Abstract
This study aimed to evaluate the protein expression of glutathione peroxidase 4 (GPX4) in resected non-small cell lung cancer (NSCLC). The clinical relevance and prognostic significance of GPX4 expression were analyzed. We reviewed patients with resected NSCLCs at Taipei Veterans General Hospital between September 2002 and January 2018. Available paraffin-embedded specimens were retrieved for immunohistochemistry (IHC) staining to detect GPX4 expression. The cutoff value for defining GPX4 positivity was determined according to the percentage of tumor stained in the microscopic field. The correlation between immune expression, clinicopathologic data, overall survival (OS), and disease-free survival (DFS) were analyzed. A total of 265 NSCLC specimens were retrieved for IHC staining. GPX4 expression positive was in 192 (72.5%) according to a cutoff value of 5%. GPX4 was a significant prognostic factor for OS and DFS on multivariate analysis at both 5% and 25% cutoff values. GPX4 expression was associated with poor OS and DFS, especially in lung adenocarcinoma (p = 0.008, and 0.027, respectively). In conclusions, IHC analysis revealed that GPX4 expression was associated with poor survival outcomes in patients with resected lung adenocarcinoma. Further research is needed to understand the role of GPX4 in tumorigenesis and the underlying mechanism responsible for survival outcomes in patients with resected lung adenocarcinoma.
Similar content being viewed by others
Introduction
Lung cancer remains the leading cause of cancer death1. Non-small cell lung cancer (NSCLC) accounts for 85% of cases of lung cancer, of which lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) are the most common subtypes. LUAD is the most common type of NSCLC and accounts for approximately 40% of lung cancers2. We have seen substantial progress in targeted therapy and immunotherapy for lung cancer with a more individualized approach in the past decades3. Despite advances in treatment options for lung cancer in the past two decades, the prognosis for patients with lung cancer is still unsatisfactory4. The identification of predictive and prognostic biomarkers that can help define the most appropriate treatment for NSCLC patients remains an unmet medical need.
Oxidative stress, an imbalance between reactive oxygen species (ROS) and antioxidant defense mechanisms in the cell, is involved in several physiological and pathological processes, including carcinogenesis. Glutathione peroxidases (GPXs) are an enzyme family with peroxidase activity that protects organisms from oxidative damage by reducing lipid hydroperoxides and free hydrogen peroxide5. The GPX family member glutathione peroxidase 4 (GPX4), the only isoenzyme that can reduce phospholipid hydroperoxide6, was considered one of the most important antioxidant enzymes in mammals7. There are few studies that suggest that the role of GPX4 in human cancer is complex and paradoxical8,9,10,11,12,13,14,15,16,17. It can act as a tumor suppressor8,9,10,11,12 and an oncogene13,14,15,16,17, according to published reports.
In NSCLC, recent reports suggest that human lung cancer cell growth can be inhibited by GPX4-related ferroptosis, a type of cell death characterized by lethal accumulation of lipid-based ROS17,18. In other words, GPX4 could help cancer cells evade death by eliminating oxidative stress, subsequently causing progression and poor prognosis. A recent report showed that high GPX4 mRNA expression correlated with poor prognosis in LUAD19. However, it is not clear if GPX4 can serve as a biomarker with clinical relevance to stratify high-risk patients, and predict their outcomes. In this study, we evaluated the association between protein expression of GPX4 and prognosis in patients with resected NSCLC.
Methods
Patients
Tumor specimens were obtained from 265 patients with NSCLC who underwent lung resection between September 2002 and January 2018 at Taipei Veterans General Hospital. There were 91 females and 174 males, with a mean age of 65 years (range 35–90). These patients were diagnosed with clinical stage I-III, not pretreated with neoadjuvant chemotherapy or radiotherapy and underwent lung resection as a cure. The clinicopathologic data, tumor grade, tumor size, lymph node involvement, and the presence of distant metastases were retrospectively collected. The survival data were obtained from the Cancer Registry Database in the Taipei Veterans General Hospital (VGH). Overall survival (OS) and disease-free survival (DFS), defined as the time from operation to death and recurrence, respectively, were used as surrogates of prognosis. This study was approved by the institutional review board (IRB) of the Taipei VGH, which waived the requirement for individual written patient consent (IRB approval No. 2019-11-011AC, on Nov 20, 2019). All methods were carried out in accordance with relevant guidelines and regulations.
Immunohistochemical (IHC) staining
IHC staining was performed on 4-μm-thick sections of formalin-fixed, paraffin-embedded tissue. After deparaffinization and rehydration, all sections were treated with microwaves in 10 mmol/L citrate buffer (pH 6.0) for 10 min for antigen retrieval. Following the blocking of endogenous peroxidase activity, the specimens were incubated at 4 °C overnight with the primary antibody to GPX4 (1:200, ab125066; Abcam, Cambridge, MA, USA). After washes, sections were subsequently incubated with biotinylated secondary antibodies (K4065; Dako, USA) for 30 min at room temperature. Slides were stained using 3,3′-diaminobenzidine chromogen (DAB) solution, and counterstained with hematoxylin, followed by mounting.
Evaluation of IHC staining was conducted by a board-certified pathologist who was blinded to the patients’ clinical data. Since there is no consensus regarding the cutoff value of GPX4 protein expression, we determined negative if less than 5% of tumor cell were stained. On the contrary, the expression was determined positive if ≥ 5% of tumor cells were IHC-stained positive. For those with positive GPX4 expression, we categorized “GPX4+” if 5% to 24% of cells were positive, “GPX4++” if 25% to 75% of cells were positive, and “GPX4+++” if more than 75% of cells were positive after IHC staining. The IHC staining patterns are shown in Fig. 1.
Immunohistochemical staining patterns of glutathione peroxidase 4 (GPX4) in resected non-small cell lung cancer specimens (original magnification × 400). Expression was marked and is shown in the left upper corner of each photograph as “GPX4−” if less than 5% of cells were positive (A), as “GPX4+” if 5–25% of cells were positive (B), as “GPX4++” if 25–75% of cells were positive (C), and as “GPX+++” if more than 75% of cells were positive (D) in the microscopic field.
Statistical analysis
The correlations between IHC staining results and clinicopathological variables were analyzed by Pearson’s χ2 test. Survival curves were estimated by the Kaplan–Meier method, and groups were compared for outcome by the log rank test. Univariate and multivariate analysis were performed with the Cox regression model. Factors in univariate analysis with a p-value < 0.05 would be included for multivariate analysis with Enter Method. A p-value < 0.05 was significant. All calculations were performed with SPSS version 22 (IBM Corp., Armonk, NY, USA).
Results
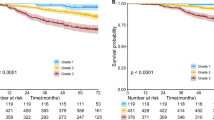
In the cohort of 265 patients with a mean age of 65.46 ± 10.94 years (range 35–90), the overall 5-year survival was 62.6%, with a median follow-up of 65 months. Demographic data and survival analysis grouped by clinicopathological characteristics are presented in Table 1. Two-third of patients were male (65.7%). According to patients’ clinical condition, pneumonectomy, bilobectomy, lobectomy, and sublobar resections were performed in 6 (2.3%), 8 (3.0%), 211 (79.6%), 40 (15.1%) patients, respectively, at operating surgeons’ discretion. After surgery, 145 (54.7%), 36 (13.6%), and 73 (27.5%) patients were in their pathological stage I, II, and III, respectively. However, 11 (4.2%) patients were upstaged to stage IV because of intraoperatively-found pleural seedings (M1) which remained resectable. Among then, 5 lobectomies and 6 sublobar resections were still performed as curative intent. The 265-formalin-fixed, paraffin-embedded surgical specimens were immunohistochemically stained to determine the GPX4 expression level. The pathologic tumor stage was determined according to the 7th edition of the American Joint Committee on Cancer staging system. For patients with pathological stage IA and IB with tumor < 3 cm, no further treatment was given. For patients with pathological stage IB with tumor ≥ 3 cm, daily oral chemotherapy with Ufur (Tegafur 100 mg + Uracil 224 mg) would be given as adjuvant therapy for 2 years. For patients with stage II and above, adjuvant chemotherapy ± radiotherapies were given. Tyrosine-kinase inhibitors (TKIs) were selectively given to patients with advanced diseases or those having disease recurrence after primary treatments. In total, 104 (39.8%) patients had adjuvant therapies after pulmonary resections, 136 (51.3%) patients had disease recurrence during follow-ups, and 112 (42.3%) patients had post-recurrence therapies. Of the 265 resected lung specimens, there were 197 adenocarcinomas versus 68 squamous cell carcinomas. In all, 29 (10.9%), 122 (46.0%), and 114 (43.0%) of the 265 tumors were respectively well, moderately, and poorly differentiated. Protein expression of GPX4 ≥ 5% of tumor cell detected by IHC staining was defined “positive” and observed in 192 (72.5%) specimens. In total, we have 65 GPX4+++, 68 GPX4++, 59 GPX4+, and 73 GPX4- according the definition described earlier. We have demonstrated GPX4 expression was significantly linked to 5-year survival (p = 0.005) (Fig. 2). There was no significant correlation between GPX4 expression and clinicopathologic factors in resected lung specimens at a cutoff value of 5%. However, we observed male patients (p = 0.001), smoker (p < 0.001), and LUSC (p < 0.001) were associated with positive GPX4 expression as we defined GPX4 expression at a cutoff value of 25% (Table 2). For clinicopathological factors, older age (p = 0.001), male patients (p < 0.001), smoker (p = 0.002), sublobar resections (p = 0.002), Tumor grade (p = 0.012), “T” (p = 0.005), “N” (p < 0.001), “M” (p = 0.006), and GPX4 (p = 0.017 and 0.002, at 5% and 25% cutoff, respectively) were associated with poor OS in univariate analysis. In multivariate analyses, only age, surgical resection, tumor grade, “T”, “N”, and GPX4 expression remained significant prognostic factors for OS with GPX4 cutoff value at both 5% and 25% (Table 3). As our definition of GPX4 expression, patients with GPX positive had worse OS and DFS than those with GPX negative (Fig. 3A,D, log rank p = 0.013 and 0.023, respectively). We further sub-grouped our cohort into LUSC and LUAD. In patients with resected LUSC, survival analysis showed that patients with positive or negative GPX4 expression had similar survival and recurrence outcomes (Fig. 3B,E, log rank p = 0.735 and 0.682, respectively). However, in patients with resected LUAD, patients with positive GPX4 expression had a significantly worse OS and DFS than those with negative GPX4 expression (Fig. 3C,F, log rank p = 0.008 and 0.027, respectively).
Glutathione peroxidase 4 (GPX4) expression with a 5% cutoff value was significantly associated with OS and DFS in patients with resected non-small cell lung cancer (A and D, log rank p = 0.013 and 0.023, respectively). In patients with resected lung squamous cell carcinoma, survival analysis showed that with or without GPX4 expression they had similar survival outcomes (B and E, log rank p = 0.735 and 0.682, respectively). However, in patients with resected lung adenocarcinoma, GPX4 expression had a significantly worse OS and DFS than those with negative GPX4 expression (C and F, log rank p = 0.008 and 0.027, respectively).
Discussion
GPXs have been known to catalyze the reduction of H2O2 or organic hydroperoxide to water or the corresponding alcohols using glutathione (GSH) as a reductant. GPXs include eight members in different body tissues with different functions. In cancer, GPX1 and GPX3 are tumor suppressors, while GPX2 may have dual roles in tumorigenesis20. GPX4, a selenoprotein, is the only known antioxidant enzyme that can directly reduce peroxidized phospholipid and cholesterol in membranes, and use a wide range of reducing cofactors in addition to GSH21. Almost all normal mammalian cells show GPX4 activity, particularly abundant in testis, adipose tissue, and retina22. In tumors, the role of GPX4 has been ambiguous and paradoxical. GPX4 expression was increased in renal cell carcinoma14, hepatocellular carcinoma15, and colon cancer23 but decreased in pancreatic cancer9, breast cancer10, renal cell carcinoma24, gastric cancer25, hepatocellular carcinoma11, and human astrocytoma12. This finding means that in different types of cancer, even in the same type, GPX4 can take on different roles regulating tumorigenesis17. There are fewer reports on the role of GPX5, -6, -7, and -8 in tumorigenesis19,20.
The association between GPX4 and cancer OS has been investigated. GPX4 expression was found negatively associated with OS in cholangiocarcinoma, colon cancer, and LUSC patients. However, in patients with hepatocellular carcinoma, skin melanoma, testicular germ cell tumor, uterine carcinosarcoma, there was no significance between GPX4 and OS17. In a study investigating the association between GPX4 and OS in patients with diffuse large B cell lymphoma (DLBCL), the GPX4-positive group had a significantly poorer prognosis in OS. However, there was no significant difference in mRNA levels between the GPX4-positive group and GPX4-negative group, suggesting that post-transcriptional regulation may be involved in GPX4 protein expression in DLBCL13. Liu et al., using an online database, reported that high GPX4 mRNA expression correlated with OS in LUAD patients19. To the best of our knowledge, no study has yet assessed the relationship between GPX4 protein expression and OS in patients with resected NSCLC. In our study, 192 (72.5%) patients were positive for GPX4 protein expression, and these patients showed a significantly poor prognosis in OS and DFS. Moreover, GPX4 was an independent prognostic factor for OS and DFS. These results suggest that GPX4 expression might result in poor prognosis by a different mechanism than the existing known prognostic predictors of lung cancer, such as TNM staging.
Ferroptosis, a unique form of regulated cell death first reported by Dr. Brent R. Stockwell in 2012, describes a non-apoptotic form of iron-dependent oxidative cell death26. This lethal process is mediated by the accumulation of lipid peroxidation products derived from iron metabolism with subsequent depletion of plasma membrane components, including cholesterol and polyunsaturated fatty acids27. In the context of cancer, evidence indicates that cancer cells produce a higher level of ROS compared to normal cells, suggesting that it might be possible to eliminate cancer cells by modulating their redox status28. GPX4, a known negative regulator of ferroptosis, may play a crucial role in preventing cells from ferroptosis. The hypothesis of whether NSCLC, especially LUAD, is sensitive to ferroptosis remains an open question. If this hypothesis can be applied to our results, the poor prognosis of LUAD with GPX4 expression may be due to the suppression of ferroptosis in cancer cells. In other words, GPX4-expressing tumor cells are likely to acquire the ability to detoxify ROS, and be resistant to therapy. This theory was echoed by a study that showed erastin, a ferroptosis inducer, decreased NSCLC cell radioresistance by inducing GPX4-mediated ferroptosis18. We would need to verify this point by observing whether cell death increases under treatment with GPX4 inhibitors in future studies.
There has been no consensus regarding the cutoff values of GPX4 protein expression predicting overall survival of NSCLCs. The reason why we defined a positive GPX4 expression at a cutoff value of 5% is that it is easier to differentiate a stained specimen (≥ 5%) from a barely or non-stained one (< 5%). Technically, there is much ambiguity to differentiate among less-stained specimens (5–24%), moderately-stained specimens (25–75%), and more-stained ones (> 75%). In our study, the association between GPX4 expression and overall survival at a 25% cutoff value (p = 0.010) was statistically stronger than it was at a 5% cutoff value (p = 0.048) (Table 3). Although, it seemed reasonable to set GPX4 expression at a 25% cutoff value in our study when it comes to better differentiating survival outcomes, we were not eligible to say which cutoff was the best.
In our study, GPX4 expression was not prognostic in LUSC patients. We have not yet known the reason. However, we noted that smoker, male patients, and LUSC were associated with positive GPX4 expression when we defined GPX4 positive at a cutoff value of 25% (Table 2). Since smoking was known to be associated with ferroptosis and GPX4 expression29, we believed that the association between smoking and LUSC may be one of the potential reasons why no correlation could be observed between GPX4 expression and LUSC in this study.
There are still several unresolved problems and limitations in this study. First, this study was unable to elucidate the underlying mechanism causing the poor prognosis in patients with GPX4 positive NSCLC. GPX4, an antioxidant enzyme, may play an important role in carcinogenesis in the context of lung cancer. However, the exact roles of oxidative stress and antioxidant in carcinogenesis have been controversial because oxidative stress can promote or suppress tumor development in different contexts30. The relationships between oxidative stress and the redox system in cancer can be complex. There is no consensus on the relationship between GPX4 expression and patient survival in lung cancer. Second, ferroptosis prevented by GPX4 might be one of the mechanisms that explain the relative immortal nature of cancer cells that contributed to poor prognosis in patients with GPX4-positive NSCLC in this study. However, the causal relationship between ferroptosis and survival is not yet proven herein. Third, the prognostic role of GPX4 for LUSC was not demonstrated in this study. It can be explained by a relatively small group of LUSC patients recruited for analysis or explained by the interaction between smoking and GPX4 expression in LUSC group. Fourth, the cohort of the present study included only patients with resected NSCLCs. The results cannot be well-applied to whole NSCLC groups in which the majority was unable to have lung resection as a treatment option.
In conclusion, using IHC analysis, we found that GPX4 expression is independently associated with poor OS and DFS in NSCLC, especially in LUAD. GPX4 is a prognostic predictor and has the potential to become an anticancer drug target for NSCLC treatment. Further investigation is needed to understand the roles of GPX4 in lung cancer tumorigenesis and its underlying mechanisms responsible for survival.
Data availability
All data analysed during this study are included in this published article.
References
Bade, B. C. & Dela Cruz, C. S. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin. Chest Med. 41, 1–24 (2020).
Duma, N., Santana-Davila, R. & Molina, J. R. Non-small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clin. Proc. 94, 1623–1640 (2019).
Naylor, E. C., Desani, J. K. & Chung, P. K. Targeted therapy and immunotherapy for lung cancer. Surg. Oncol. Clin. N. Am. 25, 601–609 (2016).
Lemjabbar-Alaoui, H., Hassan, O. U., Yang, Y. W. & Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta 1856, 189–210 (2015).
Jiao, Y., Wang, Y., Guo, S. & Wang, G. Glutathione peroxidases as oncotargets. Oncotarget 8, 80093–80102 (2017).
Imai, H. & Nakagawa, Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic. Biol. Med. 34, 145–169 (2003).
Yant, L. J. et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 34, 496–502 (2003).
Heirman, I. et al. Blocking tumor cell eicosanoid synthesis by GP x 4 impedes tumor growth and malignancy. Free Radic. Biol. Med. 40, 285–294 (2006).
Liu, J. et al. Suppression of the malignant phenotype in pancreatic cancer by overexpression of phospholipid hydroperoxide glutathione peroxidase. Hum. Gene Ther. 17, 105–116 (2006).
Cejas, P. et al. Phospholipid hydroperoxide glutathione peroxidase (PHGPx) expression is downregulated in poorly differentiated breast invasive ductal carcinoma. Free Radic. Res. 41, 681–687 (2007).
Rohr-Udilova, N. et al. Impact of glutathione peroxidase 4 on cell proliferation, angiogenesis and cytokine production in hepatocellular carcinoma. Oncotarget 9, 10054–10068 (2018).
Yen, H. C. et al. Alterations of the levels of primary antioxidant enzymes in different grades of human astrocytoma tissues. Free Radic. Res. 52, 856–871 (2018).
Kinowaki, Y. et al. Glutathione peroxidase 4 overexpression inhibits ROS-induced cell death in diffuse large B-cell lymphoma. Lab. Investig. 98, 609–619 (2018).
Su, Y. et al. Effect of GPX4 on proliferation and metastasis of renal clear cell carcinoma and its relationship with expression of IGF-1R and COX-2. Zhonghua Bing Li Xue Za Zhi 48, 955–960 (2019).
Guerriero, E. et al. GPX4 and GPX7 over-expression in human hepatocellular carcinoma tissues. Eur. J. Histochem. 59, 2540 (2015).
Lee, J. R. et al. Overexpression of glutathione peroxidase 1 predicts poor prognosis in oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 143, 2257–2265 (2017).
Zhang, X. et al. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. J. Cell. Physiol. 235, 3425–3437 (2020).
Pan, X. et al. Erastin decreases radioresistance of NSCLC cells partially by inducing GPX4-mediated ferroptosis. Oncol. Lett. 17, 3001–3008 (2019).
Liu, K., Jin, M., Xiao, L., Liu, H. & Wei, S. Distinct prognostic values of mRNA expression of glutathione peroxidases in non-small cell lung cancer. Cancer Manag. Res. 10, 2997–3005 (2018).
Brigelius-Flohe, R. & Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 1830, 3289–3303 (2013).
Aumann, K. D., Bedorf, N., Brigelius-Flohe, R., Schomburg, D. & Flohe, L. Glutathione peroxidase revisited-simulation of the catalytic cycle by computer-assisted molecular modelling. Biomed. Environ. Sci. 10, 136–155 (1997).
Roveri, A., Maiorino, M. & Ursini, F. Enzymatic and immunological measurements of soluble and membrane-bound phospholipid-hydroperoxide glutathione peroxidase. Methods Enzymol. 233, 202–212 (1994).
Yagublu, V. et al. Expression of selenium-containing proteins in human colon carcinoma tissue. Anticancer Res. 31, 2693–2698 (2011).
Rudenko, E. et al. Aberrant expression of selenium-containing glutathione peroxidases in clear cell renal cell carcinomas. Exp. Oncol. 37, 105–110 (2015).
Lan, X. et al. Decreased expression of selenoproteins as a poor prognosticator of gastric cancer in humans. Biol. Trace Elem. Res. 178, 22–28 (2017).
Dixon, S. J. et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Xie, Y. et al. Ferroptosis: Process and function. Cell Death Differ. 23, 369–379 (2016).
Trachootham, D., Alexandre, J. & Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach?. Nat. Rev. Drug Discov. 8, 579–591 (2009).
Ou, Z. et al. Cigarette smoking is associated with high level of ferroptosis in seminal plasma and affects semen quality. Reprod. Biol. Endocrinol. 27, 55 (2020).
Harris, I. S. & Brugge, J. S. Cancer: The enemy of my enemy is my friend. Nature 527, 170–171 (2015).
Acknowledgements
We sincerely thank Ya-Hui Tsai, PhD, research specialist in the Division of Pediatric Surgery, Department of Surgery, Far-Eastern Memorial Hospital for editing the figures in the manuscript.
Funding
This study was supported by grants from the Far-Eastern Memorial Hospital National Yang Ming Chiao Tung University Joint Research Program (109DN17).
Author information
Authors and Affiliations
Contributions
C.-Y.L.: Conception and design; Drafting of the manuscript. C.-C.L.: Critical revision of the manuscript. A.F.-Y.L.: Analysis and interpretation of data; Administrative, technical or material support. T.-W.H.: Acquisition of data; Analysis and interpretation of data; Administrative, technical or material support. J.-H.L.: Acquisition of data; Analysis and interpretation of data; Administrative, technical or material support. S.-C.H.: Conception and design. H.-S.H.: Conception and design; Administrative, technical or material support; Supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, CY., Liu, CC., Li, A.FY. et al. Glutathione peroxidase 4 expression predicts poor overall survival in patients with resected lung adenocarcinoma. Sci Rep 12, 20462 (2022). https://doi.org/10.1038/s41598-022-25019-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25019-2
This article is cited by
-
Clobetasol propionate, a Nrf-2 inhibitor, sensitizes human lung cancer cells to radiation-induced killing via mitochondrial ROS-dependent ferroptosis
Acta Pharmacologica Sinica (2024)
-
Prognostic signature based on mitochondria quality control proteins for the prediction of lung adenocarcinoma patients survival
Cell Death Discovery (2023)
-
GPX4 overexpressed non-small cell lung cancer cells are sensitive to RSL3-induced ferroptosis
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.