & Huang, L. Effects of grazing exclusion on biomass growth and species diversity among various grassland types of the Tibetan Plateau. Sustainability 11(6), 1705 (2019).
Simpson, A. C., Zabowski, D., Rochefort, R. M. & Edmonds, R. L. Increased microbial uptake and plant nitrogen availability in response to simulated nitrogen deposition in alpine meadows. Geoderma 336, 68–80 (2019).
Qasim, S. et al. Influence of grazing enclosure on vegetation biomass and soil quality. Int. Soil Water Conserv. 5(1), 62–68 (2017).
Hirobe, M. et al. Effects of livestock grazing on the spatial heterogeneity of net soil nitrogen mineralization in three types of Mongolian grasslands. J. Soils Sediment. 13, 1123–1132 (2013).
Luo, Y. K., Wang, C. H., Shen, Y., Sun, W. & Dong, K. H. The interactive effects of mowing and N addition did not weaken soil net N mineralization rates in semiarid grassland of Northern China. Sci. Rep. 9, 13457 (2019).
Wu, H. et al. Feedback of grazing on gross rates of N mineralization and inorganic N partitioning in steppe soils of Inner Mongolia. Plant Soil. 340(1–2), 127–139 (2011).
Xu, Y. Q., Li, L. H., Wang, Q. B., Chen, Q. S. & Cheng, W. X. The patterns between nitrogen mineralization and grazing intensities in an Inner Mongolian typical steppe. Plant Soil. 300, 289–300 (2007).
Wang, X. et al. Grazing improves C and N cycling in the Northern Great Plains: A meta-analysis. Sci. Rep. 6, 33190 (2016).
Pang, R., Sun, Y., Xu, X. L., Song, M. H. & Ouyang, H. Effects of clipping and shading on 15NO3− and 15NH4+ recovery by plants in grazed and ungrazed temperate grasslands. Plant Soil. 433(1–2), 339–352 (2018).
Sun, Y., Schleuss, P. M., Pausch, J., Xu, X. L. & Kuzyakov, Y. Nitrogen pools and cycles in Tibetan Kobresia pastures depending on grazing. Biol. Fert. Soils. 54(5), 569–581 (2018).
Andrioli, R. J., Distel, R. A. & Didone, N. G. Influence of cattle grazing on nitrogen cycling in soils beneath Stipa tenuis, native to central Argentina. J. Arid. Environ. 74(3), 419–422 (2010).
Norman, J. S., Lin, L. & Barrett, J. E. Paired carbon and nitrogen metabolism by ammonia-oxidizing bacteria and archaea in temperate forest soils. Ecosphere 6(10), 1–11 (2016).
Mukhtar, H., Lin, Y. P., Lin, C. M. & Petway, J. R. Assessing thermodynamic parameter sensitivity for simulating temperature responses of soil nitrification. Environ. Sci.-Proc. Imp. 21(9), 1596–1608 (2019).
Rütting, T., Schleusner, P., Hink, L. & Prosser, J. I. The contribution of ammonia-oxidizing archaea and bacteria to gross nitrification under different substrate availability. Soil Biol. Biochem 160, 108353 (2021).
Pan, H. et al. Management practices have a major impact on nitrifier and denitrifier communities in a semiarid grassland ecosystem. J. Soils Sediment. 16, 896–908 (2016).
Szukics, U. et al. Management versus site effects on the abundance of nitrifiers and denitrifiers in European mountain grasslands. Sci. Total Environ. 648, 745–753 (2019).
Chen, Q., Hooper, D. U. & Lin, S. Shifts in species composition constrain restoration of overgrazed grassland using nitrogen fertilization in Inner Mongolian steppe, China. PLoS ONE 6(3), e16909 (2011).
Raison, R. J., Connell, M. J. & Khanna, P. K. Methodology for studying fluxes of soil mineral-N in situ. Soil Biol. Biochem. 19, 521–530 (1987).
Kurola, J., Salkinoja-Salonen, M., Aarnio, T., Hultman, J. & Romantschuk, M. Activity, diversity and population size of ammonia-oxidizing bacteria in oil-contaminated land farming soil. FEMS Microbiol. Lett. 250, 33–38 (2005).
Tran, H. T. et al. Bacterial community progression during food waste composting containing high dioctyl terephthalate (DOTP) concentration. Chemosphere 265, 129064 (2021).
Hook, P. B. & Burke, I. C. Evaluation of a method for estimating net nitrogen mineralization in a semiarid grassland. Soil Sci. Soc. Am. J. 59, 831–837 (1995).
Liu, T. Z., Nan, Z. B. & Hou, F. J. Grazing intensity effects on soil nitrogen mineralization in semi-arid grassland on the Loess Plateau of northern China. Nutr. Cyc. Agroecosyst. 91(1), 67–75 (2011).
Li, J. P., Ma, H. B., Xie, Y. Z., Wang, K. B. & Qiu, K. Y. Deep soil C and N pools in long-term fenced and overgrazed temperate grasslands in northwest China. Sci. Rep. 9, 16088 (2019).
Di, H. J. et al. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geosci. 2(9), 621–624 (2009).
Li, J. P., Zheng, Z. R., Xie, H. T., Zhao, N. X. & Gao, Y. B. Increased soil nutrition and decreased light intensity drive species loss after eight years grassland enclosures. Sci. Rep. 7, 44525 (2017).
Luo, C. Y. et al. Effect of warming and grazing on litter mass loss and temperature sensitivity of litter and dung mass loss on the Tibetan plateau. Glob. Change Biol. 16, 1606–1617 (2010).
Shahzad, T. et al. Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biol. Biochem. 80, 146–155 (2015).
Xie, Z. et al. Identifying response groups of soil nitrifiers and denitrifiers to grazing and associated soil environmental drivers in Tibetan alpine meadows. Soil Biol. Biochem. 77, 89–99 (2014).
Clark, I. M., Hughes, D. J., Fu, Q. L., Abadie, M. & Hirsch, P. R. Metagenomic approaches reveal differences in genetic diversity and relative abundance of nitrifying bacteria and archaea in contrasting soils. Sci. Rep. 11, 15905 (2021).
He, J. Z. et al. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol. 9, 2364–2374 (2007).
Meyer, A. et al. Influence of land use intensity on the diversity of ammonia oxidizing bacteria and archaea in soils from grassland ecosystems. Microb. Ecol. 67(1), 161–166 (2014).
Zhu, X. X. et al. Effects of warming, grazing/cutting and nitrogen fertilization on greenhouse gas fluxes during growing seasons in an alpine meadow on the Tibetan Plateau. J. Agric. Meteorol. 214–215, 506–514 (2015).
Jia, Z. J. & Cornrad, R. Bacteria rather than archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 11(7), 1658–1671 (2009).
Verhamme, D. T., Prosser, J. I. & Nicol, G. W. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J. 5, 1067–1071 (2011).
Zhou, X. H. et al. Diversity, abundance and community structure of ammonia-oxidizing archaea and bacteria in riparian sediment of Zhenjiang ancient canal. Ecol. Eng. 90, 447–458 (2016).
Martens-Habbena, W., Berube, P. M., Urakawa, H., de la Torre, J. R. & Stahl, D. A. Ammonia oxidation kinetics determine niche separation of nitrifying archaea and bacteria. Nature 461, 976–979 (2009).
Clark, D. R. et al. Mineralization and nitrification: Archaea dominate ammonia-oxidising communities in grassland soils. Soil Biol. Biochem. 143, 107725 (2020).
Long, X. N., Chen, C. R., Xu, Z. H., Linder, S. & He, J. Z. Abundance and community structure of ammonia oxidizing bacteria and archaea in a Sweden boreal forest soil under 19-year fertilization and 12-year warming. J. Soils Sediment. 12, 1124–1133 (2012).
Wessén, E. & Hallin, S. Abundance of archaeal and bacterial ammonia oxidizers-possible bioindicator for soil monitoring. Ecol. Indic. 11, 1696–1698 (2011).
Yang, Y. et al. Responses of the functional structure of soil microbial community to livestock grazing in the Tibetan alpine grassland. Glob. Change Biol. 19(2), 637–648 (2013).
Zhang, C. J. et al. Impacts of long-term nitrogen addition, watering and mowing on ammonia oxidizers, denitrifiers and plant communities in a temperate steppe. Appl. Soil Ecol. 130, 241–250 (2018).
Alves, R. J. E., Minh, B. Q., Urich, T., Haeseler, A. V. & Schleper, C. Unifying the global phylogeny and environmental distribution of ammonia-oxidising archaea based on amoA genes. Nat. Commun. 9, 1517 (2018).
DeLong, E. F. Everything in moderation archaea as ‘non extremophiles’. Curr. Opin. Genet. Dev. 8(6), 649–654 (1998).
Jia, Z. J. et al. Evidence for niche differentiation of nitrifying communities in grassland soils after 44 years of different field fertilization scenarios. Pedoshpere 30(1), 87–97 (2019).
Wang, X. L. et al. Long-term fertilization effects on active ammonia oxidizers in an acidic upland soil in China. Soil Biol. Biochem. 84, 28–37 (2015).
Li, Y. Y., Chapman, S. J., Nicol, G. W. & Yao, H. Y. Nitrification and nitrifiers in acidic soils. Soil Biol. Biochem. 116, 290–301 (2018).
Olivera, N. L., Prieto, L., Bertiller, M. B. & Ferrero, M. A. Sheep grazing and soil bacterial diversity in shrub lands of the Patagonian Monte, Argentina. J. Arid. Environ. 125, 16–20 (2016).
Funding
This work was funded by the National Natural Science Foundation of China (No. 31971437, 31300386, 41807334).
Author information
Authors and Affiliations
Contributions
Q.C.: Investigation, Methodology, Resources, Writing—Original Draft; Y.T.S.: Investigation, Formal analysis; R.Z.: Methodology, Software; Q.L.B.: Validation, Writing—review and editing, Supervision, Funding acquisition; S.L.: Conceptualization and Review. All authors participated and approved the final manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Q., Shang, Y., Zhu, R. et al. Long-term enclosure at heavy grazing grassland affects soil nitrification via ammonia-oxidizing bacteria in Inner Mongolia. Sci Rep 12, 21464 (2022). https://doi.org/10.1038/s41598-022-25367-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25367-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.