Abstract
Phytic acid (PA) is an abundant natural plant component that exhibits a versatility of applications benefited from its chemical structure, standing out its use as food, packing and dental additive due to its antimicrobial properties. The capacity of PA to chelate ions is also well-established and the formation and thermodynamic properties of different metallic complexes has been described. However, research studies of these compounds in terms of chemistry and biological features are still demanded in order to extend the application scope of PA complexes. The main goal of this paper is to deepen in the knowledge of the bioactive metal complexes chemistry and their bactericide activity, to extend their application in biomaterial science, specifically in oral implantology. Thus, this work presents the synthesis and structural assessment of two metallic phytate complexes bearing the bioactive cations Zn2+ and Sr2+ (ZnPhy and SrPhy respectively), along with studies on the synergic biological properties between PA and cations. Metallic phytates were synthesized in the solid-state by hydrothermal reaction leading to pure solid compounds in high yields. Their molecular formulas were C6H12024P6Sr4·5H2O and C6H12024P6Zn6·6H2O, as determined by ICP and HRES-TGA. The metal coordination bond of the solid complexes was further analysed by EDS, Raman, ATR-FTIR and solid 13C and 31P-NMR spectroscopies. Likewise, we evaluated the in vitro ability of the phytate compounds for inhibiting biofilm production of Streptococcus mutans cultures. Results indicate that all compounds significantly reduced biofilm formation (PA < SrPhy < ZnPhy), and ZnPhy even showed remarkable differences with respect to PA and SrPhy. Analysis of antimicrobial properties shows the first clues of the possible synergic effects created between PA and the corresponding cation in different cell metabolic processes. In overall, findings of this work can contribute to expand the applications of these bioactive metallic complexes in the biotechnological and biomedical fields, and they can be considered for the fabrication of anti-plaque coating systems in the dentistry field.
Similar content being viewed by others
Introduction
Myo-inositol hexaphosphoric acid, commonly named phytic acid (PA), is an abundant natural-occurring vitamin-B related compound that constitutes the 1–5% by dry weight of most edible legumes, cereals and seeds, and represents the major phosphorous reservoirs of plants1. The unique chemical structure of PA, composed of six phosphate groups that contain twelve replaceable protons, confers to the molecule a strong interaction with multivalent cations and proteins1,2. Moreover, the dephosphorylated species of PA by phytase enzymes play a key role in the regulation of the cellular metabolism of plants and mammalians3,4. In this sense, the high reactivity of PA provides a multifunctional versatility of applications in a wide range of medical treatments and daily used products since it was considered as “generally recognized as safe” (GRAS) by the Food and Drug Administration of the United States in 19972,3,5. PA has been employed as a promising agent for the treatment of colonic cancer6 and the prevention of heart disease, renal calculi and Parkinson disease7; it has been implemented in several oral care products8, applied over metal surfaces as anticorrosion and flame-retardant coating9, and used as an additive for the preservation of food and packaging materials due to its capacity for inhibiting iron-catalysed oxidative reactions and microbial growth10.
Increasing evidence of the potential antimicrobial effect of PA has been reported in the last years. The ability of PA for inhibiting the proliferation of several foodborne bacterial pathogens5,11 has been described suppressing the growth of a broad spectrum of microbial species comprising gram-positive and gram-negative bacteria, some fungus, and even decreasing the production of bacterial biofilm12,13,14,15. The biofilm disruption ability is of particular relevance since the formation of biofilms implies the creation of a protective shell for bacteria isolating them from the host immune system and even from antibiotic treatments leading to a permanent infection16. These findings have attracted researches attention in order to expand the application of PA to the field of implants surface modification aiming to minimize implants failures associated with postoperative-related infections. Recently, metallic substrates have been functionalized with PA and exhibited promising results for decreasing the adhesion of gram-positive strains17 and titanium-PA systems also showed a bacteriostatic effect over Porphyromonas gingivalis cultures18.
Likewise, the high affinity of PA with divalent cations enables its preparation as carrier of bioactive cations in the form of metallic complexes that may tune the native properties of PA. The ability of PA to form complexes with several transition metals, alkali and alkali-hearth metals, describing the chemistry of the solution complexation equilibriums are well documented, but the molecular formulas calculated for the solid complexes are still mostly uncertain and studies lack of any type of in vitro analysis19,20,21,22. We hypothesise that the selective synthesis of metallic phytate complexes could modulate the bioactivity of PA regarding the cation bonded. Thus, we propose the synthesis and evaluation of two metallic derivatives of PA bearing Zn2+ and Sr2+ (named as ZnPhy and SrPhy respectively). The selected cations have been applied on regenerative therapies exhibiting a significant promotion of bone and cartilage tissues formation in vivo23,24,25. Zn2+ is a bioactive cation involved in several key metabolic processes and owns powerful antimicrobial, antioxidant and anti-inflammatory activities26,27. Besides, Zn2+ has been successfully incorporated on metallic surfaces or as an additive in hydroxyapatite and montmorillonite materials, exhibiting in all cases an inhibitory effect of bacterial adhesion and a reduction on the number of viable cells27,28,29,30. It is worth mentioning that we have recently reported the synthesis and evaluation of folic acid (vitamin B9) metallic complexes with Zn2+ and Sr2+, and found that their application as cell signalling factors provides tailored osteogenic properties in terms of ALP activity, matrix mineralization and expression of some osteogenic bone-like gens31. In view of all data presented, the incorporation of bioactive cations may contribute with novel properties or an enhancement of the native demonstrated potential of PA, and thus, the in vitro biological characterization of phytate complexes is of special interest for their further application in biomedicine and as food or packing additives.
Antecedents reporting spectroscopic and chemical characterization of Zn-phytate complexes in the solid-state22,32,33,34 and in the solution equilibrium chemistry35,36,37 have been described in the literature, but as far as we know, published papers on Sr-phytate complexes are scarce and in general they lack empiric data, and molecular formula in solid-state and solution complexation ability has been succinctly reported21. Generally speaking, it can be said that the chemistry of bioactive metallic complexes lacks empiric data, and molecular formula, either in solid-state or solution complexation, and have been succinctly reported. In this frame, the novelty of the present work lies in providing valuable knowledge in the chemistry of these complexes as well as in their biological properties, to extend their application in the biomedical field. Thus, specifically, our paper deepens in the structural chemical analysis of two bioactive phytate complexes and in their bactericide properties, and proposes their application in dental implantology.
Therefore, in this work we aim to carry out a deep physic-chemical characterization of ZnPhy and SrPhy in the solid-state, ZnPhy and SrPhy, that will provide further empirical spectroscopic data of interest, contributing as well to the assessment of their chemical structure and coordination mode, and to propose their application in the dentistry field for the fabrication of anti-plaque coating systems11,18. To this end, the determination of the molecular formula is carried out by ICP and HRES-TGA, and spectroscopic characterization is further analysed via 31P-NMR, 13C-NMR, ATR-FTIR and EDX spectroscopies. Furthermore, we carried out an in vitro approximation of the potential of PA, ZnPhy and SrPhy for inhibiting the production of bacterial biofilm by Streptococcus mutans (S. mutans) cultures. This property was measured through the count of viable cells and crystal violet staining. Additionally, we also analyzed the growth curves of S. mutans cultures obtained under phytate treatment by optical density measurements.
Results and discussion
Synthesis and physic-chemical characterization of phytate compounds
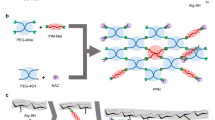
The synthetic procedure followed for the preparation of SrPhy and ZnPhy derivatives is displayed in Fig. 1. Both compounds were synthesized by the hydrothermal reaction of the commercial sodium salt of PA with the respective chloride metal salt, SrCl2 and ZnCl2, and obtained in high yields (> 90%). Precipitation of metal complexes was carried out by dropping each chloride metal solution over the respective PA solutions at a pH value of 7.4 in a final proportion M2+:PA of 6:1. The pure phytate complexes obtained were used in their solid form for all the physic-chemical characterization. The reaction scheme showed in Fig. 1 represents a conformational change of the phytate molecule when the metallic complexes are formed as it has been previously reported by other authors38 and it will be discussed below in this work. Figure 1 shows a generic formula for both complexes. Nevertheless, based on reported works regarding the coordination bonds formed between the cations and the corresponding phosphate group coordination modes, and attending to the charge and the number of cations, it can be assumed that divalent cations were bonded preferably as a bridge between adjacent phosphate groups39.
Compositional analysis and thermal degradation
The empiric molecular formula of the precursor and the metallic phytates are shown in Table 1. The amount of sodium, strontium and zinc coordinated to phosphate anions was quantified by ICP, and the content of water molecules was calculated from the HRES-TGA analysis. EDS spectroscopy confirmed the presence of characteristic peaks for Sr (1.80 keV), and Zn (1.01 keV), in the SrPhy and ZnPhy derivatives respectively, and for P (2.01 keV) in all compounds; EDS results also revealed that phytate complexes were obtained purely without chloride impurities, Fig. 2A. The compositional analysis of phytate complexes made by ICP and elemental microanalysis reveals that 4 and 6 metal atoms of Sr2+ and Zn2+ respectively were coordinated to phytate rings reaching P/M2+ molar ratios of ≈ 1.6 and 1.09 for SrPhy and ZnPhy and a P/Na+ molar ratio of ≈ 1 for PA. Atomic content in C and H were found as: PA 8.1%C, 2.6%H; SrPhy 6.1%C, 2.2%H; ZnPhy 6.2%C, 2.3%H. These results indicate the presence of C6 in the molecular formula of all compounds, and H12, H10 and H6 for PA, SrPhy and ZnPhy respectively. Besides, the addition of Sr2+ to the PA occurs in conjunction with the precipitation of Sr4Phy at pH 7.4 in a similar manner that has been described for Ca-Phy40, while Zn2+ forms Zn6Phy precipitates22,33.
(A) EDS spectra registered for PA, SrPhy, and ZnPhy with assigned characteristic peaks and (B) HRES-TGA diagrams of PA, SrPhy and ZnPhy obtained under inert atmosphere. The dashed lines in the thermograms are shown to indicate the different regions of decomposition comprising water loss, phytate rings and organic matter.
When the metallic complexes were formed, the results of the HRES-TGA curves obtained in inert atmosphere exhibited different degradation profiles respect to that of PA, Fig. 2B. HRES-TGA thermograms obtained under air atmosphere are shown in Fig. S1. Up to 150 °C, PA suffered a weight loss of ≈ 5% due to the release of water molecules (TMAX 92 °C). In contrast, water loss from SrPhy and ZnPhy started at early times (TMAX 82 °C and 75 °C respectively) and it was faster, displaying more pronounced mass decrease compared to PA, due to their higher water content, finally resulting in 3, 5 and 6 units of water molecules coordinated to PA, SrPhy and ZnPhy structures respectively. The main degradation step has been associated with the carbonization and dehydration of hydroxyl groups33,41. The decomposition of phytate anions in the metal complexes (130–290 °C for both compounds) took place at lower temperature than for PA (190–380 °C). In the final degradation step, further decomposition of carbon structure occurred at ≈ 380 °C for PA and it was close to 300 °C for the phytate complexes. Once again, the presence of divalent cations coordinated with the phosphate groups produces a decrease in the thermal stability of the compounds. As expected, the residue obtained at the maximum temperature evaluated (600 °C) had a greater mass for SrPhy (80%) and ZnPhy (81%) than for PA (77%) due to the presence of the non-degradable metallic components.
Some authors have determined the complexation ability of PA with several transitions metals and found a different binding capacity attending to the phase state analysed, the solution complexation or the solid formation19,20,22. Overall results exhibited that under PA excess conditions, soluble species with 1:1 stoichiometry (M2+:P) predominated at low pH values. However, when metal cations were in excess, the precipitation of solid phytate complexes took place for which there is some diversity in the final content of water molecules and cations coordinated to phytate anion. Ermanno et al. reported that zinc-phytate compounds synthesized under cation excess conditions are composed of one water molecule, deduced by TGA, and six coordinated cations, calculated via ICP22. Comel et al. found that zinc complexes synthesized in the same conditions and bearing the same amount of cations per molecule, lead to six coordinated water molecules33, while Champagne et al. informed that Zn/phytate ratio was initially 4, and decreased to 3.5 after 24 h, monitored by31P NMR32. For the strontium complex, Gancheff et al. found a 5:1 stoichiometry between cation and phytate anion determined by elemental analysis, and owing 16 water units per molecule, for an initial mixture 5:1 (M2+:PA)21. Interestingly, the methodologies followed for the synthesis of Zn-phytate complexes employed an acidified PA solution contrary to the methodology described in this work, which may influence the coordination number of the isolated solids.
Raman and ATR-FTIR characterization
The conformational state 5-axial/1-equatorial of the inositol ring displayed in the reaction scheme of Fig. 1 is in agreement with the work published by Isbrandt et al.42. To evaluate this, Isbrant and coworkers performed a combined analysis of Raman spectroscopy, 31P-NMR and 13C-NMR with sodium phytate complexes. Raman results (Fig. 3A) have demonstrated that C–C–H and O–C–H bending vibrational bands found in the range of 1250 and 1400 cm−1 have a maximum intensity at 1380 cm−1 when PA has a 5-axial/1-equatorial arrangement, and thus, it has been assumed that all phytate compounds have the same conformation as is represented in Fig. 1. Detailed bands assignment of Raman spectra is collected in Table 2 and it is in accordance with previous reports of other authors39,42,43. Signals of SrPhy and ZnPhy shifted to greater or lower wavenumber when compared to those of PA bands attending to the coordinated cation. Interestingly, numerous reports have demonstrated that in the liquid equilibrium of phytic acid, the conformational state adopted below pH values of 9 corresponds to the 1-axial/5-equatorial form, conversely to the evidences obtained for the solid-state of the phytate complexes explored in this study and for other solid phytate salts38,42.
ATR-FTIR spectroscopy analysis provided confirmation of the metal complexation of each compound. Expanded regions of the spectra obtained for each compound are shown in Fig. 3B and the assignment of the main vibrational mode bands in the whole spectra is collected in Table 343,44. All compounds showed a broad band around 3300 cm−1 and a single peak centered in 1640 cm−1 which were attributed to the stretching and bending vibrational modes of O–H bonds respectively, coming either from coordinated water molecules or unbounded P–O–H bonds39. The main vibrational modes of the C(O)PO3 groups are found in the region of 750–1300 cm−1. The spectra obtained in this region for each compound are shown in Fig. 3B. SrPhy and ZnPhy spectra displayed peak shifts to higher wavenumber in all these bands in comparison to those of PA. This effect has been reported for similar metallic phytate complexes and it was attributed to a change in the P-O strength bond due to the modification of the chemical structure of PA in which the formation of the coordination bond takes place. The spectrum of the PA sodium salt employed in this study (C6H12O24P6·6Na.3H2O) exhibited a peak at 1190 cm−1 that corresponds to the asymmetric stretching of P–O bonds in protonated HPO3− groups 39. Nevertheless, in the spectra obtained for the metallic compounds this band is overlapped with the symmetric stretching vibrational mode of P-O bonds. This behavior is explained by the disappearance of protonated HPO3− groups due to the establishment of the coordination bond with the divalent cation39,44. Besides, the band centred at 917 cm−1 in PA spectra splits into a double peak for ZnPhy (924—980 cm−1), and a triplet peak in the case of SrPhy (1000–960–926 cm−1) as expected since the conjugation of the metal cation is different in each complex34,39.
31P and 13C NMR analysis
The coordination bond between zinc or strontium with phytate anion was analyzed by solid 31P NMR and 13C spectroscopy, and the results are shown in Fig. 4. For the three compounds, each 31P NMR spectrum presented a broad peak for all the phosphorus atoms and two symmetrical spinning sidebands (Fig. 3A). It can be observed that signals obtained for SrPhy and ZnPhy compounds moved towards lower chemical shift when compared to those of the precursor PA. This migration can be explained by the formation of a coordination bond created between Sr2+ or Zn2+, and phosphate groups, leading to a decrease in the electric dipole moment of the bridge oxygen atom, and the consequent displacement of the signals to lower chemical shifts. Similar effect has been previously reported for metallic phytate compounds in the solid state finding that both the type of metal and the number of metal-phytate bonds influence the chemical shift of the phosphorous atoms and the spinning sidebands45. In the solid 13C NMR spectra of PA, SrPhy and ZnPhy (Fig. 3B) a single peak centered at 75.7 ppm for each compounds was displayed, denoting that there is not a direct interaction between the respective metals with the carbon atoms of the molecule42.
In overall, at sight of the published results and those obtained in the present work regarding the structural characterization of the metallic phytates, it can be concluded that there exists a relationship between the synthetic procedure and the coordination number of the as-obtained metallic complex. The complexation ability of PA highly depends on the nature of the cation, the ratio PA:M2+, the pH reaction values and the ionic strength of the medium. The variability of these parameters leads to the obtaining of solid phytate complexes with different number of coordinated cations. Physical and spectroscopic data obtained for SrPhy and ZnPhy are comparable to those previously reported for similar complexes differing mainly just in the molecular formulas33,40,43.
Antimicrobial activity
Biofilm inhibition ability
The antimicrobial potential of phytate compounds was assessed as a function of their capability to impair the growth and production of biofilm by S. mutans cultures. This strain has been described as one of the main cariogenic bacterial species of the oral microbiota and it is considered as an important risk factor in the development of dental caries46,47,48. S. mutans synthesizes glucans that promote the biofilm formation and the acidification of the buccal environment47,48,49,50,51,52 which leads to the proliferation of other biofilm bacterial species. Therefore, strategies to inhibit the proliferation and formation of S. mutans in dental plaque are key for cariogenic prevention49,53. In this vein, biofilm formed was studied by means of CV staining and colony forming unit counts (CFU) in agar-BHI solid plates. CV is a protein dye commonly used for the identification of all biofilms that stains the extracellular matrix of polysaccharides and negatively charged surface molecules54. This method implies an improvement in the determination of total biofilm and not just functional biofilm, since CV can dye both viable and dead cells together with extracellular matrix55. Phytate compounds were dissolved in a mixture of BHI:Tris–HCl 50 mM (1:1) at 100 µg/mL, and tested at a final concentration of 50 µg/mL. As control sample, bacteria were treated with a mixture of BHI:Tris–HCl 50 mM (1:1) emulating the same culture conditions established for the experimental samples but without phytate supplementation. The concentration of the Tris–HCl buffer employed was reported to not affect bacterial growth56. Moreover, based on the thermodynamics equilibriums previously established for both phytate complexes, the expected phytate species found in solution at the experiment pH value (≈7) are Zn2+ (83%) and ZnH3Phy7− (17%) for Zn-containing complexes; and Sr2+ (≈71%) and SrH5Phy5 (≈29%)for strontium phytate compounds21,33.
The quantification of CV found in the biofilms formed is represented as the optical density in Fig. 5. Inhibition capacity was composition-dependent regarding the cation bonded in each PA-complex. All phytate compounds were able to significantly reduce the biofilm produced by S. mutans (***p < 0.001), and interestingly, cells treated with ZnPhy exhibited a remarkably improved anti-biofilm activity with respect to PA and SrPhy samples (**p < 0.005). In parallel, the count of viable bacteria found in both the biofilm matrix and the planktonic supernatant were determined. There is only a significant bactericidal effect in the resuspended biofilm matrix (nearly 1 log) for ZnPhy sample (*p < 0.005) (see Supplementary Fig. S2), which is in accordance with the biofilm disruption assessment (Fig. 5). On the contrary, the CFU counts found in the planktonic solution did not show significant differences, suggesting that the antimicrobial activity when phytate treatment was applied is mainly effective for the biofilm formation. Therefore, our results demonstrate the effectiveness of two metallic phytate-complexes bearing Sr2+ and Zn2+ (SrPhy and ZnPhy) to prevent the S. mutans biofilm.
However, the metabolic role of PA in the disaggregation of bacterial biofilms remains poorly understood. It is believed that the antibacterial mechanism of PA is based in the disruption of the cell membrane integrity due to the high negative charges of its chemical structure, and thus, cellular morphology and intracellular ATP levels may be affected8,11,12. This theory is supported by the broad spectrum of both gram-positive and gram-negative bacteria for which PA has demonstrated antibacterial potential, and also by the rapid action required to achieve antibacterial activity15. Another explanation of the mechanism of action could be associated with the iron-chelating properties of PA since there are some reports that support the anti-biofilm ability for other iron-chelating agents43,44,45.
The greater disaggregation of the polymeric biofilm observed for Sr/Zn-bearing phytate complexes can be understood as a possible synergic effect between the positive cation and the negative charges of PA that may hinder the aggregation of proteins required for the adhesion of biofilm-forming polysaccharides presumably by the establishment of a ternary protein-metal-phytate complex46,47. For its part, other authors have explored the combined effect exhibited by antibiotic-based systems including Zn2+. The formation of Zn2+ complexes with kefzol (a commercial antibiotic) has been reported to remarkably improve the antibacterial activity exhibited by kefzol treatment alone48. The system zinc citrate/triclosan was also analysed and a similar synergic antimicrobial effect against S. mutans was detected, attributed to the presence of Zn2+49, which agrees with our findings. Zinc has been reported for affecting S. mutans metabolism, at mM concentration, via multiple inhibitory actions comprising the modulation of oxoenzymes, the inhibition of glycolysis, alkali production, the function of the phosphoenolpyruvate system (sugar phosphotransferase, PTS) and F-ATPase49,50,51,52, which allow biofilm growth to be controlled. However, the antimicrobial effect of Zn2+ by itself seems to be bacteriostatic since the inhibition of glycolysis, PTS and F-ATPase were reversible processes50. Thus, Zn2+ is expected to only may enable bacteria killing in combination with other bactericidal agents50, though an improvement of their intrinsic potential was noticed as described above, and also supported by our results48,49. On the contrary, a recent study found equal bacteriostatic and bactericidal properties for zinc sulphate and zinc acetate salts (tested in the range of µg/mL) against S. mutans cultures53. In our work, a low concentration of Zn2+ (≈18 µg/mL) enabled to reduce the production of biofilm by S. mutans.
Growth curves
Growth curves of S. mutans cultures under PA-compounds treatment were recorded by automatic measurement of OD600 each 20 min during 16.5 h, and the results obtained are displayed in Fig. 6. Ten-fold serial dilutions (10−1–10−4) were obtained from an initial culture at OD600 0.2. Diluted samples were growth under constant phytate compounds treatment at a fixed final concentration (50 µg/mL), due to the limited solubility of phytate complexes (Fig. 6A–D). The profile of the growth curves obtained for 10−1 diluted samples from cultures treated with different PA-compounds did not show any significative difference when compare to the untreated control sample (Fig. 6A). Nevertheless, we detected an increase in the duration of the lag phase in cultures treated with PA compounds at dilution factors 10−2–10−4 (Fig. 6B–D). Precisely, the most significant effects on the lag phase of growth were observed for cultures treated with ZnPhy and SrPhy complexes (Fig. 6D). These results could suggest a bacteriostatic effect of the PA-compounds based on their ability to increasing the time needed for bacterial population to adapt to a new environment as reported57,58. Interestingly, SrPhy and ZnPhy treated samples also exhibited this effect when 10−2 and 10−3 sample dilutions were tested, which suppose an enhancement of the intrinsic bacteriostatic properties registered for PA.
Bacteria induce the biofilm formation in response to environmental signals. The processes by which S. mutans undergoes the formation of biofilms are highly conditioned by the quorum sensing (QS) system59. QS is activated in response to the release of autoinducer molecules or pheromones in a cell density-dependent manner and confers a bacteria population the ability to alter their physiology and behaviour as a group unit instead of single entities59,60. In this sense, QS enables a collective response of bacterial populations when they are exposed to any environmental stress by the regulation of different physiological processes including sporulation, antibiotic production, competence development and biofilm differentiation, among others60,61. In our experiments, phytate supplementation altered the levels of biofilm production and the number of viable bacteria embedded in polymeric biofilm, perhaps associated by their role in the modulation of the QS transduction system. In fact, the significantly decreased of CFU found in the biofilm of ZnPhy samples (Supplementary Fig S2) is in accordance to its higher biofilm disaggregation ability (Fig. 5). Furthermore, it could be speculated that the higher OD600 values obtained for ZnPhy in Fig. 6 are tentatively attributed to the synergic role between the cation and phytate in S. mutans metabolism, which drives the proliferation of viable CFU to the planktonic solution since biofilm formation is expected to be unfavoured as was highly inhibited in Fig. 5. This work demonstrates the possibilities of applying these type of formulations in cariogenic prevention strategies. In fact, the inhibition of the proliferation of key strains such as S. mutants in dental plaque, supports further validation for testing these compounds in vivo.
Conclusions
Two metallic phytate-complexes bearing Sr2+ and Zn2+ (SrPhy and ZnPhy) have been successfully prepared in high yields. Their deep compositional analysis in the solid-state by spectroscopic techniques (ICP, EDS, Raman, ATR-FTIR, solid 13C NMR and 31P NMR) along with thermal degradation evaluation, confirmed the metal coordination bond and allowed to define their molecular formula as Sr4C6H10O24P6·5H2O and Zn6C6H6O24P6·6H2O, in a 5-axial/1-equatorial conformation. In vitro bactericidal studies results provided evidences about the capacity of phytate complexes for modulating the intrinsic antimicrobial properties of PA in terms of biofilm disruption and growth trend of S. mutans. The highest anti-biofilm activity was exhibited by ZnPhy, followed by SrPhy. This synergic effect between PA and the corresponding cation might affect other metabolic processes, and thus, is of special interest for the evaluation of their biological properties in other aspects. In general, findings of this work envision the potential of the two bioactive metallic complexes to be applied in the biotechnological and biomedical fields.
Methods
Synthesis of metallic phytate derivatives
Phytic acid sodium salt hydrate (C6H18O24P6.xNa.yH2O) was purchased from Sigma-Aldrich, strontium chloride hexahydrate (SrCl2.6H2O) from Acros Organics and zinc chloride (ZnCl2 anhydrous) from Fluka. All were used as received without further purification. SrPhy and ZnPhy derivatives were synthesized by reaction of PA with the corresponding metal chloride salt, SrCl2 or ZnCl2 respectively, following an adapted method from Fernandez-Villa et al.31. Briefly, an aqueous solution of phytic acid (0.2 M, pH adjusted to 7.4 with NaOH 0.1 M) was heated at 50 °C in a round bottom flask connected to a reflux system for 2 h. Then, 25 mL of SrCl2.6H2O or ZnCl2 solution (1.2 M, ethanol/water, 1:1, v:v) was dropped onto the previous PA solution in a 1:6 phytic acid:metal (P:M2+) molar ratio, forming a white precipitate. The reaction was further stirred for 1 h and quenched down in an ice bath. The solid formed was collected and purified by two hot vacuum-filtrations with a mixture of ethanol/water (300 mL, 1:1, v:v). Finally, the product was dried under vacuum at 50 °C until constant weight and milled to a fine white powder. All compounds were stored at room temperature under anhydrous conditions. The reaction yields were 90% and 96% for SrPhy and ZnPhy respectively.
Physic-chemical characterization methodologies
Before all the analysis, samples were dried at 50 °C and kept in a desiccator until the HR-TGA analysis, in order to prevent the uptake of water from ambient humidity. The atomic composition of phytate complexes was determined by emission spectroscopy analysis using an inductively coupled plasma (ICP) optical emission Perkin-Elmer 430DV. A given weight of the complex was dissolved in HCl (2% p/v) and the solution was made to volume in a measuring flask. The experimental water content for each molecular formula was obtained by high resolution thermogravimetric analysis (HRES-TGA) using a TGA Q500 apparatus from TA instruments under a inert nitrogen atmosphere or air at a heating rate of 10 °C/min in a range of 40 – 700 °C. Likewise, the chemical composition of phytate complexes was determined by energy-dispersive X-ray spectroscopy (EDS) using a Hitachi SU8000 equipment and elemental microanalysis using a Eurovector EA 3000 equipment.
The structural characterization of the compounds was analyzed by Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) spectroscopy (Perkin- Elmer Spectrum One spectrophotometer); Raman spectroscopy (Renishaw inVia Raman Microscope, laser wavelength 785 nm, objective 100 × 0.85 and spectral resolution of 1200 lines/mm); and solid 13C and 31P nuclear magnetic resonance (NMR) (Bruker AV-400-WB with 4 mm triple channel probe with ZrO rotors, Kel-F plug at room temperature, working frequency 161.97 MHz for 31P and 100.32 MHz for 13C, and rotation speed 10 kHz in both cases).
Cell cultures
Biofilm inhibition capacity of phytate compounds was assessed semi-quantitatively by crystal violet (CV, Sigma Aldrich) staining and through the count of viable bacteria (colony forming unit, CFU) found in both the planktonic solution and bacterial biofilm. S. mutans CECT 479 was grown in brain heart infusion (BHI, NutriSelect® Plus, Sigma Aldrich) broth medium. Experiments were performed in triplicate with five different inoculums coming from a bacterial solution with an optical density of 0.1 registered at 600 nm (OD600 0.1), recorded in a spectrophotometer model Ultrospec 10 cell density meter (Amersham Biosciences). Bacteria stock inoculum was storage at − 80 °C in 15% (v:v) glycerol solution. PA, ZnPhy and SrPhy were solved at 100 µg/mL in Tris–HCl buffer 50 mM, pH ≈ 7. To do this, the phytate complexes were solved at 2 mg/mL in Tris–HCl buffer 1 M overnight. Subsequently, 5 mL of this solution were diluted in 100 mL of deionised water. The resulting solutions were adjusted to a final concentration of metal complexes of 100 µg/mL, 50 mM of Tris–HCl, and pH close to neutral. Finally, samples were sterilised by filtration with a 0.22 nylon filters. The control samples of these experiments was a S. mutans culture in BHI:Tris–HCl buffer 50 mM (1:1) without phytate compounds treatment.
Biofilm inhibition and viable bacteria
For antimicrobial assays, 10 µL of bacteria solution OD600 0.1 (2.0 × 107 CFU/mL) were inoculated in 10 mL of BHI broth and were cultured aerobically at 37 °C in static conditions until OD600 ≈ 0.6 (1.2 × 108 CFU/mL). Then, bacteria were diluted 1:50 in BHI medium and 100 µL of the latter dilution were placed in a flat bottom 96-well plate. Subsequently, 100 µL of the corresponding phytate (100 µg/mL) or control solutions were added to each well and the plate was incubated in static conditions for 5 h at 37 °C. For the quantification of viable cells, 20 µL aliquots were extracted from the supernatant and serial dilutions in BHI medium were prepared to range 10−1–10−6. Then, the rest of the planktonic solution phase was carefully removed and the biofilm deposited on the wells was disrupted by scratching in a 200 µL solution mixture of BHI:Tris–HCl (1:1). Ten-fold serial dilutions (10−1–10−6) of these bacterial suspension were prepared. The CFU number, from both the resuspended biofilm and the planktonic, were determined by depositing 10 µL droplets of the diluted bacterial solutions (10−3–10−6) on Agar-BHI plates, followed by incubation overnight at 37 °C. On its turned, for the semi-quantification of the biofilm produced, after incubation time the supernatant was removed and 50 µL of CV solution (1% w:w) were added to each well and the plate was left to react at room temperature for 15 min. Subsequently, the dye was removed and the wells were washed three times with 200 µL of deionised water. Finally, the remaining stain was solved in 200 µL of ethanol 96% and the absorbance of the samples was measured at 595 nm (A595) in a VersaMax microplate absorbance reader (Molecular Devices, USA). Wells without bacteria were stained and washed following the same procedure to establish the basal levels of the dye retained in the walls. Data were expressed as the optical density.
Results were statistically analysed by an ANOVA test between all groups evaluated at significant levels **p < 0.005 and ***p < 0.001 (Tukey Test).
Determination of growth curves
The growth profiles of S. mutans cultures under phytate treatment were assessed spectroscopically by measuring OD600 over time. S. mutans was grew in BHI medium until OD600 0.2 (5.3 × 107 CFU/mL). Serial dilutions of this bacterial solution were prepared from 10–1 to 10−4, using a ten-fold dilution factor, and 100 µL aliquots were placed in a 96-well plate along with 100 µL of the corresponding phytate compound (100 µg/mL) or control solution (BHI:Tris–HCl, 1:1). The plate was incubated at 37 °C and shaken for 5 s before each measure. OD600 was automatically recorded every 20 min for 16.5 h in the VersaMax microplate absorbance reader.
Data availability
Materials, data and associated protocols are available to readers without undue qualifications in material transfer agreements.
References
Nissar, J., Ahad, T., Naik, H. R. & Hussain, S. Z. A review phytic acid: As antinutrient or nutraceutical. J. Pharmacogn. Phytochem. 6, 1554–1560 (2017).
Zhou, J. R. & Erdman, J. W. Phytic acid in health and disease. Crit. Rev. Food Sci. Nutr. 35, 495–508. https://doi.org/10.1080/10408399509527712 (1995).
Feil, B. Phytic acid. J. New Seeds 3, 1–35 (2001).
Shears, S. B. Inositol pentakis- and hexakisphosphate metabolism adds versatility to the actions of inositol polyphosphates: Novel effects on ion channels and protein traffic. Subcell. Biochem. 26, 187–226 (1996).
Zhang, H. et al. Phytic acid enhances biocontrol efficacy of Rhodotorula mucilaginosa against postharvest gray mold spoilage and natural spoilage of strawberries. LWT—Food Sci. Technol. 52, 110–115. https://doi.org/10.1016/j.lwt.2012.01.027 (2013).
Bazzano, L. A., He, J., Ogden, L. G., Loria, C. M. & Whelton, P. K. Dietary fiber intake and reduced risk of coronary heart disease in US men and women. Arch. Intern. Med. 2003, 163. https://doi.org/10.1001/archinte.163.16.1897 (1897).
Xu, Q., Kanthasamy, A. G. & Reddy, M. B. Neuroprotective effect of the natural iron chelator, phytic acid in a cell culture model of Parkinson’s disease. Toxicology 245, 101–108. https://doi.org/10.1016/j.tox.2007.12.017 (2008).
Nassar, M. et al. Phytic acid: Properties and potential applications in dentistry. Front. Mater. 8, 1–17. https://doi.org/10.3389/fmats.2021.638909 (2021).
Graf, E. Applications of phytic acid. J. Am. Oil Chem. Soc. 60, 1861–1867 (1963).
Graf, E. & Eaton, J. W. Antioxidant functions of phytic acid. Free Radic. Biol. Med. 8, 61–69. https://doi.org/10.1016/0891-5849(90)90146-A (1990).
Zhou, Q., Zhao, Y., Dang, H., Tang, Y. & Zhang, B. Antibacterial effects of phytic acid against foodborne pathogens and investigation of its mode of action. J. Food Prot. 82, 826–833. https://doi.org/10.4315/0362-028X.JFP-18-418 (2019).
Kim, N. H. & Rhee, M. S. Phytic acid and sodium chloride show marked synergistic bactericidal effects against nonadapted and acid-adapted Escherichia coli O157: H7 strains. Appl. Enviromental Microbiol. 82, 1040–1049. https://doi.org/10.1128/AEM.03307-15.Editor (2016).
Kim, N. H. & Rhee, M. S. Synergistic bactericidal action of phytic acid and sodium chloride against Escherichia coli O157: H7 cells protected by a biofilm. Int. J. Food Microbiol. 227, 17–21. https://doi.org/10.1016/j.ijfoodmicro.2016.03.026 (2016).
Boukhris, I. et al. Towards understanding the antagonistic activity of phytic acid against common foodborne bacterial pathogens using a general linear model. Plos One 15, 1–15. https://doi.org/10.1371/journal.pone.0231397 (2020).
Nassar, R. et al. Antimicrobial activity of phytic acid: An emerging agent in endodontics. Front. Cell. Infect. Microbiol. 11, 1–8. https://doi.org/10.3389/fcimb.2021.753649 (2021).
Asensio, G., Vázquez-Lasa, B. & Rojo, L. Achievements in the topographic design of commercial titanium dental implants: Towards anti-peri-implantitis surfaces. J. Clin. Med. https://doi.org/10.3390/jcm8111982 (2019).
Córdoba, A. et al. Direct covalent grafting of phytate to titanium surfaces through Ti-O-P bonding shows bone stimulating surface properties and decreased bacterial adhesion. ACS Appl. Mater. Interfaces 8, 11326–11335. https://doi.org/10.1021/acsami.6b02533 (2016).
Liu, Y., Wu, J., Zhang, H., Wu, Y. & Tang, C. Covalent immobilization of the phytic acid-magnesium layer on titanium improves the osteogenic and antibacterial properties. Colloids Surf B Biointerfaces 203, 111768. https://doi.org/10.1016/j.colsurfb.2021.111768 (2021).
Torres, J. et al. Solution behaviour of myo -inositol hexakisphosphate in the presence of multivalent cations. Prediction of a neutral pentamagnesium species under cytosolic/nuclear conditions. J. Inorg. Biochem. 99, 828–840. https://doi.org/10.1016/j.jinorgbio.2004.12.011 (2005).
Bebot-Brigaud, A., Dange, C., Fauconnier, N. & Gérard, C. 31P NMR, potentiometric and spectrophotometric studies of phytic acid ionization and complexation properties toward Co2+, Ni2+, Cu2+, Zn2+ and Cd2+. J. Inorg. Biochem. 75, 71–78. https://doi.org/10.1016/S0162-0134(99)00041-0 (1999).
Torres, J. et al. Interaction of myo -inositol hexakisphosphate with alkali and alkaline earth metal ions : Spectroscopic, potentiometric and theoretical studies. J. Mol. Struct. 874, 77–88. https://doi.org/10.1016/j.molstruc.2007.03.035 (2008).
Ermanno, V. et al. Complex formation between phytic acid and divalent metal ions: A solution equilibria and solid state investigation. Anal. Bioanal. Chem. 374, 173–178. https://doi.org/10.1007/s00216-002-1469-6 (2002).
Jiménez, M., Abradelo, C., San Román, J. & Rojo, L. Bibliographic review on the state of the art of strontium and zinc based regenerative therapies. recent developments and clinical applications. J. Mater. Chem. B. 7, 1974–1985. https://doi.org/10.1039/c8tb02738b (2019).
Martin-del-campo, M. et al. Strontium folate loaded biohybrid scaffolds seeded with dental pulp stem cells induce in vivo bone regeneration in critical sized defects. Biomater. Sci. https://doi.org/10.1039/c6bm00459h (2016).
Asensio, G. et al. Biomimetic gradient Scaffolds containing hyaluronic acid and Sr / Zn folates for osteochondral tissue engineering. Polymers 14(1), 12 (2022).
Prasad, A. S. Zinc is an antioxidant and anti-inflammatory agent: Its role in human health. Front. Nutr. 1, 1–10. https://doi.org/10.3389/fnut.2014.00014 (2014).
Sirelkhatim, A. et al. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 7, 219–242. https://doi.org/10.1007/s40820-015-0040-x (2015).
Malachová, K., Praus, P., Rybková, Z. & Kozák, O. Antibacterial and antifungal activities of silver, copper and zinc montmorillonites. Appl. Clay Sci. 53, 642–645. https://doi.org/10.1016/j.clay.2011.05.016 (2011).
Petrini, P. et al. Antibacterial activity of zinc modified titanium oxide surface. Int. J. Artif. Organs 29, 434–442. https://doi.org/10.1177/039139880602900414 (2006).
Thian, E. S. et al. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 24, 437–445. https://doi.org/10.1007/s10856-012-4817-x (2013).
Fernández-Villa, D. et al. Vitamin B9 derivatives as carriers of bioactive cations for musculoskeletal regeneration applications: Synthesis, characterization and biological evaluation. Eur. J. Med. Chem. 212, 113–152 (2021).
Champagne, E. T. & Fisher, M. S. Binding differences of Zn (II) and Cu (II) ions with phytate. J. Inorg. Biochem. 38, 217–223. https://doi.org/10.1016/0162-0134(90)84014-G (1990).
Comel, J., Meux, E., Leclerc, N. & Diliberto, S. Use of phytic acid for selective precipitation of undesirable metals (Al, Fe, Pb) contained in the leachates from hydrometallurgical processes. J. Environ. Chem. Eng. https://doi.org/10.1016/j.jece.2021.105450 (2021).
Sakai, H., Ikemoto, Y., Kinoshita, T., Moriwaki, T. & Yoshida, K. T. Fourier-transform spectra of metal salts of phytic acid in the mid- to far-infrared spectral range. Vib. Spectrosc. 92, 215–219. https://doi.org/10.1016/j.vibspec.2017.07.003 (2017).
Veiga, N. et al. Potentiometric and spectroscopic study of the interaction of 3 d transition metal ions with inositol hexakisphosphate Nicol a. J. Mol. Struct. 1098, 55–65. https://doi.org/10.1016/j.molstruc.2015.05.034 (2015).
Veiga, N. et al. Coordination, microprotonation equilibria and conformational changes of myo-inositol hexakisphosphate with pertinence to its biological function. R. Soc. Chem. 43, 16238–16251. https://doi.org/10.1039/c4dt01350f (2014).
Kremer, C., Torres, J., Bianchi, A., Savastano, M. & Bazzicalupi, C. myo -inositol hexakisphosphate : Coordinative versatility of a natural product. Coord. Chem. Rev. 419, 213403. https://doi.org/10.1016/j.ccr.2020.213403 (2020).
He, Z., Zhong, J. & Cheng, H. N. Conformational change of metal phytates : Solid state 1D 13 C and 2D 1 H- 13 C NMR spectroscopic investigations. J. food Agric. Environ. 11, 965–970 (2013).
He, Z., Honeycutt, C. W., Zhang, T. & Bertsch, P. M. Preparation and FT-IR characterization of metal phytate compounds. J. Environ. Qual. 35, 1319–1328. https://doi.org/10.2134/jeq2006.0008 (2006).
Crea, F., Crea, P., De Robertis, A. & Sammartano, S. Speciation of phytate ion in aqueous solution. characterisation of Ca-phytate sparingly soluble species. Chem. Speciat. Bioavailab. 16, 53–59. https://doi.org/10.3184/095422904782775090 (2004).
Daneluti, A. L. M., Matos, J. & do R.,. Study of thermal behavior of phytic acid. Braz. J. Pharm. Sci. 49, 275–283. https://doi.org/10.1590/S1984-82502013000200009 (2013).
Isbrandt, L. R. & Oertel, R. P. Conformational states of myo-inositol hexakis(phosphate) in aqueous solution. A 13C NMR, 31P NMR, and Raman spectroscopic investigation. J. Am. Chem. Soc. 102, 3144–3148. https://doi.org/10.1021/ja00529a043 (1980).
Dymińska, A. Z. L. et al. Spectroscopic properties and molecular structure of copper phytate complexes : IR, Raman, UV – Vis, EPR studies and DFT calculations. JBIC J. Biol. Inorg. Chem. 24, 11–20. https://doi.org/10.1007/s00775-018-1622-0 (2019).
Guan, X., Shang, C., Zhu, J. & Chen, G. ATR-FTIR investigation on the complexation of myo-inositol hexaphosphate with aluminum hydroxide. J. Colloid Interface Sci. 293, 296–302. https://doi.org/10.1016/j.jcis.2005.06.070 (2006).
He, Z. et al. Solid-state fourier transform infrared and 31P nuclear magnetic resonance spectral features of phosphate compounds. Soil Sci. 172, 501–515. https://doi.org/10.1097/SS.0b013e318053dba0 (2007).
Hamada, S. & Slade, H. D. Biology, immunology, and cariogenicity of streptococcus mutans. Microbiol. Rev. 44, 331–384. https://doi.org/10.1128/mr.44.2.331-384.1980 (1980).
Valm, A. M. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J. Mol. Biol. 431, 2957–2969. https://doi.org/10.1016/j.jmb.2019.05.016 (2019).
Tanner, A. C. R., Kressirer, C. A., Rothmiller, S., Johansson, I. & Chalmers, N. I. The caries microbiome: Implications for reversing dysbiosis. Adv. Dent. Res. 29, 78–85. https://doi.org/10.1177/0022034517736496 (2018).
Lin, Y., Chen, J., Zhou, X. & Li, Y. Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides. Crit. Rev. Microbiol. 47, 667–677. https://doi.org/10.1080/1040841X.2021.1915959 (2021).
Guo, L. et al. Investigating acid production by Streptococcus mutans with a surface-displayed pH-sensitive green fluorescent protein. Plos One 8, 1–10. https://doi.org/10.1371/journal.pone.0057182 (2013).
Klein, M. I., Hwang, G., Santos, P. H. S., Campanella, O. H. & Koo, H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front. Cell. Infect. Microbiol. 5, 1–8. https://doi.org/10.3389/fcimb.2015.00010 (2015).
Ben-Zaken, H. et al. Isolation and characterization of Streptococcus mutans phage as a possible treatment agent for caries. Viruses https://doi.org/10.3390/v13050825 (2021).
Mosaddad, S. A. et al. Oral microbial biofilms: An update. Eur. J. Clin. Microbiol. Infect. Dis. 38, 2005–2019. https://doi.org/10.1007/s10096-019-03641-9 (2019).
Xu, Z. et al. Crystal violet and XTT assays on staphylococcus aureus biofilm quantification. Curr. Microbiol. 73, 474–482. https://doi.org/10.1007/s00284-016-1081-1 (2016).
Pitts, B., Hamilton, M. A., Zelver, N. & Stewart, P. S. A microtiter-plate screening method for biofilm disinfection and removal. J. Microbiol. Methods 54, 269–276. https://doi.org/10.1016/S0167-7012(03)00034-4 (2003).
Mackeen, P. C., Person, S., Warner, S. C., Snipes, W. & Stevens, S. E. Silver-coated nylon fiber as an antibacterial agent. Antimicrob. Agents Chemother. 31, 93–99 (1987).
Li, B., Qiu, Y., Shi, H. & Yin, H. The importance of lag time extension in determining bacterial resistance to antibiotics. Analyst 141, 3059–3067. https://doi.org/10.1039/c5an02649k (2016).
Navarro-Pérez, M. L., Fernández-Calderón, M. C. & Vadillo-Rodríguez, V. Decomposition of growth curves into growth rate and acceleration: A novel procedure to monitor bacterial growth and the time-dependent effect of antimicrobials. Appl. Ind. Microbiol. https://doi.org/10.1128/aem.01849-21 (2022).
Utsumi, R. Bacterial Signal Transduction: Networks and Drug Targets; 2008; Vol. 361; ISBN 9780387788845.
Matsumoto-Nakano, M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 54, 22–29. https://doi.org/10.1016/j.jdsr.2017.08.002 (2018).
Suntharalingam, P. & Cvitkovitch, D. G. Quorum sensing in streptococcal biofilm formation. Trends Microbiol. 13, 3–6. https://doi.org/10.1016/j.tim.2004.11.009 (2005).
Acknowledgements
This work has been funded by CAM (projects IND2018/BMD-9485 and P2018/NMT4389). The authors are grateful to Jose David Gómez from the Characterization Service of ICTP, CSIC; to Esperanza Benito from the Characterization Service of ICTP, CSIC; and to Tamara María Díez Rodríguez for the assistance in the work. The authors thank to Luis Bartolomé for technical and human support provided by SGIker (UPV/EHU/ ERDF, EU) in the microanalysis. M.A.P., L.R., B.V.L. and A.M.H.A. are members of the Technological Interdisciplinary Platform SUSPLAST+ from the Spanish National Research Council (CSIC). This research work was performed in the framework of the Nanomedicine CSIC HUB (ref 202180E048).
Author information
Authors and Affiliations
Contributions
G.A. and M.M. performed all the physic-chemical experiments and contributed to the analysis and discussion of the results. G.A. and A.M.H.A. carried out the antimicrobial assays and contributed to the discussion of the results obtained. G.A. wrote the main draft manuscript text. L.R., B.V.L., M.A.P. and A.M.H.A. reviewed the manuscript. L.R. and B.V.L. managed the design of the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asensio, G., Hernández-Arriaga, A.M., Martín-del-Campo, M. et al. A study on Sr/Zn phytate complexes: structural properties and antimicrobial synergistic effects against Streptococcus mutans. Sci Rep 12, 20177 (2022). https://doi.org/10.1038/s41598-022-24300-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24300-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.