Abstract
Luobuma (Apocynum venetum, Poacynum pictum, and P. hendersonni) are perennial herbs widely used in the textile and medical industries and ecological restoration. In the summer of 2020, reddish-brown or off-white sunken shape necrotic lesions were observed on the stems and shoots of seven Luobuma ecotypes grown in the field in Yuzhong County, Gansu province of China, which is a limiting factor that affects the growth, function and application of Luobuma. To make clear whether the new symptoms were caused by a novel pathogen, a combined research in field and greenhouse was conducted. Based on the morphological and molecular analysis results, the pathogen causing the necrotic lesions was identified as Boeremia exigua var. rhapontica. The incidence and disease index of the seven ecotypes in the field ranged from 11.49 to 33.68% and 6.63 to 23.01, respectively, from 2020 to 2021. The results showed that the disease severity gradually increased with the growing season. According to the pathogenicity analysis of the eight ecotypes in the greenhouse, the ecotypes Pp-BMK and Pp-BMH were susceptible, while ecotype Pp-BMQ was resistant to Boeremia exigua var. rhapontica infection. Thus, the present study provides a theoretical basis for preventing and controlling the stem and leaf necrotic lesions disease on Luobuma by planting resistant varieties/ecotypes. To our knowledge, this is the first report of stem necrotic lesions and leaf spots on Luobuma caused by B. exigua var. rhapontica.
Similar content being viewed by others
Introduction
Luobuma plants are popular traditional herbs belonging to the family Apocynaceae1 and are widely distributed in Central Asia (Caspian Sea, Kazakhstan, Turkmenistan, Uzbekistan, and Northwest China)2. Similar to the wild Luobuma, which are mostly found in steppes, semi-deserts, and deserts zones2, cultivated Luobuma are also adapted to arid climates or dry areas with moderate salinity2. Luobuma plants are increasingly becoming important economic crops worldwide due to their wide usage in tea, medicine, and textiles1.
Luobuma plants include two genera with three species; Apocynum venetum, Poacynum pictum, and P. hendersonii3. In 2017, eight Luobuma ecotypes were introduced to Yuzhong, Gansu province from Xinjiang, China4. Taxonomic and phenotypic identification of the eight ecotypes was conducted by Gao et al.5. Among these ecotypes, P. hendersonii plants got extinct in 2019, leaving only seven ecotypes in Yuzhong, Gansu province, China, to date. A novel necrotic lesions symptom has been frequently observed on the stem of the different ecotypes in Yuzhong in 2020. The fungal isolations from these stems have been identified as Boeremia exigua (synonym: Phoma exigua)6, based on their morphology, spores, and hypha of fungus colony.
Boeremia exigua is a plant pathogen causing serious leaf (petioles and rachises), stem, or root lesion diseases on various plants worldwide. Examples of diseases caused by this pathogen include leaf necrotic lesions disease of Fraxinus excelsior in Poland7, snags rotten leaves disease of Morus malba in China8, leaf spot of Hydrangea paniculate in Italy9, black root rot of Cichorium intybus var. sativum in Chile10, and leaves necrotic lesions of Cycas circinalis in India11. Boeremia exigua species has 11 varieties, which are B. exigua var. coffeae, B. exigua var. exigua, B. exigua var. heteromorpha, B. exigua var. pseudolilacis, B. exigua var. linicola, B. exigua var. forsythiae, B. exigua var. viburni, B. exigua var. gilvescens, B. exigua var. populi, B. exigua var. inoxydabilis, and B. exigua var. rhapontica12,13. These varieties are mainly distinguished based on their host specificity and pathogenicity12, and molecular methods are often used to support the morphological identifications12,13. Previous studies have found that the genus Boeremia could be successfully identified based on its actin, beta-tubulin, calmodulin, elongation factor, and ITS genes7,12,13.
Stem necrotic lesions disease was observed in Luoboma fields in 2020, with Luobuma ecotypes with severe disease symptoms. Thus, the present study aimed to (i) identify the pathogens causing the necrosis lesion symptoms of Luobuma and (ii) test the pathogenicity and occurrence of the pathogen on different ecotypes in the field.
Results
Symptoms of stem necrotic lesions disease on Luobuma in the field
Leaf symptoms caused by the pathogen Boeremia exigua were not observed on Luobuma plants; however, serious necrotic lesion symptoms were observed on the stem between 2020 and 2021. The initial symptom on stems and shoots began as small reddish-brown spots, with an unclear or clear borderline between infected and healthy tissues. The spot sizes later enlarged, forming irregular, rhomboid, diamond, or sunken shapes, with white or lighter coloured centers. However, when the stem necrotic lesions became severe, the spots turned white or off-white with ripe pycnidia girdling the stems forming depressions, which then withered and died (Fig. 1).
Isolation frequency of Boeremia exigua strain
We isolated 38 strains from the 70 plants of the seven ecotypes with stem necrotic lesion symptoms. The isolation frequency of the strains from the infected stem tissues and pycnidia on the infected stem tissues were 42.5% and 100%, respectively. The pathogen was not found in the root tissues (without symptoms) of the infected plants but was isolated successfully from the soil. Thus, eight strains (seven from the seven ecotypes and one from the soil) were screened for further analysis (Table 1).
For each strain, a spore was transferred onto a fresh PDA plate under a compound microscope (YS2-H, NIkon, China) to obtain purified pathogens for further analysis.
Morphological and biological characteristics
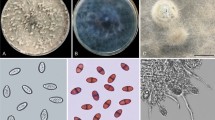
The morphological characteristics of the eight pathogenic strains were consistent. The colonies grew to 39 mm in diameter on OA, 44 mm on PDA, and 24 mm on PCA 5 days after inoculation. After 10 days, the colonies had 53 mm diameter on OA, 69 mm on PDA, and 48 mm on PCA; however, those on PCA were irregular, salmon with light brown aerial mycelium, and had scattered growth with visible fascicular branches. Colonies on OA were regular, white/olivaceous to olivaceous with olivaceous aerial mycelium, while those on PDA were regular and tan/light salmon panniform-like colonies with developed white/olivaceous aerial mycelium. However, the colonies had irregular whitish-grey lobed margins on PCA, OA, and PDA (Fig. 2). Conidia on PCA were hyaline, smooth, oblong with obtuse ends, aseptate, straight, sometimes guttulate, and measured 4.94–10.13 μm × 2.03–3.01 μm (average 2.67 μm × 6.42 μm) (Fig. 3a,b). Moreover, the hyphae on PCA were brown, septate, synaxillary branches which were mostly binary at acute angles, with node-like expansion (Fig. 3c). Thus, the pathogen was preliminarily identified as Boeremia exigua based on the morphological characteristics.
Molecular identification and phylogenetic analysis
To verify the accuracy of the morphological identification, we sequenced 8 strains screened in this study and obtained the sequences of 24 strains (6 species and 11 varieties) including Phoma herbarum as the outgroup from NCBI for the phylogenetic analysis (Table 2).
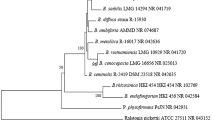
In the single gene analysis, we found that the eight strains causing stem necrotic lesions disease on Luobuma could not be distinguished by the single loci ITS (Fig. S1), ACT (Fig. S2), and TUB (Fig. S3) but could be identified by loci TEF (Fig. S4). Moreover, the strains were closely related to B. exigua var. rhapontica, which was originally reported in Rhaponticum repens. The phylogenetic tree (generated through multiple sequence alignment) also showed that the eight strains clustered with B. exigua var. rhapontica strain (Fig. 4). Based on these findings, the pathogen was identified to be Boeremia exigua var. rhapontica.
Assessment of the disease development in the field
The incidence and disease index of the seven ecotypes differed significantly (P < 0.05) in the field. The maximum incidence of the seven ecotypes ranged from 19.43% to 33.68%, and the maximum disease index ranged from 8.39 to 23.01 in 2020 (Figs. 5, 6), while in 2021, the ranges were 11.49% to 18.43% and 6.63 to 10.24 in 2021, respectively (Figs. 7, 8). Although the stem necrotic lesions of Luobuma first occurred from Mid-July to Mid-August in 2020–2021, the disease occurrence in 2020 was earlier and more severe than that in 2021. The disease index and incidence during the two-year growing season were the highest for Pp-BMK and Pp-BMH, and the lowest for Pp-BMQ (Fig. 9).
Pathogenicity on the leaf tissues
To explore whether B. exigua var. rhapontica infects leaves, we separately inoculated conidial suspensions of strains AvLM-Be and Av-Be on the leaves of the eight ecotypes. Five days after inoculation, the strains caused similar symptoms with B. exigua var. rhapontica, which resulted in discolored spots closer to the leaf edges and leaf tips (Fig. 10a). However, 13 days after inoculation, the necrosis lesions gradually expanded along the leaf margins and tips, and the disease spots presented unclear or clear concentric rings, which were irregularly shaped lesions after a few spots merged. This resulted in withering and death of some leaves (Fig. 10b). The leaves of P. hendersonni had clear concentric rings of the disease spots (Fig. 10c,d), while the leaves of A. venetum and P. pictum had unclear concentric rings (Fig. 10e). No symptoms were observed on the corresponding controls. Furthermore, the pathogen was re-isolated from the symptomatic leaves, and their identification was confirmed by morphology and molecular analysis. The pathogenicity analysis showed that under greenhouse conditions, B. exigua var. rhapontica could infect Luobuam leaves and induce slightly different symptoms for 8 ecotypes of the three species of Luobuma.
The symptoms of leaves necrotic lesions disease in inoculated leaves of Luobuma. (a) leaf symptoms of A. venetum 5 days after inoculation, (b) leaf symptoms of A. venetum 13 days after inoculation, (c,d) the clear wheel disease spots on the leaves of P. hendersonni and (e) unclear wheel disease spots on the leaves of A. venetum and P. pictum.
The leaf incidence of the eight ecotypes was investigated in triplicate from late September to early October under greenhouse conditions. The collected data showed that ecotype Pp-BMK was the highest incidence rate on Sept. 20 and Sept. 28 (8.34% and 10.42%, respectively), while the ecotype Pp-BMH had the lowest incidence rate (2.78% and 2.78%, respectively) (Fig. 11; P < 0.05). However, 21 days after inoculation, the incidence rate of ecotype Av-LHM was the largest (15.97%), while that of Pp-BMH was the lowest (4.86%).
Pathogenicity on the stem tissues
To confirm Koch’s postulates, we inoculated the two strains (AvLM-Be and Av-Be) on the stem of the eight ecotypes. After 5 days, the stems of all inoculated ecotypes developed necrosis lesions, which had brown spots with wedge-shaped indentations at the beginning (Fig. 12a). However, 13 days after inoculation, the symptomatic stems and shoots withered and died (Fig. 12b–d). There were no differences in lesion shape on the stems of the eight ecotypes; however, the degree of necrosis lesion spots and the wedge-shaped indentations were more severe on the thick stems (Fig. 12a,e). No symptoms were observed on the corresponding controls. The morphological and molecular analysis of the re-isolated pathogen showed that the pathogen was B. exigua var. rhapontica. Moreover, pathogenicity tests showed that B. exigua var. rhapontica could infect stems of Luobuam under greenhouse conditions, and the resulting stem necrosis lesions shape was similar to those observed in the field; however, the lesion color differed from those under the field conditions.
The pathogenicity of B. exigua var. rhapontica had significant differences (P < 0.05) in stem tissues of the eight ecotypes. The necrosis lesion spot length of the eight ecotypes ranged from 5.20 mm (Pp-BMQ) to 14.18 mm (Pp-BMK) 13 days after inoculation. Therefore, these pathogenicity results showed that ecotype Pp-BMQ had higher resistance to the B. exigua strains, while ecotype Pp-BMK had lower resistance than the other ecotypes (Fig. 13).
Discussion
The present study reported a novel and severe stem necrotic lesions disease on Luobuma caused by Boremeria exigua. The pathogenic fungus, B. exigua, also infects many other plants as their hosts. For example, 11 varieties of B. exigua reportedly infect 45 plant species belonging to 31 genera and 19 families13. Although the infection is host-dependent, the pathogen isolated from one host could also infect other different hosts. B. exigua var. forsythiae has previously been reported on Forsythia of the Oleaceae family in the Netherlands6 and has been shown to successively infect Hydrangea macrophylla13. Therefore, the nomenclature of the Boeremia varieties was based on different hosts and the combination of their morphology and molecules. In our study, B. exigua var. rhaponticai infected eight ecotypes belonging to three Luobuma species, which were differentiated based on the phylogeny of four loci (ITS, ACT, TUB, and TEF) coupled with their morphological characteristics. To our knowledge, this is the first report of B. exigua var. rhapontica causing stem necrotic lesions and leaf spots in Luobuma.
Boeremia exigua var. rhapontica has previously been identified at the species level on Russian knapweed of the family Asteraceae in Turkey in 200314, and its variety B. exigua var. rhapontica was only identified in 2015 based on host association analysis13. In 2015, Berner13 described the colony, conidia, and pycnidia characteristics of B. exigua var. rhapontica isolated from Russian knapweed (Rhaponticum repens) on agar. Boeremia exigua var. rhapontica was found to be genetically closer to B. exigua var. coffeae, originally identified on coffee plants (Coffea arabica)6,13. However, the B. exigua var. rhapontica was proposed based on the molecular and biological characteristics analysis13. The present study cultured the B. exigua var. rhapontica on three different culture media (PCA, OA, and PDA), and its colony characteristics were described. The colonies had irregular whitish-grey lobed margins on PCA, OA, and PDA. The morphological characteristics of the conidia and hyphae on PCA were also described, and these descriptions provide an important reference for B. exigua var. rhapontica identification in future studies.
Previous studies recommended that Boeremia be identified based on a combined phylogeny analysis using ITS, actin, beta-tubulin, calmodulin, and translation elongation factor 1-alpha genes, rather than using any of the single genes13,15. Therefore, our study used the four genes (ITS, ACT, TUB, and TEF) to identify the strains. The phylogenetic tree showed that the eight strains clustered closely with B. exigua var. rhapontica. Moreover, in the single gene analysis, the strains were comparable with B. exigua var. rhapontica using only the loci TEF. Therefore, based on the molecular and biological data obtained in this study, the strains that infected the leaves and stems of three Luobuma species were identified as B. exigua var. rhapontica.
Boeremia exigua reportedly infects leaves10,16,17, fruits17, tubers18, roots19, and stems10 in the field. For instance, B. exigua has been reported as a leaf spot pathogen of Japanese ginseng (Panax japonicus) in China, affecting approximately 95% of plants in the field and causing severe leaves necrotic lesions with elliptical and flavicant margins, which are irregularly shaped at the leaf margins or tips16. The pathogen also causes leaves and fruit spots in okra (Abelmoschus esculentus) and results in brown to brownish black and sunken cavities lesions with an incidence rate of 35%–55% in different fields17. Other studies have shown that B. exigua causes underground plant diseases; for example, the pathogen reportedly causes tuber rots in sweet potatoes (Ipomoea batatas)18. B. exigua also causes black root rot of the industrial chicory (Cichorium intybus var. sativum) in Chile, exhibiting dark and firm sunken lesions, which turn into black-colored cavities on the crown, with yield losses of up to 31%19. The stem and leaf spot disease of the common speedwell (Veronica officinalis) is also caused by B. exigua in Switzerland20. The present study reported the first B. exigua var. rhapontica infection cases of A. venetum, P. pictum, and P. hendersonni. The pathogen infected Luobuma stems under natural conditions, but the leaves were not infected. However, the leaves inoculated with B. exigua var. rhapontica conidial suspensions showed visible symptoms of necrosis lesion spots with unclear or clear concentric rings, which caused withering and death of the Luobuma.
The absence of the symptomatic leaves in the field could be linked to Luobuma rust disease caused by Melampsora apocyni, an aggressive causal agent in the field, with an incidence rate of up to 90% and whose urediospores could cover the whole leaves in severe cases21. The rust disease of Luobuma usually occurs from late June to early July in Yuzhong; however, the stem necrotic lesions disease of Luobuma began in late July to early August. Therefore, M. apocyni or its metabolites could have occupied the infection sites; thus, B. exigua var. rhapontica had no space to invade. Meanwhile, the leaf disease caused by B. exigua var. rhapontica was not prevalent in the 2-year field study, and this necessitates further investigation. Furthermore, our results demonstrated that the different ecotypes had slight differences in their disease symptoms, especially in the color of the disease spots. This may be associated with the stem color of the different ecotypes5.
Since we observed that the pathogen B. exigua var. rhapontica was prevalent on Luobuma plants, including the two species (A. venetum and P. pictum), we further investigated the disease index and occurrence of the stem necrotic lesions disease on the seven ecotypes of the two species in the same fields in the year 2020 and 2021. This was to reveal the relationship between species or ecotypes and disease development. The results demonstrated that the effect of the pathogen on the two species had no difference at the species level but differed at the host plant (the ecotypes) level. Moreover, the pathogen was also isolated successfully from the soil, indicating that soil might be one of the overwintering pathogen sites, acting as a primary source of infection for the subsequent growing seasons.
The susceptibility of cultivars/ecotypes to B. exigua infection varied. For instance, potato (Solanum sp.) varieties had varying resistance levels to the invasion of B. exigua var. foveata22. A pathogenicity trial of the lima bean (Phaseolus lunatus) cultivars inoculated with B. exigua var. exigua also showed that the cultivars had different susceptibility levels to B. exigua var. exigua23. In our study, consistent differences in stem disease occurrence on the different ecotypes were observed in the greenhouse and field conditions. Stems and leaves of the different ecotypes exhibited different responses to B. exigua var. rhapontica infection.
Conclusions
To the best of our knowledge, this is the first report of stem necrotic lesions and leaf spots disease on Luobuma caused by B. exigua var. rhapontica. The disease incidence rate in the field was up to 34%, and its prevalence and damage were second to the rust disease and spot blight caused by Septoria apocyni24. The present study provides useful information for identifying and determining the pathogenicity of B. exigua var. rhapontica, and the occurrence of the stem necrotic lesions disease. However, further studies are required to determine the control measure of the disease.
Methods
Site establishment
A seed-breeding field composed of 8 Luobuma ecotypes (Av-LHM, Pp-BMQ, Pp-BMB, Pp-BMH, Pp-BMZ, Pp-BMX, Pp-BMK, and Ph-DBM) was established in 2017 in Yuzhong County, China (35° 34′ 20″–36° 26′ 30″ N, 103° 49′ 15″–104° 34′ 40″ E). The seeds were provided by Altay Gaubao Tea Co., LTD., located in Altay Prefecture of the Xinjiang Uyghur Autonomous Region, China. Plant rows were 1 m apart, while the spacing within the rows was 0.5 m apart. Each ecotype had four plots (5 by 10 m2 each). Among the planted ecotypes, Ph-DBM degraded gradually and eventually died in 2019; thus, only seven ecotypes were studied.
Disease survey
We performed successive disease surveillance during the growth season of Luobuma from 2020 to 2021 to determine the field the incidence and disease index of stem necrotic lesions of the seven ecotypes. The disease symptom was observed and recorded, and the severity of the stem necrotic lesion was visually estimated as the percentage of observed stems covered by necrotic lesions with/without pycnidia. The disease severity scale included 0. for no infection signs; 1. for small reddish-brown disease spot; 2. for long strip or elliptical reddish-brown disease spot; 3. for necrotic lesions with larger black-brown-patched margins, the white or off-white centers with fewer pycnidia or without pycnidia; 4. for necrotic lesions with depression and larger white or off-white areas at the center with a mass of ripe pycnidia; 5. for necrotic lesions with girdling depressions and withered stems, or dead stems (Fig. 14). The incidence and disease index of the stem necrotic lesions were investigated in five locations for each ecotype. All stems from 5 Luobuma plants, one from each location, were analyzed for the symptoms, and those with many necrosis lesions per stem were recorded to have a maximum severity level.
Sample collection and isolation
The stems were collected from the 7 ecotypes of Luobuma for the necrotic lesion symptoms analysis. The roots of the plants with severe stem necrotic lesions were also collected from ten plants of each ecotype to determine whether the pathogen also infected the roots. Two isolation methods were used in the present study to accurately identify the pathogen causing the stem necrotic lesions disease. Briefly, the infected stems and healthy root tissues were surface-sterilized in 75% ethanol for 45 s, rinsed thrice with sterile distilled water, and air-dried on sterile filter paper. Ten segments (0.4 cm × 0.3 cm) of the symptomatic stem and healthy root tissues were cut from 10 random plants and were cultured in a Petri dish containing potato dextrose agar (PDA) (one Petri dish per plant) and incubated at 23 ± 1 °C for 5 days. For the other isolation method, the pycnidia from stem tissues were cultured on PDA under the same conditions. To determine whether the pathogen exists in the soil, we collected soil samples (5 cm–10 cm soil layer) from four locations of each ecotype containing Luobuma with severe stem necrotic lesion symptoms. The soil samples were frozen at 4 °C for 2 weeks, and the soil microorganisms were isolated based on the plate dilution method25.
Morphological and biological characteristics analysis
The mycelial plugs (4 mm diameter) from the edge of the growing colonies were excised and cultured in a fresh media (PCA, OA, and PDA) and incubated at 25 °C in the dark for 3 weeks. The morphological characteristics, including the colony colour and outline, mycelium structure, spores size, and the presence/absence of pycnidium, were determined using 3-week-old cultures. The spore size was measured for three strains using an Olympus BX-51 microscope (Olympus Corporation, Japan) at × 50 magnification. Moreover, the same microscope was used to observe the spore and mycelium structures. Relevant data were compared via Tukey’s test (P < 0.05) using IBM SPSS Statistics 19.0.
We also measured the effect of temperature on the pathogen growth after culturing the plates containing the mycelial plugs (4 mm diameter) were incubated at 5 °C, 10 °C, 15 °C, 20 °C, 25 °C, 30 °C, and 35 °C in the dark for 3 weeks. Colony diameter (4 replicates) was measured after 10 days using the method by Han et al.26.
DNA extraction, polymerase chain reaction (PCR) amplification, and sequencing
Total genomic DNA was extracted from mycelia (about 60 mm diameter), scraped from one-week colonies cultured on PDA, using Fungal Mini Kit (Omega bio-Tek, Doraville, CA), according to the manufacturer’s protocol with slight modifications. The purity and concentration of the obtained DNA were assessed using a NanoDrop ®ND-1000 UV (NanoDrop Technologies, USA), and the DNA was stored at − 20 °C for further analysis. The presence of rDNA internal transcribed spacer (ITS) region, actin (ACT), β-tubulin (TUB), and translation elongation factor-1 alpha (TEF) genes in the extracted DNA was determined by conventional PCR. The sequences of primers used are displayed in Table 3.
The PCR reactions were conducted on a DLAB PCR TC1000-G Thermo cycler in a final volume of 25 μL, which included 9 μL of ddH2O, 1 μL of each primer (forward and reverse), 1.5 μL of the template DNA, and 12.5 μL of DNA polymerase. The PCR amplification process consisted of an initial denaturation at 95 °C for 5 min followed by 35 cycles of denaturation at 95 °C for 30 s, a 2 min annealing at 53 °C for ITS, 56 °C for the ACT, 57 °C for TUB, and 55 °C for TEF, elongation at 72 °C for 2.5 min, and extension at 72 °C for 30 s. The final extension was at 72 °C for 8 min. The effectiveness of the PCR and the length of the amplified DNA fragments were verified by 1.5% agarose gel electrophoresis. DNA sequencing was then performed by Sangon Biotech (Wuhan, China).
Phylogenetic analysis
Sequences of all four target regions (ITS, ACT, TUB, and TEF) obtained from Boeremia species were individually aligned using the Clustal X program of the MEGA software version 7, and the parameters were manually adjusted to allow for maximum sequence similarity. Then the four loci were combined using the Sequence Matrix 1.8. Bayesian inference trees were then constructed using MrBayes v3.2. The Markov chain was run for a maximum of 1 million generations, in which every 50 generations were sampled and the first 25% of Markov chain Monte Carlo (mcmc) samples were discarded as burn-in.
Pathogenicity tests
The pathogenicity test was conducted using the pot experiment. The seeds of three Luobuma species used in the present study were collected in 2016 from a nursery comprising eight ecotypes, established in 2009 at Alakak township, Xinjiang Uyghur Autonomous Region, China (87° 33′ 50′′ E, 47° 42′ 41′′ N). The 8 ecotypes are the same as those grown in Yuzhong, Gansu Province. The seeds were sterilized with 75% alcohol and germinated in Petri dishes containing two layers of sterilized filter papers, previously soaked in 5 mL of sterile distilled water. Five days after germination, the seedlings were transplanted to planting trays containing vermiculite, which was autoclaved twice at 121 ℃ for 6 h. In April 2019, the seedlings were transplanted into pots (20 cm diameter and 60 cm height and made of artificial PVC pipe material) containing a mixture of loess, sand, and rotten chicken manure (volume ratio, 10:3:1). Each pot was transplanted with 4 seedlings, and the average greenhouse temperature was 25 °C during the day and 18 °C at night, while the average relative humidity (RH) was 72% during the day and 81% at night.
Since the six strains isolated from infected Luobuma stems had the same morphological and biological characteristics, we used AvLM-Be isolated from symptomatic Luobuma stems as a representative strain for Koch’s postulates analysis. Additionally, the Av-Be strain isolated from the soil was also used in the present study to determine the pathogenicity of the soil strains. The two strains (AvLM-Be and Av-Be) were cultured on PCA at 25 °C for one month to produce the pycnidium. Two groups of the pathogen inoculums were then re-prepared for inoculating the leaves and stems of Luobuma plants grown 60 days in pots. The inoculum suspension containing 1 × 107 spore was mixed with two drops of Tween 80, and 20 mL of the spore suspension was sprayed on the leaves of the eight Luobuma ecotypes. Four pots were set for each ecotype. The other pathogen inoculum was filtered by two layers of sterilized gauze, and the hypha segments and the colonized medium particles trapped on the gauze were used to inoculate the Luobuma stems. Three holes, about 0.2 mm depth, were created on the lower parts of the stems using a 0.3 mm diameter needle and were inoculated by placing the hypha segments or the colonized medium particles on the wounded parts of the stems. The plants were then covered with black plastic bags for 48 h after inoculation. After 5 days, the lengths of superficially visible stem necrotic lesions were measured, and the disease incidence of leaves was assessed and recorded.
Data analysis
The incidence and disease index data were analyzed using a numerical taxonomy and multi-variate analysis systems NTSYS-pc 2.1b (Exeter Software, Setauket, New York, USA), to clarify the resistant and susceptible ecotypes, as described by Villarino et al.30.
The significant ranges were: confirmed incidence of the stems necrosis lesions on Luobuma (< 1%; 1–2%; 2–3%; 3–4%; 4–5%; 5–6%; 6–7%; 7–8%; 8–9%; 9–10%; 10–11%; 11–12%; 12–13%; 13–14%; 14–15%; 15–16%; 16–17%; 17–18%; 18–19%; 20–21%; 21–22%; 22–23%; 23–24%; 24–25%; 25–26%; 26–27%; 27–28%; 28–29%; 29–30%; 30–31%; 31–32%; 32–33%; 33–34%; 34–35%); disease index of the stems necrosis lesions on Luobuma (< 1; 1–2; 2–3; 3–4; 4–5; 5–6; 6–7; 7–8; 8–9; 9–10; 10–11; 11–12; 12–13; 13–14; 14–15; 15–16; 16–17; 17–18; 18–19; 20–21; 21–22; 22–23; 23–24; 24–25).
Data availability
All data generated or analyzed during this study are included or specifed in this published article. The seeds were provided by Altay Gaubao Tea Co., LTD., located in Altay Prefecture of the Xinjiang Uyghur Autonomous Region, China. Plant materials were collected according to the relevant institutional, national, and international guidelines and legislation.
References
Xie, W. Y., Zhang, X. Y., Wang, T. & Hu, J. J. Botany, traditional uses, phytochemistry and pharmacology of Apocynum venetum L. (Luobuma): A review. J. Ethnopharmacol. 141, 1–8. https://doi.org/10.1016/j.jep.2012.02.003 (2012).
Thevs, N. et al. Apocynum venetum L. and Apocynum pictum Schrenk (Apocynaceae) as multi-functional and multi-service plant species in Central Asia: A review on biology, ecology, and utilization. J. Appl. Bot. Food Qual. 85, 159–167 (2012).
Jiang, Y. & Li, B. T. China Botanical Records (Beijing Science Press, 1977).
Lan, Y. R., Li, T., Duan, T. Y. & Gao, P. Effects of pappus removal and low-temperature short-term storage on interspecific and intraspecific variation in seed germination of Luobuma. Seed Sci. Technol. 47, 13–24. https://doi.org/10.15258/sst.2019.47.1.02 (2019).
Gao, P. et al. Characteristics photosynthetic physiology and growth with 8 Luobuma ecotypes in the Apocynum and Poacynum from Altay of Xinjiang, China. Acta Bot. Boreali Occid. Sin. 35, 2069–2077 (2015).
Aveskamp, M. M., de Gruyter, J., Woudenberg, J. H. C., Verkley, G. J. M. & Crous, P. W. Highlights of the Didymellaceae: A polyphasic approach to characterize Phoma and related pleosporalean genera. Stud. Mycol. 65, 1–60. https://doi.org/10.1080/21501203.2010.519156 (2010).
Kowalski, T., Kraj, W., Bednarz, B. & Rossa, R. The association of Boeremia lilacis with necrotic lesions on shoots and leaf petioles and its pathogenicity towards Fraxinus excelsior. Eur. J. Plant Pathol. 154, 961–974. https://doi.org/10.1007/s10658-019-01715-0 (2019).
Wei, H. L. Identification of pathogen species of mulberry snags rotten leaves disease (Guang xi University, 2015).
Garibaldi, A., Gilardi, G., Matic, S. & Gullino, M. L. First report of a leaf spot caused by Boeremia exigua var. exigua on Hydrangea paniculata in Italy. Plant Dis. 102, 1670–1671. https://doi.org/10.1094/PDIS-01-18-0064-PDN (2018).
Grinbergs, D., France, A. & Varrelmann, M. First Report of Boeremia exigua var. exigua (syn. Phoma exigua var. exigua) causing black root rot on Industrial Chicory (Cichorium intybus var. sativum) in Chile. Plant Dis. 100, 2328–2329. https://doi.org/10.1094/PDIS-05-16-0591-PDN (2016).
Banerjee, A. & Panja, B. First report of Boeremia exigua var. exigua as a pathogen of Cycas circinalis in India. J. Plant Pathol. 102, 935–936. https://doi.org/10.1007/s42161-020-00514-5 (2020).
Aveskamp, M. M. et al. Development of taxon-specific sequence characterized amplified region (SCAR) markers based on actin sequences and DNA amplification fingerprinting (DAF): A case study in the Phoma exigua species complex. Mol. Plant Pathol. 10, 403–414. https://doi.org/10.1111/j.1364-3703.2009.00540.x (2009).
Berner, D. et al. Assessment of Boeremia exigua var. rhapontica, as a biological control agent of Russian knapweed (Rhaponticum repens). Biol. Control 81, 65–75. https://doi.org/10.1016/j.biocontrol.2014.11.009 (2015).
Tunali, B., Eskandari, F. M., Berner, D. K., Farr, D. F. & Castlebury, L. A. First report of leaf blight caused by Phoma exigua on Acroptilon repens in Turkey. Plant Dis. 87, 1540. https://doi.org/10.1094/PDIS.2003.87.12.1540C (2003).
Hou, L. W. et al. The phoma-like dilemma. Stud. Mycol. 96, 309–396. https://doi.org/10.1016/j.simyco.2020.05.001 (2020).
You, J. M. et al. First report of Phoma exigua causing leaf spot on Japanese ginseng (Panax japonicus) in China. Plant Dis. 100, 534. https://doi.org/10.1094/PDIS-03-15-0355-PDN (2016).
Zhao, Q., Xie, X. W., Shi, Y. X., Chai, A. L. & Li, B. J. Boeremia leaf and fruit spot of okra caused by Boeremia exigua in China. Can. J. Plant Pathol. 38, 395–399. https://doi.org/10.1080/07060661.2016.1216896 (2016).
Gai, Y. P., Ma, H. J., Chen, X. L., Chen, H. H. & Li, H. Y. Boeremia tuber rot of sweet potato caused by B. exigua, a new post-harvest storage disease in China. Can. J. Plant Pathol. 38, 243–249. https://doi.org/10.1080/07060661.2016.1158742 (2016).
Grinbergs, D. E. & France, R. A. Black root rot of industrial chicory (Cichorium intybus L. var. sativum) in Chile caused by Boeremia exigua var. exigua. Phytopathology 104, 47–47 (2014).
Michel, V. V., Daepp, M., Woudenberg, J. H. C., de Gruyter, J. & de Wit, P. J. G. M. First report of Boeremia exigua var. exigua causing stem and leaf spot on Common Speedwell in Switzerland. Plant Dis. 102, 440–441. https://doi.org/10.1094/PDIS-05-17-0677-PDN (2018).
Gao, P. et al. Factors influencing rust (Melampsora apocyni) intensity on cultivated and wild Apocynum venetum in Altay Prefecture, China. Phytopathology 109, 593–606. https://doi.org/10.1094/PHYTO-04-18-0145-R (2019).
Kadir, S. & Umaerus, V. Varietal differences to infection of potato stems by the gangrene pathogen Phoma exigua var. foveata. Potato. Res. 30, 1–8. https://doi.org/10.1007/BF02357679 (1987).
Gorny, A. M. et al. Tan spot of lima bean caused by Boeremia exigua var. exigua in New York State, USA. Can. J. Plant Pathol. 37, 523–528. https://doi.org/10.1080/07060661.2015.1105873 (2015).
Gao, P., Duan, T. Y., Nan, Z. B. & O’Connor, P. J. First report of Septoria apocyni causing spot blight on the species of Apocynum venetum and Poacynum pictum in China. Plant Dis. 98, 1429. https://doi.org/10.1094/PDIS-02-14-0133-PDN (2014).
Johnson, L. F., Curl, E. A., Bond, J. H. & Fribourg, H. A. Method for studying soil microflora-plant disease relationships. Burgess Publ. Co. 22, 178 (1959).
Han, Y. C. et al. Distribution and characteristics of Colletotrichum spp. associated with anthracnose of strawberry in Hubei, China. Plant Dis. 100, 996–1006. https://doi.org/10.1094/PDIS-09-15-1016-RE (2016).
White, T. J., Bruns, T. D., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications (eds Innis, M. A. et al.) 315–322. (Academic Press, 1990).
Carbone, I. & Kohn, L. M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91, 553–556. https://doi.org/10.2307/3761358 (1999).
Glass, N. L. & Donaldson, G. C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microb. 61, 1323–1330. https://doi.org/10.1128/AEM.61.4.1323-1330.1995 (1995).
Villarino, M., Melgarejo, P. & De Cal, A. Growth and aggressiveness factors affecting Monilinia spp. survival peaches. Int. J. Food Microbiol. 227, 6–12. https://doi.org/10.1016/j.ijfoodmicro.2016.02.011 (2016).
Acknowledgements
This research was financially supported by Key Project of Science and Technology Department of Xinjiang Autonomous Region, China (2016E02015, 2016A03006).
Author information
Authors and Affiliations
Contributions
Writing of original draft: Y.L.; funding acquisition and revising of original draft: T.D. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lan, Y., Duan, T. Characterization of Boeremia exigua causing stem necrotic lesions on Luobuma in northwest China. Sci Rep 12, 21609 (2022). https://doi.org/10.1038/s41598-022-25125-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25125-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.