Abstract
Our aim was to validate and analyze the prognostic impact of the novel International Association for the Study of Lung Cancer (IASLC) Pathology Committee grading system for invasive pulmonary adenocarcinomas (IPAs) in Chinese patients and to evaluate its utility in predicting a survival benefit from adjuvant chemotherapy (ACT). In this multicenter, retrospective, cohort study, we included 926 Chinese patients with completely resected stage I IPAs and classified them into three groups (Grade 1, n = 119; Grade 2, n = 431; Grade 3, n = 376) according to the new grading system proposed by the IASLC. Recurrence-free survival (RFS) and overall survival (OS) were estimated by the Kaplan–Meier method, and prognostic factors were assessed using univariable and multivariable Cox proportional hazards models. All included cohorts were well stratified in terms of RFS and OS by the novel grading system. Furthermore, the proposed grading system was found to be independently associated with recurrence and death in the multivariable analysis. Among patients with stage IB IPA (N = 490), the proposed grading system identified patients who could benefit from ACT but who were undergraded by the adenocarcinoma (ADC) classification. The novel grading system not only demonstrated prognostic significance in stage I IPA in a multicenter Chinese cohort but also offered clinical value for directing therapeutic decisions regarding adjuvant chemotherapy.
Similar content being viewed by others
Introduction
According to the predominant histologic pattern, the 2015 WHO classification system categorized invasive pulmonary adenocarcinoma (IPA) into three groups: low grade (lepidic predominant), intermediate grade (acinar or papillary predominant), and high grade (solid or micropapillary predominant)1. Previous studies showed that the classification according to the predominant pattern was a prognostic factor and predictor of response to adjuvant chemotherapy (ACT)2,3. In our previous study, we demonstrated that high grade, as determined by the adenocarcinoma classification, could predict recurrence-free survival (RFS) and the benefit from ACT in patients with stage IB IPAs4.
Recently, by considering the cribriform and fused gland patterns indicative of high-grade IPA, the International Association for the Study of Lung Cancer (IASLC) Pathology Committee proposed a novel grading system for IPA: well-differentiated (lepidic predominant containing no or less than 20% high-grade patterns), moderately differentiated (acinar or papillary predominant containing no or less than 20% high-grade patterns), poorly differentiated (any tumor containing 20% or more high-grade patterns)5. The prognostic impact of this novel grading system on RFS and overall survival (OS) has been subsequently confirmed in patients with stages I-III and III-IV IPAs5,6.
However, to date, the utility of this grading system in predicting a survival benefit from ACT has not been estimated, especially in patients with upgraded IPA. Thus, in this study, we aimed to provide an external validation of the newly proposed grading system and investigate its predictive value for ACT benefit in Chinese cohorts.
Materials and methods
Study cohorts
This multicohort study was approved by the institutional review boards of the participating institutions: the Shanghai Pulmonary Hospital (SPH), the Second Affiliated Hospital of Soochow University (SAH), and Ningbo No.2 Hospital (NNH). We reviewed the pathologic reports of all patients who underwent complete surgical resection of non-small-cell lung cancer between January 2015 and December 2015 at the three institutions. Overall, data on 3725 patients with a pathologic diagnosis of IPA were retrieved. Patients who satisfied the following criteria were excluded: (a) incomplete clinicopathologic and follow-up information (n = 341); (b) synchronous or metachronous lung cancer (n = 478); (c) stage II or higher stage lesions (n = 1685); (d) lack of complete pathologic slices for histologic re-evaluation (n = 137); and (e) diagnosis of invasive mucinous adenocarcinoma or other variants (n = 158). Finally, according to the 8th edition of the TNM classification of malignant tumors7, 926 patients (SPH cohort, n = 661; SAH cohort, n = 134; NNH cohort, n = 131) with stage I IPA were included in this study (Fig. S1). We set the follow-up endpoints as RFS and OS, which were defined as the duration from the date of surgery to that of recurrence and the duration from surgery until death or the last follow-up, respectively.
Histologic evaluation and grading criteria
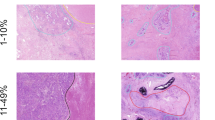
The formalin-fixed and paraffin-embedded tissue specimens of all included patients were concurrently re-evaluated by two pathologists (Likun Hou and Chunyan Wu) using a multiheaded microscope, and cases were discussed until an agreement on diagnosis was made. Histologic patterns of IPA were classified according to the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) multidisciplinary classification of lung adenocarcinoma (Fig. S2); the percentage of each pattern was recorded in 5% increments8. Based on the percentage of histologic patterns, all patients were categorized into three grades based on the new grading system proposed by the IASLC Pathology Committee: grade 1, lepidic-predominant tumor, containing less than 20% high-grade patterns (micropapillary, solid, complex glandular patterns); grade 2, acinar- or papillary-predominant tumor, containing less than 20% high-grade patterns; and grade 3, any tumor containing greater than or equal to 20% high-grade patterns. For comparison, we also recorded the grades according to the ADC classification, which were as follows: grade 1, lepidic predominant; grade 2, acinar or papillary predominant; and grade 3, solid or micropapillary predominant.
Statistical analysis
Continuous variables are presented as the mean and standard deviation and were compared using Student’s t test; categorical variables are presented as counts with percentages and were compared using Pearson’s chi-squared test. The survival outcomes of different pattern groups or treatment groups were estimated with the Kaplan–Meier method, and the log-rank test was performed to test significance. Univariable and multivariable Cox proportional hazards regression analyses were performed to identify the independent prognostic factors. All Kaplan–Meier analyses were conducted using the “survival” package in R software (version 3.5.3, http://www.R-project.org). The comparison of baseline information and Cox proportional hazards analyses were performed using SPSS (version 20.0, IBM, Armonk, NY, USA). A two-sided p value less than 0.05 was considered statistically significant for all analyses.
Results
Baseline characteristics
The clinicopathologic characteristics of the 926 included patients from three institutions are illustrated in detail in Table 1. According to the new grading system, 63 (9.5%), 320 (48.4%), and 278 (42.1%) patients in the SPH cohort were stratified into grade 1, grade 2, and grade 3, respectively. The SAH and NNH cohorts had more patients with grade 1 but fewer patients with grade 2 and grade 3 tumors (p < 0.001). Moreover, the SPH cohort contained more patients with stage IB disease (p < 0.001) and positive visceral pleural invasion (p = 0.015). The remaining characteristics, including age, sex, smoking status, surgery strategy, and lymphovascular invasion, were similar among the three cohorts.
Prognostic performance of the grading system
Of all 926 patients from three institutions, significant differences were found in RFS (Fig. 1A, p < 0.001) and OS (Fig. 1B, p < 0.001) among grade 1, grade 2, and grade 3, as defined by the newly proposed grading system. In the SPH cohort, the prognoses of patients stratified into grade 3 by the new grading system were significantly worse than those of patients in grade 2 and grade 1 (Fig. S3-left panel). The 5-year RFS and OS rates in the SPH cohort were 96.7% and 96.8% in grade 1, 85.6% and 90.5% in grade 2, and 76.4% and 82.2% in grade 3, respectively (both p < 0.001). Similar prognostic trends were well validated in the SAH and NHH cohorts, in which the grading system clearly discriminated the survival difference among the three grades (Fig. S3-middle and right panels). For patients from SAH, the 5-year RFS and OS rates were 92.4% and 92.6% for grade 1, 76.1% and 77.4% for grade 2, and 74.5% and 78.5% for grade 3, respectively (p = 0.003 and 0.023 for RFS and OS, respectively); the 5-year RFS and OS of patients from NNH were 93.0% and 93.1% for grade 1, 78.0% and 81.4% for grade 2, and 72.2% and 75.7% for grade 3, respectively (p < 0.001 and 0.003 for RFS and OS, respectively).
The univariable analysis found that all included clinicopathologic factors, except for smoking history (p = 0.728) and surgery strategy (p = 0.837), were significantly associated with patients’ RFS and OS (Table 2, all p < 0.05). In particular, for the predominant subtypes, the survival outcomes did not significantly differ between patients whose tumors had predominant complex glandular patterns and those whose tumors had micropapillary or solid patterns (p = 0.311 and 0.115 for RFS, p = 0.468 and 0.117 for OS). After multivariable adjustment by statistically significant clinicopathological variables (Table 2), the proposed grading system remained a powerful and independent predictor of RFS (HR for grade 1: 0.165, 95% CI: 0.067–0.409, p < 0.001; HR for grade 2: 0.526, 95% CI: 0.383–0.722, p < 0.001) and OS (HR for grade 1: 0.134, 95% CI: 0.042–0.428, p = 0.001; HR for grade 2: 0.482, 95% CI: 0.287–0.639, p < 0.001). In addition, the presence of visceral pleural invasion (p < 0.001 for RFS and p = 0.004 for OS) and lymphovascular invasion (p < 0.001 for RFS and p = 0.002 for OS) might each serve as an independent factor associated with increased risk of recurrence and death. We also found that age (p = 0.004) was another important and independent prognostic factor in determining OS rates. In the subgroups stratified by pathologic stage and age, those patients with grade 3 tumors exhibited significantly worse RFS and OS than those with grade 2 or grade 1 tumors (all p < 0.001, Fig. 2 and Fig. S4), which demonstrates that the new grading system could be a strong and stable prognostic factor.
Predictive implication of the new grading system for ACT benefit
Of 490 patients with stage IB IPA, 298 (60.8%) patients were treated with ACT, while another 192 (39.2%) patients did not receive any adjuvant therapy. Table 3 summarizes and compares the baseline information of the two groups, which indicates that patients younger than 65 years (p < 0.001), with a history of smoking (p < 0.001), higher pathological grades (p < 0.001), visceral pleural invasion (p = 0.025) and lymphovascular invasion (p = 0.034) tended to receive ACT. With the adenocarcinoma classification and proposed grading system, 36 (7.3%), 407 (83.1%), and 47 (9.6%) patients were categorized into grade 1, grade 2, and grade 3, respectively. However, among patients categorized as grade 2 according to the ADC classification (n = 407), 171 patients (42.0%) were upgraded to grade 3 under the new grading system (Table S1).
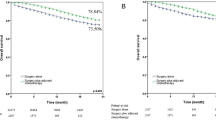
We noted that ACT did not enhance survival in all 407 patients with previous grade 2 disease (Fig. 3-left panel, p = 0.220 and 0.150 for RFS and OS, respectively). We further found that while the adoption of ACT showed no association with improved survival in 236 patients in the previous and newly proposed grade 2 category (Fig. S5-left panel, p = 0.39 for RFS and p = 0.48 for OS, respectively), a significant added benefit of ACT (Fig. 3-middle panel, both p < 0.001 for RFS and OS) was found for 171 patients who were upgraded from grade 2 to the newly proposed grade 3. In addition, all patients with previous or newly proposed grade 3 disease (Fig. S5-right panel and Fig. 3-right panel, all p < 0.001) showed significantly prolonged survival after treatment with ACT. The multivariable analysis revealed a significant benefit from ACT in patients with grade 3 tumors (HR for RFS: 0.373, 95% CI: 0.236–0.589, p < 0.001; HR for OS: 0.340, 95% CI: 0.198–0.584, p < 0.001) but not in those with grade 2 tumors (p = 0.387 for RFS and p = 0.479 for OS), in accordance with the newly proposed grading system.
Discussion
In this study, we provide a large external validation of the novel grading system for IPA proposed by the IASLC Pathology Committee regarding its prognostic value in stage I disease and its ability to predict ACT benefit. Our results demonstrated that stage I patients classified into grade 3 by the new grading system had significantly worse outcomes than those classified as grades 1 and 2. The multivariable analysis indicated that pathological grading was an independent prognostic factor for both RFS and OS. We further found that while stage IB patients in the overall cohort showed no survival difference with or without ACT, those defined as grade 3 by the proposed grading system showed a significant favorable response to ACT. In particular, patients upgraded from grade 2 to the newly proposed grade 3 could obtain a significant added benefit from ACT.
The IASLC recently proposed a grading system for IPA that considers the prognostic impact of traditional histological growth patterns and their proportions in addition to other pathologic factors, such as tumor spread through air spaces (STAS), nuclear grade, and mitotic grade5. After the initial research was published, Weng et al.6 applied this grading system to 136 cases with advanced stage IPA (stages IIIA, IIIB, and IV) and demonstrated that patients with high-grade tumors had significantly shorter progression-free survival, but not OS, than those with intermediate-grade and low-grade tumors. However, the treatment strategy and prognostic behavior varied between advanced IPAs diagnosed by needle biopsy and early-stage disease9, and for the latter, the clinical relevance of the new grading system needs further validation. Based on a large Japanese cohort, Rokutan-Kurata et al.10 validated the prognostic significance of the new histologic grading system in all-stage disease and compared this system with two previous conventional histologic systems. Subsequently, Kagimoto et al.11 also confirmed the effectiveness of the grading criteria for prognostic stratification based on a cohort of all-stage patients. They revealed in greater detail that the new grading system could achieve RFS stratification in the subgroup of pathologic stage I patients but not in those with stage II or III disease, which seemed to be slightly inconsistent with the previous study. In our study, prognostic validation of the newly proposed grading system was performed in multi-institutional cohorts of stage I IPA. Our findings were consistent with the subgroup analysis reported by Kagimoto et al.11, which supports that the proposed grade was a significant and robust predictor of RFS and OS in stage I disease (p < 0.05 in all cohorts). In addition, we expanded the validation by demonstrating that the same prognostic effect was seen in the stage IA and IB IPA subgroups.
Notably, the complex glandular patterns, which used to be classified as high-grade acinar12, were formally considered a poorly differentiated histologic subtype by the new grading system. The grading proposal5 and previous relative studies13,14 emphasized that complex glandular patterns were associated with poor prognosis, similar to traditional high-grade patterns (solid and micropapillary). Our analyses were consistent with the findings of these researchers, which demonstrates that patients who had tumors with predominant complex glandular patterns showed no significant difference in survival outcomes compared with those whose tumors had micropapillary or solid patterns (all p > 0.05). Thus, it is substantially meaningful to classify tumors with complex glandular patterns as a new type of high-grade IPA to advance clinical management in terms of either prognostic assessment or treatment decisions.
Previous studies5,6,11 focused on the prognostic value of the new grading system but lacked further discussions related to the administration of adjuvant therapy. The treatment guidelines published in 2017 and several earlier studies provided data that did not support the addition of ACT following curative resection in patients with stage IA disease, whereas stage IB patients with an increased risk of recurrence might be ideal candidates for ACT15,16,17,18. However, the selected criteria of high-risk patients with stage IB lesions as defined by the eighth edition of the TNM staging system are still ambiguous, and an efficient predictive indicator to identify those who might benefit from ACT is warranted.
Pathologic grading has been recognized as a traditional predictor of postoperative prognosis and adjuvant treatment guidance19,20,21. Many studies have shown that the effect of ACT on RFS was significantly different in acinar/papillary- and micropapillary/solid- predominant IPAs, with the latter showing a benefit2,16,22. However, high-grade tumors defined by predominant patterns only account for 10%-25% of all tumors16,23. Additionally, the newly proposed grading system determined that any tumor containing 20% or more poorly differentiated patterns was high grade, which was associated with a worse prognosis similar to those with predominantly poorly differentiated patterns5. Based on prognosis, we added to these findings by demonstrating that stage IB patients stratified into grade 3 using the new grading system were more likely to benefit from ACT in terms of both RFS and OS (p < 0.001), but we also report a nonsignificant trend of survival benefits for those in the proposed grade 2 category. Notably, we demonstrated a significant survival benefit of ACT for those patients upgraded to the newly proposed grade 3 but stratified into grade 2 by their predominant tumor patterns. Given that the proposed grading system covers a larger population of high-grade patients than the predominant pattern only, this new system might guide the identification of more suitable candidates with stage IB IPA for ACT.
Several limitations of our study should be noted. The first is the retrospective data collection and the small sample size of patients with grade 1, even though overall, 926 cases from three different institutions were included in this study. This suggests that our findings should be validated in larger well-designed prospective studies. Second, the present study only examined the prognostic impact of the new grading system in Chinese and Asian cohorts, and greater efforts are urgently needed to validate this system in different ethnic populations. Third, the decision about the use of ACT in stage IB disease was made by clinicians and/or patients and was not randomized, which also increases the risk of selection biases. The validity of grading for ACT benefit prediction should be further verified in future clinical trials of stage IB IPA with a prospective and random design.
In conclusion, our study supported that the newly proposed grading system proposed by the IASLC Pathology Committee in 2020 could serve as a strong and effective predictor of survival evaluation in patients with stage I IPA. Moreover, histologic grading criteria might be a useful predictive tool to identify which patients might benefit from ACT. Thus, the grading system potentially offers clinical value in directing therapeutic regimen selection for patients with stage I IPA.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to confidentiality but are available from the corresponding author on reasonable request.
References
Travis, W. D. et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 10, 1243–1260 (2015).
Tsao, M. S. et al. Subtype classification of lung adenocarcinoma predicts benefit from adjuvant chemotherapy in patients undergoing complete resection. J. Clin. Oncol. 33, 3439–3446 (2015).
Ujiie, H. et al. Solid predominant histologic subtype in resected stage I lung adenocarcinoma is an independent predictor of early, extrathoracic, multisite recurrence and of poor postrecurrence survival. J. Clin. Oncol. 33, 2877–2884 (2015).
Ma, M. et al. Micropapillary or solid pattern predicts recurrence free survival benefit from adjuvant chemotherapy in patients with stage IB lung adenocarcinoma. J. Thorac. Dis. 10, 5384–5393 (2018).
Moreira, A. L. et al. A grading system for invasive pulmonary adenocarcinoma: a proposal from the international association for the study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 15, 1599–1610 (2020).
Weng, C.-F. et al. New International Association for the Study of Lung Cancer (IASLC) Pathology Committee grading system for the prognostic outcome of advanced lung adenocarcinoma. Cancers 12, 3426 (2020).
Goldstraw, P. et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 11, 39–51 (2016).
Travis, W. D. et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 6, 244–285 (2011).
Campos-Parra, A. D. et al. Relevance of the novel IASLC/ATS/ERS classification of lung adenocarcinoma in advanced disease. Eur. Respir. J. 43, 1439–1447 (2014).
Rokutan-Kurata, M. et al. Validation study of the international association for the study of lung cancer histologic grading system of invasive lung adenocarcinoma. J. Thorac. Oncol. 16, 1753–1758 (2021).
Kagimoto, A. et al. Utility of newly proposed grading system from International Association for the Study of Lung Cancer for invasive lung adenocarcinoma. JTO Clin. Res. Rep. 2, 100126 (2021).
Travis, W. D., Brambilla, E., Burke, A. P., Marx, A. & Nicholson., A. G. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart (International Agency for Research on Cancer, Lyon, 2015).
Kuang, M. et al. Clinical significance of complex glandular patterns in lung adenocarcinoma: clinicopathologic and molecular study in a large series of cases. Am. J. Clin. Pathol. 150, 65–73 (2018).
Moreira, A. L., Joubert, P., Downey, R. J. & Rekhtman, N. Cribriform and fused glands are patterns of high-grade pulmonary adenocarcinoma. Hum. Pathol. 45, 213–220 (2014).
Xie, D. et al. Radiomics nomogram for prediction disease-free survival and adjuvant chemotherapy benefits in patients with resected stage I lung adenocarcinoma. Trans. Lung Cancer Res. 9, 1112–1123 (2020).
Hung, J. J. et al. Adjuvant chemotherapy improves the probability of freedom from recurrence in patients with resected stage IB lung adenocarcinoma. Ann. Thorac. Surg. 101, 1346–1353 (2016).
Zhang, P. et al. Influence of adjuvant chemotherapy on survival for patients with stage IB and IIA non-small cell lung cancer. Thorac. Cancer 12, 30–39 (2021).
Kris, M. G. et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non–small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline update. J. Clin. Oncol. 35, 2960–2974 (2017).
Sica, G. et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am. J. Surg. Pathol. 34, 1155–1162 (2010).
von der Thüsen, J. H. et al. Prognostic significance of predominant histologic pattern and nuclear grade in resected adenocarcinoma of the lung: potential parameters for a grading system. J. Thorac. Oncol. 8, 37–44 (2013).
Zhao, Z. R. et al. Prognostic impact of pattern-based grading system by the new IASLC/ATS/ERS classification in Asian patients with stage I lung adenocarcinoma. Lung Cancer 90, 604–609 (2015).
Qian, F. et al. Prognostic significance and adjuvant chemotherapy survival benefits of a solid or micropapillary pattern in patients with resected stage IB lung adenocarcinoma. J. Thorac. Cardiovasc. Surg. 155, 1227–1235.e1222 (2018).
Park, S. et al. Volume doubling times of lung adenocarcinomas: correlation with predominant histologic subtypes and prognosis. Radiology 295, 703–712 (2020).
Funding
This work was partly supported by the National Natural Science Foundation of China (91959126, 8210071009), Shanghai Science and Technology Commission (21YF1438200), Shanghai Hospital Development Center (SHDC2020CR3047B), Clinical Research Foundation of Shanghai Pulmonary Hospital (FK1937), 2020–2021 Innovation Group Project (Chen Chang), Projects of the Science and Technology Commission of Shanghai Municipality (18411962900, 20035800100), the Science-Technology Foundation for Young Scientists of Gansu Province (Nos.: 18JR3RA305, 21JR1RA107); and the Natural Science Foundation of Gansu Province (No:21JR1RA118, 21JR1RA092).
Author information
Authors and Affiliations
Consortia
Contributions
L.H., T.W., and D.C. performed study concept and design; L.H., Y.S., J.D., M.Z., and Yifan Zhong performed development of methodology and writing, review, and revision of the paper; D.C., M.Y., M.M., G.Z., Y.C., Yuming Zhu and C.W. provided acquisition, analysis and interpretation of data, and statistical analysis; T.W., X.D. and Yu Zhang provided technical and material support. Q.C. C.W., and C.C. performed supervision and data curation. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Approval from the institutional review board of the participating institutions and a waiver for informed consent were obtained for this retrospective study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hou, L., Wang, T., Chen, D. et al. Prognostic and predictive value of the newly proposed grading system of invasive pulmonary adenocarcinoma in Chinese patients: a retrospective multicohort study. Mod Pathol 35, 749–756 (2022). https://doi.org/10.1038/s41379-021-00994-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00994-5
This article is cited by
-
PET/CT-based deep learning grading signature to optimize surgical decisions for clinical stage I invasive lung adenocarcinoma and biologic basis under its prediction: a multicenter study
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
A pretreatment prediction model of grade 3 tumors classed by the IASLC grading system in lung adenocarcinoma
BMC Pulmonary Medicine (2023)
-
The solid component within part-solid nodules: 3-dimensional quantification, correlation with the malignant grade of nonmucinous pulmonary adenocarcinomas, and comparisons with 2-dimentional measures and semantic features in low-dose computed tomography
Cancer Imaging (2023)
-
Radiomic and quantitative-semantic models of low-dose computed tomography for predicting the poorly differentiated invasive non-mucinous pulmonary adenocarcinoma
La radiologia medica (2023)
-
An ordinal radiomic model to predict the differentiation grade of invasive non-mucinous pulmonary adenocarcinoma based on low-dose computed tomography in lung cancer screening
European Radiology (2023)