Abstract
Prey-specialised spiders are adapted to capture specific prey items, including dangerous prey. The venoms of specialists are often prey-specific and less complex than those of generalists, but their venom composition has not been studied in detail. Here, we investigated the venom of the prey-specialised white-tailed spiders (Lamponidae: Lampona), which utilise specialised morphological and behavioural adaptations to capture spider prey. We analysed the venom composition using proteo-transcriptomics and taxon-specific toxicity using venom bioassays. Our analysis identified 208 putative toxin sequences, comprising 103 peptides < 10 kDa and 105 proteins > 10 kDa. Most peptides belonged to one of two families characterised by scaffolds containing eight or ten cysteine residues. Toxin-like proteins showed similarity to galectins, leucine-rich repeat proteins, trypsins and neprilysins. The venom of Lampona was shown to be more potent against the preferred spider prey than against alternative cricket prey. In contrast, the venom of a related generalist was similarly potent against both prey types. These data provide insights into the molecular adaptations of venoms produced by prey-specialised spiders.
Similar content being viewed by others
Introduction
Venom is a trait used for various purposes, most notably predation and defence. It is a very successful trait, having evolved independently in a remarkably large proportion of animals on the tree of life1. Venoms represent complex mixtures of tens to thousands of compounds, including peptides, proteins and low molecular mass compounds2,3. While the complexity of venom composition can be driven by multiple factors4, diet is likely to have a strong effect on species that use venom for prey capture5. It has generally been assumed that predators with a narrower range of prey in their diets do not have to possess a complex venom arsenal. Indeed, it has been shown that specialised predators, like some spiders and cone snails, display a less complex repertoire of venom components compared to generalist predators6,7. Alternatively, predator–prey arms races are a very prominent factor determining the complexity of predatory venoms8 and may have a greater effect on specialists. As prey may evolve resistance to some venom components, predators may respond by recruiting additional toxins and thereby increasing venom complexity. In studies on arachnids, the venoms of specialist feeders are reported to be very potent on their focal prey, but less potent on alternative prey9. This suggests high prey-specificity of the compounds present in their venoms.
Spiders represent an ideal model group of predators for studying venom specificity. Although most spiders are generalist predators, approximately 5% of spiders are prey specialists10. However, spider-venomics research so far has focused predominantly on large species and those that are medically important, whereas many taxa have been neglected11. Although there is extensive literature on the diversity of disulfide-rich peptides found in araneomorph spider venoms, their venoms remain relatively unstudied compared with the vast diversity of araneomorph spiders, which represent ~ 90% of extant spider species.
Lamponidae is a relatively small spider family comprising less than 200 described species, mostly occurring in Australia and surrounding islands12,13. The most well-known are species of the genus Lampona, often called the white-tailed spiders. They are fairly large and often synanthropic. Due to frequent encounters, their capacity to bite through human skin, and their potent venom, these spiders have accrued an insidious reputation. They have been blamed for causing necrotic lesions, despite the complete lack of clinical evidence14.
In spite of their reputation, the venoms of Lamponidae have not been studied in detail. The pharmacological and enzymatic activities of venom from male and female Lampona cylindrata L. Koch, 1866 were shown to be different15,16, but the venom composition was not described comprehensively. Lampona spiders have been reported to prey on other spiders12. Our recent investigation of the prey-capture behaviour of Lampona murina L. Koch, 1873 revealed the presence of specific morphological and behavioural adaptations for handling spider prey17. Notably, L. murina relied on its venom to immobilise spider prey, suggesting that its venom may have arachnid-specific effects and may have been exposed to predator–prey arms races. Our previous study6 using proteomic profiling based on molecular mass revealed several dominant bands/peaks in Lampona murina venom, but the composition of the venom was not analysed in detail.
Here, our aim was to further investigate the venom composition and toxicity of Lampona spiders with a view to investigating the taxon-specificity of the venom. We hypothesised that Lampona venom is more potent on its focal spider prey, and that the spider-specific efficacy is due to specific venom toxins (either peptides or proteins). We employed proteo-transcriptomic methods to characterise the proteomic composition of venom produced by Lampona spiders, and compared its taxon specificity with that of a related generalist from the genus Gnaphosa (Gnaphosidae). We report that Lampona venom contains numerous and abundant peptides similar to those of other araneomorph spiders, as well as larger proteins. We confirmed that Lampona venom is more efficient toward spider prey than alternative prey.
Results
Proteo-transcriptomics
We collected Lampona spiders belonging to two species: Lampona murina and Lampona sp. indet. The venom of both species was pooled due to the low amount of milked venom, and subsequent venom activity was investigated at the genus level. Two separate transcriptomes were produced, corresponding to each species. Transcriptomic analysis recovered 152.3 million reads for the L. murina transcriptome and 165.3 million reads for the Lampona sp. transcriptome. The quality of the transcriptome was high as judged by a large portion of orthologs of the assembled transcripts mapping to two groups (Arthropoda: 60 species, 1066 orthologs; Metazoa: 65 species, 978 orthologs). The proportion of BUSCO orthologs identified by the assembled transcriptomes was 87% (Arthropoda) and 90.2% (Metazoa) for L. murina, and 83.9% (Arthropoda) and 88% (Metazoa) for Lampona sp. A high score of mapping of original reads to the assembled transcripts proved the high quality of the assembly (98.92% of aligned reads for L. murina, 99.35% of aligned reads for Lampona sp.).
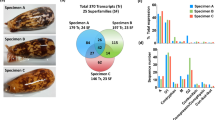
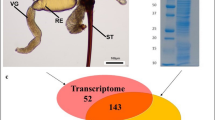
To identify peptides and proteins in Lampona venom, we combined the transcriptome from dissected venom glands with LC–MS/MS proteomic analysis of crude milked venom. We decided to use only the L. murina transcriptome for this data analysis. All reported peptide and protein sequences were therefore confirmed to be present in the L. murina venom. The combined analysis revealed 208 different putative toxin sequences in the proteome after quality filtering (Supplementary Table S1). The venom contained numerous peptides with less than 100 amino-acid residues (103 sequences) as well as larger proteins (105 sequences) (Fig. 1a). The peptide-encoding transcripts were more abundant, accounting for 74.2% of the transcripts (Fig. 1b). Putative toxin families were assigned based on sequence similarities identified using BLAST and cysteine scaffold patterns in the case of disulfide-rich peptides.
Peptides
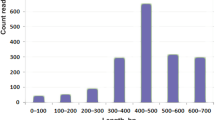
The median length of the identified venom peptides (those with mature forms < 10 kDa) was 6.7 kDa (Fig. 2). Almost all identified peptides (97%) were cysteine-rich, with a cysteine scaffold suggesting they likely represent variations on the inhibitor-cystine-knot (ICK) motif. Interestingly, there were only two peptides with the basic spider-venom ICK scaffold (–C–C–CC–C–C–, where dashes indicating intervening non-Cys residues in variable numbers), as a large proportion of peptides contained more than 6 cysteine residues: 56% of venom peptides had 10 cysteine residues and 24% had 8 cysteine residues (Fig. 3). The identified peptides were classified into 15 families. Due to the complexity of previous spider toxin nomenclature, we did not attempt to extend previous classification systems and the 'families' depicted here are delineated only with respect to the sequence similarity of their members with other members of the same venom. The two most prominent peptide families were named lampotoxin venom Family 1 and Family 2.
Lampotoxin venom Family 1
Most toxins in Family 1 have ten cysteine residues with a scaffold of the form a –C–C–CXCC–C–C–C–C–C– (Fig. 4). Many Family 1 peptides are predicted to be members of Pfam Toxin_34 or D_CTX (δ-ctenitoxins) (Supplementary Table S1). Their cysteine scaffolds are similar to groups 6 and 8 of NaV-channel targeting peptides (NaSpTx) as classified by Klint et al.18, and to groups V–VIII of venom peptides of the ctenid P. nigriventer according to the classification of Diniz et al.19 (note that families in these two classification systems are not congruent). Similar described toxins from Ctenidae, Agelenidae, and Lycosidae are thought to be ICK peptides with long C-terminal extensions and extra disulfide bonds. While many of these known peptides have extra CXC motifs that yield a framework of –C–C–CXCC–CXC–CXC–C–C–C–, only seven identified lampotoxins contain these extra CXC motifs (Fig. 4).
We identified 60 different putative lampotoxins belonging to Family 1. Lampotoxin Family 1-encoding transcripts were the most abundant sequences in the transcriptome, representing 48.0% of all transcripts. Three of these lampotoxins (U-LATX(1)-Lm14, U-LATX(1)-Lm30a, U-LATX(1)-Lm30c) each accounted for more than 5% of all transcripts (Fig. 1b). Given the BLAST similarity of Family 1 toxins to µ-ctenitoxins, δ-ctenitoxins and ω-ctenitoxins, we hypothesise they may modulate the activity of voltage-gated sodium (NaV) or calcium (CaV) channels.
Lampotoxin venom Family 2
Most Family 2 toxins possess 8 cysteine residues in a scaffold lacking the CXCC motif of Family 1 peptides. However, they have two CXC motifs after the central CC doublet, without subsequent cysteine residues, yielding a scaffold of the form –C–C–CC–CXC–CXC– (Fig. 5). We identified 26 different putative Family 2 lampotoxins, which together contribute 18.4% of all transcripts. One lampotoxin (U-LATX(2)-Lm106) accounted for more than 5% of all transcripts (Fig. 1b). Family 2 lampotoxins show sequence similarities with ω-agatoxins, araneomorph venom peptides that modulate the activity of CaV channels.
Proteins
The larger venom proteins were not as abundant as peptides, but they are very diverse, making up 57 different families (Fig. 1; Supplementary Table S1). The proteins comprised a number of putative toxins, including neprilysins, trypsins, LRR toxins, a phospholipase A2, and many others. In the terms of the number of sequences, the most numerous were toxins similar to trypsins from ctenid venoms with 12 sequences (Family 7). We also detected 12 protein sequences belonging to the neprilysin family (Family 9). The third most prominent protein family (Family 57) contained eleven protein sequences with little similarity to known toxins or proteins. Similarly, Family 3 contained five sequences with no detectable similarity to known proteins. Two additional sequences belonging to Family 3 were classified as peptides, as their molecular mass were lower than 10 kDa, and thus they were treated as such in the overall analysis (Fig. 1). Family 26 contained four different sequences similar to LRR toxins. In other protein families, only one or two sequences were identified in each family (Supplementary Table S1).
In terms of abundance, U-LATX(3)-Lm12, U-LATX(3)-Lm13 and U-LATX(4)-Lm28 were the most prominent proteins, forming 15%, 14% and 9% of protein transcripts, respectively. Proteins U-LATX(3)-Lm12 and U-LATX(3)-Lm13 belonged to Family 3, which is the most abundant protein family. The function of these proteins is not known, and they do not have close BLAST matches that could be used to infer their function.
Probably not all of 105 protein sequences in the Lampona venom are toxins. Some larger venom components are likely involved in toxin maturation (e.g. proline isomerase; U-LATX(33)-Lm184 and U-LATX(33)-Lm192; Family 33) or are venom spreading factors (e.g. hyaluronidase; U-LATX(30)-Lm108 and U-LATX(30)-Lm112; Family 30). A spermidine synthase was identified as well (U-LATX(19)-Lm157; Family 19), suggesting that the venom contains small-molecule polyamine toxins as do many other spider venoms20.
The efficacy of crude venoms
To test the relative efficacy of Lampona venom on different prey types, we injected venom into a preferred prey type (spiders) or non-preferred prey (crickets), and compared this with venom from a related generalist spider (Gnaphosidae: Gnaphosa). We observed lethal effects after 24 h and paralysing effects after 1 h (Table 1). The venom of prey-specialised Lampona was far more lethal on their preferred prey represented by a spider (LD50 = 0.07 nl/mg) than on alternative prey represented by a cricket (LD50 = 3.27 nl/mg). On the other hand, venom of the generalist predator Gnaphosa was similarly potent on both prey types (spider prey: LD50 = 0.28 nl/mg; cricket prey: LD50 = 0.26 nl/mg; Fig. 6 and Supplementary Fig. S1). The interaction between the predator species and prey type was significant (GLM-b, χ21 = 43.8, P < 0.0001; Fig. 6), suggesting that the venom of Lampona sp. is prey-specific. The venom of Lampona was almost 50-fold more lethal to spiders than to crickets (GLM-b, χ21 = 71.2, P < 0.0001), while the venom of Gnaphosa was similarly potent to both prey types (GLM-b, χ21 = 0.1, P = 0.70, Table 1).
In Lampona, the paralysing dose after 1 h was similar to the lethal dose after 24 h for spider prey (GEE-b, χ21 = 0.4, P = 0.51, Table 1); but it differed for cricket prey (GEE-b, χ21 = 13.1, P < 0.001, Table 1): some crickets injected with lower venom concentrations were able to recover from paralysis after 24 h (Supplementary Fig. S2a). In Gnaphosa, the paralysing effect after 1 h was stronger than the lethal effect after 24 h for both spider and cricket prey (GEE-b, χ21 = 52.5, P < 0.0001, Table 1), as lower venom concentrations paralysed prey, but they recovered after 24 h (Supplementary Fig. S2b).
The efficacy of Lampona venom fractions
In addition, we tested the efficacy of low-MW and high-MW venom fractions. The toxicity differed between the high- and low-MW fractions (GLM-b, χ21 = 19.8, P < 0.0001) and between prey types (GLM-b, χ21 = 12.1, P < 0.001). The low-mass compounds (< 10 kDa) caused no mortality in either spiders or crickets, while the high-mass compounds (> 10 kDa) caused mortality but at different rates: 90% for spiders and 20% for crickets (Fig. 7). The effect of venom dose (after taking into account prey mass) was not significant (GLM-b, χ21 = 0.9, P = 0.33). The mortality was similar after 1, 3 and 24 h for both combinations of fractions and prey.
Discussion
In this study, we provided the first detailed analysis of the venom composition of prey-specialised white-tailed spider of the genus Lampona. Altogether, we confirmed the presence of 208 unique peptide and protein sequences in the pooled milked venom. The most numerous and abundant components of the Lampona proteome were peptides from lampotoxin families 1 and 2. The toxins from these two families share some similarities. Specifically, some toxins from Family 1 have the same two XCX motifs as Family 2 peptides, so the families are most likely related. Many of the described spider toxins from the infraorder Mygalomorphae contain six cysteine residues and form an ICK structural motif (but more complex toxins can be found in mygalomorph venoms as well3) in which two disulfide bridges and the intervening sections of the peptide backbone form a closed loop that is bisected by the third disulfide bridge. This motif provides these peptide toxins with exceptional resistance to heat, extremes of pH, and proteases21. Other structural scaffolds found in spider toxins include the disulfide-directed β-hairpin (DDH) and Kunitz motifs22,23. The lampotoxin Families 1 and 2 contained a higher number of cysteine residues, usually 10 or 8, respectively, and had a higher mass than most reported mygalomorph toxins. This was already implied by our previous study, where MALDI-TOF MS analysis of peptides showed peaks with the highest intensity close to 4 and 7 kDa6. It is possible that toxins with a more complex structure than the typical ICK motif, and containing more than six cysteine residues, are prominent in the Araneomorphae infraorder, although such a conclusion may be premature based on the current limited sampling of araneomorph spider venoms24. Indeed, these two lampotoxin families show complex cysteine scaffolds, similar to other toxins reported to exist in araneomorph venoms24,25,26,27,28,29. Cysteine-rich venom peptides are typically neurotoxins that target various ion channels30, suggesting a similar role for these Lampona peptides. Besides the Family 1 and 2 lampotoxins, most of the Lampona peptides (97%) also have a high cysteine content, suggesting the presence of an ICK motif or other disulfide-constrained scaffolds.
The Lampona venom proteins are more diverse than the venom peptides, although they are not the main venom component in terms of abundance. SDS-PAGE analysis of proteins performed in our previous study6 showed numerous dominant bands close to 37 kDa and between 50 and 75 kDa. Here, we found that Lampona venom contains proteins similar to galectins, LRR proteins, and trypsins (S1 proteases) that might correspond to the 37 kDa band, and toxins such as numerous neprilysins (M13 proteases) that might correspond to the 50–75 kDa band. The most numerous protein sequences in this study belonged to neprilysins (lampotoxin Family 9) and proteins similar to trypsins (lampotoxin Family 7). Neprilysins are proteins with functions connected to signalling pathways or metabolism of regulatory peptides31, but they have been also described as venom components of snakes and spiders32,33,34,35,36,37. Their proposed functions in spider venoms are extracellular matrix degradation35,36 or toxicity resulting in flaccid paralysis and darkened skin areas in cricket prey37. They may act as spreading factors in Lampona venom as well, but also facilitate toxicity since the high-mass compounds caused prey paralysis in venom fraction bioassays. The proteins classified as lampotoxin Family 7 showed similarity to trypsins or serine proteases. In most cases, their closest BLAST match was a serine protease from the spider Phoneutria nigriventer (Keyserling, 1891) (U21-ctenitoxin-Pn1a). Serine proteases in spider venoms have an unclear function, but it has been suggested that they play a role in toxin maturation, prey digestion, hemostasis impairment, or tissue damage38,39. We also identified four sequences similar to LRR proteins. LRR proteins have been described from a few spiders, namely the common house spider (Parasteatoda tepidariorum (C. L. Koch, 1841)), the western black widow spider (Latrodectus hesperus Chamberlin & Ivie, 1935), and the wasp spider (Argiope bruennichi Scopoli, 1772), but their role in the venom was not specified40,41,42. The most abundant components of the protein fraction were five proteins from Family 3 with unclear putative function due to low similarity to known sequences.
Behavioural experiments with Lampona murina showed that they rely on their venom to immobilise spider prey9. In our laboratory bioassays, the Lampona venom showed stronger potency toward spider prey, indicating the possible presence of prey-specific compounds in the venom. In our study, we revealed more than 200 venom components in Lampona venom. It is currently not known which compound or compounds are responsible for the prey-specificity of Lampona venom. Several reports of prey-specific venom compounds across different taxa can be found in the literature, including mollusc-specific toxins of a cone snail43, a bird-specific toxin of a mangrove catsnake44, and crustacean- and insect-specific latrotoxins in the venom of widow spiders (genus Latrodectus)45.
Very little information is known about the venom of spiders with specific dietary habits. The venom composition of fish-hunting spiders of the genus Dolomedes (family Pisauridae)46 and the ant spider Lachesana tarabaevi Zonstein and Ovtchinnikov, 1999 (family Zodariidae)47 have been investigated , but available information on the diets of these spiders suggest that they are not true prey-specialists (sensu Pekár and Toft48). Although many pisaurid spiders consume fish, they are generalists with broad diets49. Many zodariid spiders are specialists feeding only on ants, but some are generalists, including another species of Lachesana, Lachesana insensibilis Jocqué 199150, and Cybaeodamus taim Lise, Ott & Rodrigues, 2009 with non-prey-specific venom51. Moreover, although the analysis of the venom composition of L. tarabaevi revealed interesting venom compounds with specific cytolytic activity47, there is no information on the prey specificity of these venom compounds.
The prey specificity of the toxins of prey-specialised predators has usually been indicated on a higher taxonomic level (e.g., phylum or class). Since Lampona venom was also potent against prey other than spiders, like crickets, although with lower efficacy, various compounds present in the venom may be effective against different prey taxa. Such contrasting taxon-specific potency of different toxins in a single venom has been already demonstrated in snake venom52. Alternatively, one toxin may exhibit different affinity and potency towards various prey taxa53. In our experiment, we tested the venom specificity only on one representative of the focal and alternative prey taxa due to the low amount of milked venom. The prey species utilised in this study are not part of the natural trophic niche in which Lampona venom has evolved. The prey species were selected due to their availability in high numbers. Additional experiments on the natural prey of Lampona spiders are required to further confirm the prey-specific venomic adaptations. The venom specificity of araneophagous spiders is usually not restricted to a single prey species, as they are not monophagous but indiscriminately hunt several species of spider prey51. Anecdotal observations of the white-tailed spiders suggest they are able to subdue and paralyse various spider prey taxa12. Our previous study9 has shown the paralysis latency of L. murina spiders is also longer in another cricket species (Acheta domestica (Linnaeus 1758)), confirming the pattern of lower venom susceptibility of alternative insect prey.
One limitation of this study is that we pooled the venom from two Lampona species due to the low volumes available, and analysed the venom only at the genus level. We analysed only the L. murina transcriptome against proteomic data obtained from the pooled venom sample and present it as a venom proteome of Lampona (species not defined). We note that it is technically possible that proteomic data from the unidentified Lampona species may have been used to 'misidentify' a sequence which is present in the L. murina transcriptome but not present in L. murina venom. However, considering the two species are congeneric they should share a large portion of almost identical transcripts, and we consider that the frequency of such false positives is likely to be very low in comparison to true positives. All Lampona spiders are generally considered to be araneophagous, therefore we expect similar venomic adaptations in the whole genus. Further investigation is needed to identify species differences in venom composition, spider-specific toxins and their mode of action.
Interestingly, the high-MW fraction from Lampona venom showed higher efficacy than the low-MW fraction in potency bioassays, although the peptides were more abundant than proteins in the venom as revealed by proteo-transcriptomic analysis. The discrepancy between the abundance of high-mass compounds and their efficacy might be caused by the methodology used for the venom fractionation. Specifically, due to the large size of the disulfide-rich peptides detected in Lampona venom (mean 6.7 kDa and many 9–10 kDa), and depending on the steepness and precision of the molecular weight cut-off used to separate high- and low-molecular-mass toxins, some disulfide-rich peptides may also contribute to the observed toxicity toward preferred prey, as they might not have been separated properly from the protein fraction used in the bioassays. More experiments are needed to identify the exact toxins responsible for the prey-specific efficacy of the venom, starting with a greater quantity of venom and employing chromatographic separation techniques. Some recent studies revealed that invertebrate venoms may exhibit inter-individual variability in venom composition54. Ideally, the venom from different individuals should be analysed separately. Here, we were not able to analyse Lampona venom on an individual level due to the low number of individuals and the small volume of venom obtained from each individual. Thus, we had to combine venom from several individuals for the analyses described herein. Although this is not ideal, our study still provides the first detailed insight into the venom composition of Lampona spiders.
Overall, our study revealed that besides morphological and behavioural adaptations, the white-tailed spiders also possess potent prey-specific venom to handle their focal spider prey. We identified a unique mix of novel compounds in Lampona venom. Due to its taxon-specific toxicity, Lampona venom represents an excellent model system for future studies focusing more thoroughly on the mechanisms of venom prey-specificity or possible exploitation of such toxins in applied research.
Methods
Spiders and prey
Lampona spp. spiders (Lamponidae) were collected on the Macquarie University campus, North Ryde, Sydney, Australia. Collected spiders belonged to two species (Lampona murina and Lampona sp. indet.); specimens of both species were used to analyse the venom on the genus level due to the low numbers of specimens and the small amount of milked venom. Gnaphosa lucifuga (Walckenaer, 1802) spiders (Gnaphosidae), were collected in Mohelno Serpentine Steppe in the Czech Republic. Spiders were kept singly in glass tubes (height: 6 cm, diameter: 1.5 cm) with moisturised gypsum on the bottom and stored in a chamber at room temperature (22 °C) and L:D = 16:8. Spiders were fed regularly with other spiders or crickets. Water was provided every three days.
In laboratory bioassays, we used juvenile Pardosa sp. wolf spiders (Pardosa lugubris group, N = 130, body mass: 13.0 ± 3.5 mg) and Gryllus assimilis (Fabricius, 1775) juvenile crickets (N = 125, body mass: 5.6 ± 4.0 mg) as prey. Pardosa spiders were collected in the surrounds of the Department of Botany and Zoology at Masaryk University (Brno, Czech Republic) and kept singly in punctured Eppendorf tubes placed in a bag with moisturized cotton in a chamber at low temperature (10 °C) and L:D = 16:8. Crickets were bought at a local pet store.
Venom extraction
Venom was obtained from six Lampona spiders and five Gnaphosa spiders using an electrical milking technique55,56,57. A spider was anesthetised with CO2 for 2 min, placed on a stub, covered with a mesh and the venom collected into a glass microcapillary (volume 0.5 or 1 µl) that was slid onto one of the fangs of the spider’s chelicerae. The spider was stimulated with an electric impulse to release venom into the capillary. Individual spiders were milked several times at approximately three-weekly intervals. Microcapillaries containing the venom were stored in the freezer at − 80 °C before further processing. Overall, 5 μl of Lampona venom and 6.5 μl of Gnaphosa venom was obtained by repeatedly milking the spiders.
Transcriptomics
To prepare a transcriptome library, paired venom glands were dissected from Lampona murina and Lampona sp. spiders that were milked five days before dissection to stimulate venom transcript production. To minimize contamination, the dissected glands were immediately placed in a drop of physiological solution (0.9% NaCl) and left for 5 min to wash away haemolymph and epithelial cells and other tissues on the surface of the glands. Then, glands were placed into 2 ml of TRIzol Reagent (Life Technologies, USA) in an Eppendorf tube, compressed by a tweezer and kept for 15 min at room temperature. The tube was stored at − 20 °C until further processing. Total RNA was extracted using TriReagent (MRC Holland) and RNAeasy spin columns (Qiagen). The tissue was lysed in TriReagent, and RNA was separated into an aqueous phase with 1–bromo–3–chloropropane (BCP) (MRC Holland). The aqueous phase was mixed 1:1 with 70% ethanol and transferred to an RNAeasy column. The extraction continued according to manufacturer’s protocol, including on-column DNAse digestion.
The libraries were sequenced on the Illumina Hi-Seq platform. Raw reads were quality checked (using FastQC58) and pre-processed before assembling (trimming by Trimmomatic59 and correction of sequencing errors by Rcorrector60). The following steps were repeated for both species (Lampona murina and Lampona sp.) to obtain two separate venom gland transcriptomes. Transcriptome assembly was performed using Trinity61 in three runs with different kmer setting (21, 25, 31) to optimise the results as advised by the Trinity authors. All standalone assemblies were combined and clustered based on nucleotide/amino-acid sequence similarity using the EvidentialGene toolset62. The quality of the reduced set of transcripts was assessed by mapping of pre-processed reads back to the transcripts using BOWTIE63 and by comparing them to specific ortholog databases (i.e., Arthropoda, Metazoa) using BUSCO64. Open reading frames larger than 90 bp were detected and translated by TransDecoder65 to yield a database of possible amino acid sequences to compare to mass spectrometric data.
Proteomics
Crude pooled Lampona venom (2.25 µl) was loaded onto a Vivacon 500 centrifugal filtration device with MWCO 10 kDa (Sartorius Stedim Biotech). The filter was washed with 50 μL of 50 mM NaHCO3, and the flow-through (peptidome fraction) was collected. The peptidome fraction was split into halves. One half was reduced and alkylated by using the same amounts of the same reagents as for protein concentrate (please see below) with one additional reduction step (for iodoacetamide (IAA) inactivation); the second half was analysed without any treatment. The protein concentrate retained in the Vivacon 500 device was mixed with 100 μL of 50 mM dithiothreitol and incubated for 30 min. After additional centrifugation, 100 μL of 50 mM IAA were added and the filter was incubated in the dark for 30 min. After the next centrifugation step, the filter was washed with 200 μL of 50 mM NaHCO3. Trypsin (sequencing grade, Promega) was added onto the filter and the mixture was incubated for 14 h at 37 °C. The tryptic peptides were finally eluted by centrifugation followed by two additional elutions with 50 μL of 50 mM NaHCO3. The peptides were extracted from the vials by using 25% formic acid (FA)/acetonitrile (1:1 v/v mixture) in presence of 0.001% poly(ethylene glycol) and vacuum concentrated.
The peptide mixtures were subjected to LC–MS/MS analysis by using an RSLCnano system (Thermo Fisher Scientific, Waltham, MA, USA) coupled to an Impact II Qq-Time-Of-Flight mass spectrometer (Bruker, Bremen, Germany). Prior to LC separation, peptides were online concentrated on a trap column (100 μm × 30 mm) filled with 3.5-μm X-Bridge BEH 130 C18 sorbent (Waters, Milford, MA, USA). The peptides were separated using an Acclaim Pepmap100 C18 column (3 µm particles, 75 μm × 500 mm; Thermo Fisher Scientific) using the following solvent gradient (mobile phase A: 0.1% FA in water; mobile phase B: 0.1% FA in 80% acetonitrile; 300 nl/min): the gradient elution started at 1% mobile phase B and increased to 56% during the first 50 min, then increased linearly to 80% mobile phase B over the next 5 min, followed by isocratic elution with 80% mobile phase B for 10 min. Equilibration of the trapping column and the column was done prior to sample injection. The analytical column outlet was directly connected to the CaptiveSpray nanoBooster ion source (Bruker, Bremen, Germany). MS and MS/MS spectra were acquired using a data-dependent strategy with a 3 s long cycle time. Mass range was set to m/z range of 150–2200 and precursors were selected from m/z range of 300–2000. The acquisition speed of MS and MS/MS scans was 2 Hz and 4–16 Hz, respectively. Speed of MS/MS spectra acquisition was based on precursor intensity. The pre-processing of the mass spectrometric data including recalibration, compound detection, and charge deconvolution was carried out using DataAnalysis software (version 4.2 SR1; Bruker).
Bioinformatic integration of proteomic and transcriptomic data
To identify venom peptides and proteins, mass spectral data were searched against the database of possible amino acid sequences obtained from the venom-gland transcriptome using the Paragon and ProtGroup algorithms in ProteinPilot software (SCIEX, Framingham, MA, USA) with a cut-off of 95% confidence at the protein level. We decided to use only the L. murina transcriptome in the bioinformatic data integration, but we refer to the proteome on the genus level due to possible misidentifications from unidentified Lampona sp. (see Discussion). cDNA sequences encoding the identified toxins were re-mapped to correct errors and identify variants and missing termini using Geneious software version 2019.0.4 (Biomatters, Auckland, New Zealand). Precursor sequences were then annotated using SignalP66, BLAST searches against the SwissProt database67,68, and HMMER searches against the Pfam database69,70. Because of the larger size of many of the toxins (> 5 kDa), the mature structures of very few were resolved from the native and reduced/alkylated datasets and for this reason we generated predicted mature forms for the remainder using the hidden Markov model of the SpiderP algorithm71. These 'best mature' sequences (Supplementary Table S1, column AE) were then used as a database for a new search of the mass spectral data using the Paragon and Protgroup algorithms, and redundant or undetected toxins were removed from the proteome. Toxins were named according to rational nomenclature guidelines72.
Bioassays with crude venom
Pooled crude venoms from Lampona and Gnaphosa spiders were diluted to different concentrations in 0.1 M ammonium acetate buffer, pH 6.1 for the venom toxicity bioassays57. Model prey were anesthetised with CO2 before injection of 50 nl of venom into the thorax or the prosoma using a glass microsyringe. Several venom concentrations that caused dose/weight-dependent effects were tested; 5–30 prey were used per venom concentration (Supplementary Table S2). Simultaneously, for each concentration, a control cohort of prey were injected with ammonium acetate buffer to exclude the effect of buffer and merely piercing the prey with the capillary. If there was mortality in the control group, the data for the given trial was discarded. After injection, prey were placed individually into small Petri dishes (diameter 35 mm) with a small piece of moisturized cotton. Each tested prey individual was weighed using a Kern 770 balance (Balingen, Germany) with a precision of 0.01 mg. Mobility of the prey was observed 1, 3 and 24 h after the injection. Prey were considered dead or completely paralyzed when there was no movement after a light touch with a pincer and partially paralyzed when they could not move in Petri dish normally (not able to walk, erratic movements, etc.).
Bioassays with venom fractions of Lampona
To investigate which venom components are responsible for the specific venom toxicity in Lampona, we performed bioassays with two venom fractions: smaller peptides (< 10 kDa) and larger proteins (> 10 kDa). The crude venom sample (2.25 µl) was diluted in 100 µl of 50 mM PBS and added into a Microcon 10 centrifugal filter unit (Merck Millipore, Germany) with a 10 kDa molecular weight cut-off. The low mass fraction (< 10 kDa) was obtained by centrifugation at 14,000 g. The high mass fraction (> 10 kDa), which remained on the upper part of the filter, was collected after being shaken in an additional 25 µl of 50 mM PBS.
Both fractions were diluted in ammonium acetate buffer, and one concentration for the given prey was prepared (1:50 for spider prey, corresponding venom dose: 0.56 ± 0.15 nl/mg; 1:10 for cricket prey, corresponding venom dose: 0.74 ± 0.14 nl/mg). This concentration was higher than the median lethal dose value from the previous experiment; therefore, it should induce paralysis or death in prey. Diluted venom fractions were injected into ten individuals of preferred and alternative prey types (spider and cricket) and paralysis/mortality was observed over 24 h. As a control, buffer only was injected into ten prey individuals.
Bioassays data analysis
Venom toxicities were compared using dose–response analyses (Supplementary Fig. S1) performed in the R environment73. The complementary log–log model with binomial distribution using generalised linear models (GLM-b) was used to fit the binary data. The mortality of the prey after 24 h was the response variable, log-transformed venom dose (in nl per mg) was a covariate, and venom origin and prey type were factors. Median lethal dose values (LD50) within 24 h for each combination of the venom and prey type were estimated from models using dose.p function from the MASS package74. A 95% confidence interval for each LD50 value was calculated using the formula for normal distribution75.
To evaluate the paralysing properties of each venom, effective doses (ED50) were estimated from the models (GLM-b) where the affected prey (dead or paralysed) after 1 h was used as the response variable instead of mortality. Comparison between paralysing and lethal effect for each spider was made using another model with the type of effect (paralysis/mortality) as another factor. In the latter case, Generalised Estimating Equations with a binomial distribution (GEE-b) from the geepack package76 were used instead of GLM-b, as the rate of affected prey after 1 and 24 h represents repeated measurements on prey individuals. An autoregressive correlation structure (AR1) for replicated observations over time was used to account for these temporal replications77.
The toxicity of the Lampona venom fractions were also compared using a generalised linear model with a binomial distribution (GLM-b). The mortality of the prey after 24 h was the response variable, venom concentration (in nl per mg) was a covariate, and venom fraction and prey type were factors.
Data availability
The raw reads have been submitted to GenBank, the sequence read archive (SAR) accession is SRR18933286, BioProject PRJNA832048. The Transcriptome Assembly has been deposited at DDBJ/EMBL/GenBank under the accession GJYG00000000. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD033502. Protein sequences discovered in this project were submitted to GenBank and assigned the accession numbers ON226530–ON226737. Additional data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Schendel, V., Rash, L. D., Jenner, R. A. & Undheim, E. A. The diversity of venom: The importance of behavior and venom system morphology in understanding its ecology and evolution. Toxins 11(11), 666 (2019).
Casewell, N. R., Wüster, W., Vonk, F. J., Harrison, R. A. & Fry, B. G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 28(4), 219–229 (2013).
Pineda, S. S. et al. Structural venomics reveals evolution of a complex venom by duplication and diversification of an ancient peptide-encoding gene. Proc. Natl. Acad. Sci. USA 117(21), 11399–11408 (2020).
Chippaux, J. P., Williams, V. & White, J. Snake venom variability: Methods of study, results and interpretation. Toxicon 29(11), 1279–1303 (1991).
Lyons, K., Dugon, M. M. & Healy, K. Diet breadth mediates the prey specificity of venom potency in snakes. Toxins 12(2), 74 (2020).
Pekár, S. et al. Venom gland size and venom complexity—essential trophic adaptations of venomous predators: A case study using spiders. Mol. Ecol. 27(21), 4257–4269 (2018).
Phuong, M. A., Mahardika, G. N. & Alfaro, M. E. Dietary breadth is positively correlated with venom complexity in cone snails. BMC Genom. 17(1), 401 (2016).
Holding, M. L., Biardi, J. E. & Gibbs, H. L. Coevolution of venom function and venom resistance in a rattlesnake predator and its squirrel prey. Proc. R. Soc. B. 283(1829), 20152841 (2016).
Pekár, S., Líznarová, E., Bočánek, O. & Zdráhal, Z. Venom of prey-specialized spiders is more toxic to their preferred prey: A result of prey-specific toxins. J. Anim. Ecol. 87(6), 1639–1652 (2018).
Pekár, S., Coddington, J. A. & Blackledge, T. A. Evolution of stenophagy in spiders (Araneae): Evidence based on the comparative analysis of spider diets. Evolution 66(3), 776–806 (2012).
Herzig, V., King, G. F. & Undheim, E. A. Can we resolve the taxonomic bias in spider venom research?. Toxicon: X 1, 100005 (2019).
Platnick, N. A relimitation and revision of the Australasian ground spider family Lamponidae (Araneae: Gnaphosoidea). Bull. Am. Mus. Nat. Hist. 2000(245), 1–328 (2000).
World Spider Catalog. Version 22.0. Natural History Museum Bern. http://wsc.nmbe.ch. Accessed 15 Mar 2021 (2021).
White, J. & Weinstein, S. A. A phoenix of clinical toxinology: White-tailed spider (Lampona spp.) bites. A case report and review of medical significance. Toxicon 87, 76–80 (2014).
Rash, L. D., King, R. G. & Hodgson, W. C. Sex differences in the pharmacological activity of venom from the white-tailed spider (Lampona cylindrata). Toxicon 38, 1111–1127 (2000).
Young, A. R. & Pincus, S. J. Comparison of enzymatic activity from three species of necrotising arachnids in Australia: Loxosceles rufescens, Badumna insignis and Lampona cylindrata. Toxicon 39, 391–400 (2001).
Michálek, O., Petráková, L. & Pekár, S. Capture efficiency and trophic adaptations of a specialist and generalist predator: A comparison. Ecol. Evol. 7(8), 2756–2766 (2017).
Klint, J. K. et al. Spider-venom peptides that target voltage-gated sodium channels: Pharmacological tools and potential therapeutic leads. Toxicon 60(4), 478–491 (2012).
Diniz, M. R. et al. An overview of Phoneutria nigriventer spider venom using combined transcriptomic and proteomic approaches. PLoS ONE 13(8), e0200628 (2018).
Wilson, D. et al. The aromatic head group of spider toxin polyamines influences toxicity to cancer cells. Toxins 9(11), 346 (2017).
Herzig, V. & King, G. F. The cystine knot is responsible for the exceptional stability of the insecticidal spider toxin ω-hexatoxin-Hv1a. Toxins 7(10), 4366–4380 (2015).
Wang, X. H. et al. Discovery and characterization of a family of insecticidal neurotoxins with a rare vicinal disulfide bridge. Nat. Struct. Biol. 7(6), 505–513 (2000).
Yuan, C. H. et al. Discovery of a distinct superfamily of Kunitz-type toxin (KTT) from tarantulas. PLoS ONE 3(10), e3414 (2008).
Luo, J. et al. Molecular diversity and evolutionary trends of cysteine-rich peptides from the venom glands of Chinese spider Heteropoda venatoria. Sci. Rep. 11, 3211 (2021).
Cole, J., Buszka, P. A., Mobley, J. A. & Hataway, R. A. Characterization of the venom proteome for the wandering spider, Ctenus hibernalis (Aranea: Ctenidae). J. Proteom. Bioinform. 9, 196–199 (2016).
Korolkova, Y. et al. New Insectotoxin from Tibellus Oblongus Spider venom presents novel daptation of ICK Fold. Toxins 13(1), 29 (2021).
Koua, D. et al. Proteotranscriptomic insights into the venom composition of the wolf spider Lycosa tarantula. Toxins 12(8), 501 (2020).
Liberato, T., Troncone, L. R. P., Yamashiro, E. T., Serrano, S. M. & Zelanis, A. High-resolution proteomic profiling of spider venom: Expanding the toxin diversity of Phoneutria nigriventer venom. Amino Acids 48(3), 901–906 (2016).
Oldrati, V. et al. Peptidomic and transcriptomic profiling of four distinct spider venoms. PLoS ONE 12(3), e0172966 (2017).
King, G. F. & Hardy, M. C. Spider-venom peptides: Structure, pharmacology, and potential for control of insect pests. Annu. Rev. Entomol. 58, 475–496 (2013).
Turner, A. J., Isaac, R. E. & Coates, D. The neprilysin (NEP) family of zinc metalloendopeptidases: Genomics and function. BioEssays 23(3), 261–269 (2001).
Casewell, N. R., Harrison, R. A., Wüster, W. & Wagstaff, S. C. Comparative venom gland transcriptome surveys of the saw-scaled vipers (Viperidae: Echis) reveal substantial intra-family gene diversity and novel venom transcripts. BMC Genom. 10(1), 1–12 (2009).
Tan, C. H., Tan, K. Y., Fung, S. Y. & Tan, N. H. Venom-gland transcriptome and venom proteome of the Malaysian king cobra (Ophiophagus hannah). BMC Genom. 16(1), 1–21 (2015).
Tan, K. Y., Tan, C. H., Chanhome, L. & Tan, N. H. Comparative venom gland transcriptomics of Naja kaouthia (monocled cobra) from Malaysia and Thailand: Elucidating geographical venom variation and insights into sequence novelty. PeerJ 5, e3142 (2017).
Undheim, E. A. et al. A proteomics and transcriptomics investigation of the venom from the barychelid spider Trittame loki (brush-foot trapdoor). Toxins. 5(12), 2488–2503 (2013).
do Nascimento, S. M., de Oliveira, U. C., Nishiyama-Jr, M. Y., Tashima, A. K. & Silva Junior, P. I. D. Presence of a neprilysin on Avicularia juruensis (Mygalomorphae: Theraphosidae) venom. Toxin Rev. 41(2), 370–379 (2021).
Zobel-Thropp, P. A. et al. Not so dangerous after all? Venom composition and potency of the Pholcid (daddy long-leg) spider Physocyclus mexicanus. Front. Ecol. Evol. 7, 256 (2019).
Diniz, M. R. et al. An overview of Phoneutria nigriventer spider venom using combined transcriptomic and proteomic approaches. PLoS ONE 13(8), e0200628 (2018).
He, Q. et al. The venom gland transcriptome of Latrodectus tredecimguttatus revealed by deep sequencing and cDNA library analysis. PLoS ONE 8(11), e81357 (2013).
Haney, R. A., Ayoub, N. A., Clarke, T. H., Hayashi, C. Y. & Garb, J. E. Dramatic expansion of the black widow toxin arsenal uncovered by multi-tissue transcriptomics and venom proteomics. BMC Genom. 15(1), 1–18 (2014).
Haney, R. A., Matte, T., Forsyth, F. S. & Garb, J. E. Alternative transcription at venom genes and its role as a complementary mechanism for the generation of venom complexity in the common house spider. Front. Ecol. Evol. 7, 85 (2019).
Lüddecke, T. et al. An economic dilemma between molecular weapon systems may explain an arachno-atypical venom in wasp spiders (Argiope bruennichi). Biomolecules 10(7), 978 (2020).
Fainzilber, M., Gordon, D., Hasson, A., Spira, M. E. & Zlotkin, E. Mollusc-specific toxins from the venom of Conus textile neovicarius. Eur. J. Biochem. 202(2), 589–595 (1991).
Pawlak, J. et al. Denmotoxin, a three-finger toxin from the colubrid snake Boiga dendrophila (Mangrove Catsnake) with bird-specific activity. J. Biol. Chem. 281(39), 29030–29041 (2006).
Krasnoperov, V. G., Shamotienko, O. G. & Grishin, E. V. Isolation and properties of insect and crustacean-specific neurotoxins from the venom of the black widow spider (Latrodectus mactans tredecimguttatus). J. Nat. Toxins 1, 17–23 (1992).
Xu, X. et al. A comparative analysis of the venom gland transcriptomes of the fishing spiders Dolomedes mizhoanus and Dolomedes sulfurous. PLoS ONE 10(10), e0139908 (2015).
Kuzmenkov, A. I., Sachkova, M. Y., Kovalchuk, S. I., Grishin, E. V. & Vassilevski, A. A. Lachesana tarabaevi, an expert in membrane-active toxins. Biochem. J. 473(16), 2495–2506 (2016).
Pekár, S. & Toft, S. Trophic specialisation in a predatory group: The case of prey-specialised spiders (Araneae). Biol. Rev. 90(3), 744–761 (2015).
Nyffeler, M. & Pusey, B. J. Fish predation by semi-aquatic spiders: A global pattern. PLoS ONE 9(6), e99459 (2014).
Pekár, S. & Lubin, Y. Prey and predatory behavior of two zodariid species (Araneae, Zodariidae). J. Arachnol. 37(1), 118–121 (2009).
Michálek, O., Kuhn-Nentwig, L. & Pekár, S. High specific efficiency of venom of two prey-specialized spiders. Toxins 11(12), 687 (2019).
Modahl, C. M., Mrinalini, Frietze, S. & Mackessy, S. P. Adaptive evolution of distinct prey-specific toxin genes in rear-fanged snake venom. Proc. R. Soc. B. 285(1884), 20181003 (2018).
Harris, R. J., Zdenek, C. N., Harrich, D., Frank, N. & Fry, B. G. An appetite for destruction: Detecting prey-selective binding of α-neurotoxins in the venom of Afro-Asian elapids. Toxins 12(3), 205 (2020).
Duran, L. H., Rymer, T. L. & Wilson, D. T. Variation in venom composition in the Australian funnel-web spiders Hadronyche valida. Toxicon: X 8, 100063 (2020).
Kuhn-Nentwig, L., Schaller, J. & Nentwig, W. Purification of toxic peptides and the amino acid sequence of CSTX-1 from the multicomponent venom of Cupiennius salei (Araneae: Ctenidae). Toxicon 32(3), 287–302 (1994).
Friedel, T. & Nentwig, W. Immobilizing and lethal effects of spider venoms on the cockroach and the common mealbeetle. Toxicon 27(3), 305–316 (1989).
Eggs, B., Wolff, J. O., Kuhn-Nentwig, L., Gorb, S. N. & Nentwig, W. Hunting without a web: How lycosoid spiders subdue their prey. Ethology 121(12), 1166–1177 (2015).
Andrews, S. FastQC: A quality control tool for high throughput sequence data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2015).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30(15), 2114–2120 (2014).
Song, L. & Florea, L. Rcorrector: Efficient and accurate error correction for Illumina RNA-seq reads. GigaScience 4(1), s13742–s14015 (2015).
Grabherr, M. G. et al. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 29(7), 644 (2011).
Gilbert, D. EvidentialGene: Evidence directed gene predictions for eukaryotes. Available online at: http://arthropods.eugenes.org/EvidentialGene/ (2010).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10(3), 1–10 (2009).
Seppey, M., Manni, M. & Zdobnov, E. M. BUSCO: Assessing genome assembly and annotation completeness. In Gene Prediction (ed. Kollmar, M.) 227–245 (Humana, 2019).
Haas, B. TransDecoder. Available online at: https://github.com/TransDecoder/TransDecoder (2015).
Petersen, T. N., Brunak, S., Von Heijne, G. & Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 8(10), 785–786 (2011).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25(17), 3389–3402 (1997).
UniProt. The universal protein knowledgebase in 2021. Nucleic Acids Res. 49(1), 480–489 (2021).
Eddy, S. R. A probabilistic model of local sequence alignment that simplifies statistical significance estimation. PLoS Comput. Biol. 4(5), e1000069 (2008).
Finn, R. D. et al. Pfam: The protein families database. Nucleic Acids Res. 42(1), 222–230 (2014).
Wong, E. S., Hardy, M. C., Wood, D., Bailey, T. & King, G. F. SVM-based prediction of propeptide cleavage sites in spider toxins identifies toxin innovation in an Australian tarantula. PLoS ONE 8(7), e66279 (2013).
King, G. F., Gentz, M. C., Escoubas, P. & Nicholson, G. M. A rational nomenclature for naming peptide toxins from spiders and other venomous animals. Toxicon 52(2), 264–276 (2008).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org/ (2019).
Venables, W. N. & Ripley, B. D. Random and mixed effects in Modern Applied Statistics with S 271–300 (Springer, New York, 2002).
Pekár, S. & Brabec, M. Modern Analysis of Biological Data: Generalized Linear Models in R (Masaryk University Press, 2016).
Halekoh, U., Højsgaard, S. & Yan, J. The R package geepack for generalized estimating equations. J. Stat. Softw. 15(2), 1–11 (2006).
Pekár, S. & Brabec, M. Generalized estimating equations: A pragmatic and flexible approach to the marginal GLM modelling of correlated data in the behavioural sciences. Ethology 124(2), 86–93 (2018).
Acknowledgements
Core Facility Bioinformatics of CEITEC Masaryk University is gratefully acknowledged for the obtaining of the transcriptomic data presented in this paper. A.A.W. would like to thank Alun Jones from the Queensland Bioscience Precinct for advice on mass spectral data processing.
Funding
O.M. was supported by Virtual Networking Grant (E-COST-GRANT-CA19144-7dc9bfbd) of the COST Action EUVEN (CA19144). We acknowledge financial support from the Australian National Health & Medical Research Council (Principal Research Fellowship APP1136889 to G.F.K.) and the Australian Research Council (Centre of Excellence Grant CE200100012 to G.F.K. and Discovery Grant DP200102867 to A.A.W.). CIISB, Instruct-CZ Centre of Instruct-ERIC EU consortium, funded by MEYS CR infrastructure project LM2018127, is gratefully acknowledged for the financial support of the measurements at the CEITEC Proteomics Core Facility. This article is part of a project that has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 101031131.
Author information
Authors and Affiliations
Contributions
O.M.: designed the study, performed the venom bioassays, analysed data and wrote the first draft of the manuscript; A.A.W.: performed bioinformatic integration of proteomic and transcriptomic data; O.S. and Z.Z.: performed proteomic analyses; S.P.: designed the study and collected spider specimens; G.F.K.: designed the study; all authors contributed to the text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Michálek, O., Walker, A.A., Šedo, O. et al. Composition and toxicity of venom produced by araneophagous white-tailed spiders (Lamponidae: Lampona sp.). Sci Rep 12, 21597 (2022). https://doi.org/10.1038/s41598-022-24694-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24694-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.