Abstract
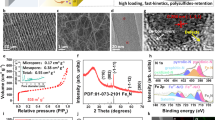
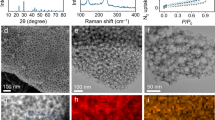
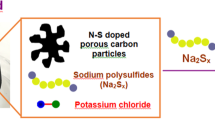
The activity of electrocatalysts for the sulfur reduction reaction (SRR) can be represented using volcano plots, which describe specific thermodynamic trends. However, a kinetic trend that describes the SRR at high current rates is not yet available, limiting our understanding of kinetics variations and hindering the development of high-power Li||S batteries. Here, using Le Chatelier’s principle as a guideline, we establish an SRR kinetic trend that correlates polysulfide concentrations with kinetic currents. Synchrotron X-ray adsorption spectroscopy measurements and molecular orbital computations reveal the role of orbital occupancy in transition metal-based catalysts in determining polysulfide concentrations and thus SRR kinetic predictions. Using the kinetic trend, we design a nanocomposite electrocatalyst that comprises a carbon material and CoZn clusters. When the electrocatalyst is used in a sulfur-based positive electrode (5 mg cm−2 of S loading), the corresponding Li||S coin cell (with an electrolyte:S mass ratio of 4.8) can be cycled for 1,000 cycles at 8 C (that is, 13.4 A gS−1, based on the mass of sulfur) and 25 °C. This cell demonstrates a discharge capacity retention of about 75% (final discharge capacity of 500 mAh gS−1) corresponding to an initial specific power of 26,120 W kgS−1 and specific energy of 1,306 Wh kgS−1.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data supporting findings from this work are available within this Article and the Supplementary Information. All other relevant data supporting findings are available from the corresponding author on request. Source data are provided with this paper.
References
Peng, L. et al. A fundamental look at electrocatalytic sulfur reduction reaction. Nat. Catal. 3, 762–770 (2020).
Zhao, C. et al. A high-energy and long-cycling lithium–sulfur pouch cell via a macroporous catalytic cathode with double-end binding sites. Nat. Nanotechnol. 16, 166–173 (2020).
Zhao, C. X. et al. Semi-immobilized molecular electrocatalysts for high-performance lithium-sulfur batteries. J. Am. Chem. Soc. 143, 19865–19872 (2021).
Zhou, G., Chen, H. & Cui, Y. Formulating energy density for designing practical lithium–sulfur batteries. Nat. Energy 7, 312–319 (2022).
Li, G., Chen, Z. & Lu, J. Lithium–sulfur batteries for commercial applications. Chem 4, 3–7 (2018).
Manthiram, A., Fu, Y., Chung, S. H., Zu, C. & Su, Y. S. Rechargeable lithium–sulfur batteries. Chem. Rev. 114, 11751–11787 (2014).
Fang, R. et al. More reliable lithium–sulfur batteries: status, solutions and prospects. Adv. Mater. 29, 1606823 (2017).
Pang, Q., Liang, X., Kwok, C. Y. & Nazar, L. F. Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 1, 16132 (2016).
Tikekar, M. D., Choudhury, S., Tu, Z. Y. & Archer, L. A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 1, 16114–116120 (2016).
Yang, X., Luo, J. & Sun, X. Towards high-performance solid-state Li–S batteries: from fundamental understanding to engineering design. Chem. Soc. Rev. 49, 2140–2195 (2020).
Yang, Y. et al. Electrocatalysis in lithium sulfur batteries under lean electrolyte conditions. Angew. Chem. Int. Ed. 57, 15549–15552 (2018).
Song, Y. et al. Rationalizing electrocatalysis of Li–S chemistry by mediator design: progress and prospects. Adv. Energy Mater. 10, 1901075 (2019).
Hua, W. et al. Selective catalysis remedies polysulfide shuttling in lithium–sulfur batteries. Adv. Mater. 33, 2101006 (2021).
Dibden, J. W., Smith, J. W., Zhou, N., Garcia-Araez, N. & Owen, J. R. Predicting the composition and formation of solid products in lithium-sulfur batteries by using an experimental phase diagram. Chem. Commun. 52, 12885–12888 (2016).
Shen, Z. et al. Cation-doped ZnS catalysts for polysulfide conversion in lithium–sulfur batteries. Nat. Catal. 5, 555–563 (2022).
Zhong, Y. R. et al. Surface chemistry in cobalt phosphide-stabilized lithium–sulfur batteries. J. Am. Chem. Soc. 140, 1455–1459 (2018).
Xue, W. et al. Intercalation-conversion hybrid cathodes enabling Li–S full-cell architectures with jointly superior gravimetric and volumetric energy densities. Nat. Energy 4, 374–382 (2019).
Du, Z. et al. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium–sulfur batteries. J. Am. Chem. Soc. 141, 3977–3985 (2019).
Zhou, G. et al. Catalytic oxidation of Li2S on the surface of metal sulfides for Li–S batteries. Proc. Natl Acad. Sci. USA 114, 840–845 (2017).
De Heer, J. The principle of Le Châtelier and Braun. J. Chem. Educ. 34, 375 (1957).
Zhang, L. et al. In situ optical spectroscopy characterization for optimal design of lithium–sulfur batteries. Chem. Soc. Rev. 48, 5432–5453 (2019).
Li, H. et al. Revealing principles for design of lean-electrolyte lithium metal anode via in-situ spectroscopy. J. Am. Chem. Soc. 142, 2012–2022 (2020).
Li, H. et al. Reversible electrochemical oxidation of sulfur in ionic liquid for high-voltage Al−S batteries. Nat. Commun. 12, 5714 (2021).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Zou, Q. & Lu, Y. C. Solvent-dictated lithium sulfur redox reactions: an operando UV-vis spectroscopic study. J. Phys. Chem. Lett. 7, 1518–1525 (2016).
Li, H. et al. Unraveling the catalyst-solvent interactions in lean-electrolyte sulfur reduction electrocatalysis for Li–S batteries. Angew. Chem. Int. Ed. 61, e202213863 (2022).
He, Q., Freiberg, A. T. S., Patel, M. U. M., Qian, S. & Gasteiger, H. A. Operando identification of liquid intermediates in lithium–sulfur batteries via transmission UV–vis spectroscopy. J. Electrochem. Soc. 167, 080508 (2020).
Hwang, J. et al. Perovskites in catalysis and electrocatalysis. Science 358, 751–756 (2017).
Singh, J. P., Park, J. Y., Chae, K. H., Ahn, D. & Lee, S. Soft X-ray absorption spectroscopic investigation of Li(Ni0.8Co0.1Mn0.1)O2 cathode materials. Nanomaterials 10, 759 (2020).
Massalski, T. B. & Okamoto, H. Binary Alloy Phase Diagrams 2nd edn (ASM International, 1990).
Kurata, H. & Colliex, C. Electron-energy-loss core-edge structures in manganese oxides. Phys. Rev. B 48, 2102–2108 (1993).
Lin, F. et al. Synchrotron X-ray analytical techniques for studying materials electrochemistry in rechargeable batteries. Chem. Rev. 117, 13123–13186 (2017).
Fan, F. Y., Carter, W. C. & Chiang, Y. M. Mechanism and kinetics of Li2S precipitation in lithium–sulfur batteries. Adv. Mater. 27, 5203–5209 (2015).
Bhargav, A., He, J., Gupta, A. & Manthiram, A. Lithium–sulfur batteries: attaining the critical metrics. Joule 4, 285–291 (2020).
Vivanco, J. P. & Rodriguez-Monroy, C. Graphene applications in the energy field: state-of-the-art and impact. In Proc. 16th LACCEI International Multi-Conference for Engineering, Education and Technology (2018).
Acknowledgements
This research was financially supported by the Australian Research Council (ARC) through the Discovery Project Program (grant numbers FL170100154 and DP220102596). Density functional theory computations were undertaken with the assistance of the National Computational Infrastructure (NCI), supported by the Australian Government. B.J. is supported by a Fellowship at the University of Wollongong. Part of this research was undertaken on the X-ray Absorption Spectroscopy (XAS), Powder Diffraction (PD) and Soft X-ray Spectroscopy (SXR) Beamlines at the Australian Synchrotron, part of ANSTO.
Author information
Authors and Affiliations
Contributions
S.-Z.Q. conceived and supervised this research. H.L. designed and carried out experiments and density functional theory computations. R.M. carried out the synthesis of the cluster catalysts and electrochemical tests. C.Y. assisted with the design of the sulfur positive electrode. A.T. assisted with soft X-ray spectroscopy and related data analyses. W.H. tested the Li−S battery performance. Q.G. assisted with the in situ synchrotron X-ray diffraction measurements. B.J. carried out X-ray adsorption spectroscopy and data analyses. X.C. captured scanning transmission electron microscope images. S.-Z.Q. and K.D. revised the paper. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Bing Joe Hwang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–32, Tables 1 and 2, Notes 1–6 and References.
Source data
Source Data Fig. 1
Source data for Fig. 1 are in sheet 1. Source Data Fig. 2 Source data for Fig. 2 are in sheet 2. Source Data Fig. 3 Source data for Fig. 3 are in sheet 3. Source Data Fig. 4 Source data for Fig. 4 are in sheet 4. Source Data Fig. 5 Source data for Fig. 5 are in sheet 5. Source Data Fig. 6 Source data for Fig. 6 are in sheet 6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Meng, R., Ye, C. et al. Developing high-power Li||S batteries via transition metal/carbon nanocomposite electrocatalyst engineering. Nat. Nanotechnol. (2024). https://doi.org/10.1038/s41565-024-01614-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41565-024-01614-4