Abstract
The correct development and activity of neurons and glial cells is necessary to establish proper brain connectivity. DYRK1A encodes a protein kinase involved in the neuropathology associated with Down syndrome that influences neurogenesis and the morphological differentiation of neurons. DYRK1A loss-of-function mutations in heterozygosity cause a well-recognizable syndrome of intellectual disability and autism spectrum disorder. In this study, we analysed the developmental trajectories of macroglial cells and the properties of the corpus callosum, the major white matter tract of the brain, in Dyrk1a+/− mice, a mouse model that recapitulates the main neurological features of DYRK1A syndrome. We found that Dyrk1a+/− haploinsufficient mutants present an increase in astrogliogenesis in the neocortex and a delay in the production of cortical oligodendrocyte progenitor cells and their progression along the oligodendroglial lineage. There were fewer myelinated axons in the corpus callosum of Dyrk1a+/− mice, axons that are thinner and with abnormal nodes of Ranvier. Moreover, action potential propagation along myelinated and unmyelinated callosal axons was slower in Dyrk1a+/− mutants. All these alterations are likely to affect neuronal circuit development and alter network synchronicity, influencing higher brain functions. These alterations highlight the relevance of glial cell abnormalities in neurodevelopmental disorders.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) encompasses a group of neurodevelopmental disorders (NDDs) with a marked genetic component that are defined by impaired social interaction and communication, and stereotypic dysfunctions. These behavioural alterations often coincide with intellectual disability (ID) or developmental delay and language disorders1. The heterogeneity in the phenotypic presentation of ASD has for long limited our understanding of the molecular pathways, cell types and neuronal circuits affected in this disorder. However, the identification of genes conferring a risk of ASD in large-scale genomic studies2,3,4,5 has made it possible to advance in this direction. As such, there is now compelling evidence that alterations to the prenatal and postnatal brain that affect cortical connectivity are pathological drivers of ASD5,6,7,8.
DYRK1A is one of the high-confidence ASD risk genes identified in NDD/ASD genomic studies3,5,8,9. Mutations in DYRK1A arise de novo, and they include dominant point mutations and small insertions or deletions (indels) that lead to loss-of-function9,10,11,12. Chromosomal rearrangements and microdeletions affecting one DYRK1A allele have also been described in several unrelated patients13,14,15. All these mutations cause characteristic features that define a well-recognizable syndrome of ID/ASD known as MRD7 (Mental Retardation Dominant 7, OMIM: 614104) or DYRK1A-intellectual disability syndrome (DYRK1A syndrome for short, ORPHA: 464306). Core symptoms of DYRK1A syndrome include general developmental delay, microcephaly, moderate to severe ID, speech delay or an absence of communicative language, motor difficulties and a distinctive facial gestalt10,16,17. Core symptoms in affected patients are often accompanied by ASD or autistic features and epilepsy10,17,18. Although the impact of DYRK1A mutations on brain morphology has not been studied systematically, the most frequent magnetic resonance image changes reported in affected cases are cortical atrophy, enlarged ventricles, a small brainstem, delayed myelination and hypoplasia of the corpus callosum (CC)19.
DYRK1A (Dual-specificity Tyrosine phosphorylation Regulated Kinase1A) encodes an evolutionary conserved kinase that regulates several cellular functions and signalling pathways through the phosphorylation of cytosolic and nuclear substrates20,21. The functions of this kinase have been studied in the developing and mature central nervous system (CNS) as DYRK1A lies on chromosome 21, the triplication of which is critical for the cognitive deficits and other neurological dysfunctions associated with Down syndrome (DS)22. In the developing CNS, DYRK1A regulates cell cycle exit of neural progenitors, dendrite and axon development, and physiological apoptosis22,23, thereby ensuring proper brain growth and neuronal connectivity. Moreover, DYRK1A haploinsufficiency in mice causes the characteristic neurological features of DYRK1A syndrome, including general developmental delay, microcephaly, learning deficits, autism-relevant behaviours, and febrile and tonic–clonic seizures12,24,25,26. The neocortex of haploinsufficient Dyrk1a+/− mice is normally laminated24 but with a surplus of excitatory neurons and alterations to the proportions of layer-specific neurons, a phenotype that results from early defects in the differentiation of cortical neural stem cells12,27. Abnormalities in the basal dendritic arbors of cortical pyramidal neurons have also been reported in Dyrk1a+/− mice28, suggesting that the defects in connectivity associated with DYRK1A syndrome may arise from alterations in both the timing of cortical neuron production and neuronal morphogenesis.
Astrocytes and oligodendroglial cells are fundamental for the correct assembly, maturation and function of neuronal circuits29. There is considerable evidence implicating glial cell dysfunction in ASD pathogenesis30,31,32. Astrocytes and oligodendrocyte precursor cells (OPCs) are generated from gliogenic neural stem cells through stereotypic developmental programs that rely on the interplay between intrinsic and external cues33,34,35. Glial development appears to be affected in DYRK1A syndrome, given the abnormalities in myelination and the CC in children with the syndrome19. Indeed, there are alterations to the expression of glial genes and genes involved in gliogenesis in the transcriptome of the early postnatal Dyrk1a+/− cerebral cortex12. For example, expression of the astrocyte marker glia fibrillary acidic protein (GFAP) is enhanced in regions of the postnatal and young adult Dyrk1a+/− mouse brain24,36.
Here, the Dyrk1a+/− mouse model was used to study the development of cortical glial cells in DYRK1A syndrome. As a result, alterations to astrogliogenesis and OPC production were detected in the developing brain of Dyrk1a+/− mice, along with a delay in the progression of the oligodendroglial lineage. Thus, these mice eventually have an excess of cortical astrocytes, hypomyelination of the CC and reduced velocities of action potential (AP) propagation along myelinated and unmyelinated callosal axons. These data indicate that glial cells contribute to the deficits in neuronal connectivity associated with DYRK1A syndrome, and that therapies aimed at promoting oligodendroglial differentiation and myelination may be beneficial for children suffering from this syndrome.
Results
Cortical astrogenesis is enhanced in Dyrk1a +/− mice
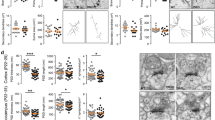
During development, the neural stem cells of the dorsal telencephalon (also referred to as radial glia cells) first generate the excitatory neurons of the neocortex and subsequently, glial cells. This switch to gliogenesis occurs at around embryonic day (E) 18 in the mouse33, with new-born astroglial cells migrating away from the germinal ventricular zone (VZ), thereafter maturing and dividing to populate the entire neocortex37. In astrocytes, the expression of GFAP and other protein markers of late differentiation commences during the first week of postnatal life33. Thus, cortical astrogenesis was evaluated in haploinsufficient Dyrk1a+/− mice by immunostaining the brain for GFAP at postnatal day (P) 5 and P7. GFAP+ cells with the characteristic astrocyte staining accumulated in the white matter and cortical layer (I) close to the meninges (Fig. 1a,b,d) of Dyrk1a+/− and Dyrk1a+/+ mice at both developmental stages. A few GFAP+ astrocytes were detected in the remaining cortical layers (II to VI) in P5 and P7 control brains, with these cells concentrated in layer (VI), next to the germinal region (Fig. 1b,d). A similar distribution of GFAP+ cells to that in the controls was seen in the P5 Dyrk1a+/− neocortex but with significantly more cells (Fig. 1c). Astrocytes were more abundant in mutant neocortices at P7 and they were more widespread across the cortical plate than in control animals (Fig. 1d,e). Hence, the production of cortical astrocytes appears to be enhanced in postnatal Dyrk1a+/− mutants.
More astrocytes in the postnatal cerebral cortex of Dyrk1a+/− mice. (a) Schematic representation of a coronal brain section indicating the corpus callosum in grey and the region of the cerebral cortex (CX) for cell quantification. (b, d) Representative brain images from P5 (b) and P7 (d) Dyrk1a+/+ (+/+) and Dyrk1a+/− (+/−) mice stained for GFAP, indicating the position of the external (V-II) and internal (VI) cortical layers. The boxed areas are magnified to show GFAP+ astrocytes. Dotted lines define the white matter (WM). (c, e) Mean number (± SEM) of GFAP+ astrocytes quantified at P5 (c) and P7 (e) in 1000 μm wide columns expanding layers II to VI. The values correspond to individual animals obtained from 1 litter in c and from 2 litters in e: *P < 0.05 and ****P < 0.0001, Student’s t-test. Scale bars: 250 μm (b); 500 μm (d); 100 μm in magnifications.
NOTCH and JAK-STAT signalling are key pathways for the initiation of gliogenesis in the developing cerebral cortex33. DYRK1A regulates these two pathways in opposite directions, restraining NOTCH signalling by limiting the capacity of its intracellular domain to sustain transcription38, while promoting JAK-STAT signalling and enhancing STAT3 transcriptional activity39. The expression of the progliogenic genes Notch2, Sox4, Sox11 and Nfib was enhanced in the cerebral cortex of new-born Dyrk1a+/− mice12, suggesting possible defects in the differentiation of the mutant progenitors at the onset of gliogenesis. Thus, the gliogenic potential of dorsal Dyrk1a+/− progenitors was evaluated by electroporating the eGFP reporter plasmid into E16.5 Dyrk1a+/+ and Dyrk1a+/− brains, and visualizing the astroglial progeny of the electroporated progenitors in the P5 cerebral cortex immunostained for GFAP (Supplementary Fig. S1a,b). The percentage of cortical GFP+ cells that expressed GFAP (GFAP+;GFP+ cells/total GFP+ cells) was similar in the two genotypes (Supplementary Fig. S1c), indicating that DYRK1A haploinsufficiency does not compromise the capacity of dorsal progenitors to differentiate into astrocytes. These progenitors were then immunolabeled in E17.5 brains using the progenitor marker SOX240, when cortical neurogenesis is almost complete in both the Dyrk1a+/− mutant and control embryos12. However, SOX2+ cells were more abundant in the dorsal germinal region of Dyrk1a+/− than control embryos (Supplementary Fig. S2). Thus, the increase in the number of cortical astrocytes in postnatal Dyrk1a+/− mutants (Fig. 1) does not seem to be a result of an increased capacity of the progenitors to differentiate into astrocytes but rather, to an accumulation of progenitors at the end of the neurogenic period.
Accordingly, we then assessed whether the abnormal number of astrocytes in Dyrk1a+/− pups was maintained in young adult (P60) animals. GFAP expression in healthy brains varies across regions and this expression is undetectable in many astrocytes by immunohistochemistry, or difficult to attribute to individual cells41. Therefore, astrocytes were labelled in adult samples using antibodies against the SOX9 transcription factor expressed by all brain astrocytes outside the neurogenic regions42. In Dyrk1a+/+ and Dyrk1a+/− cerebral cortices SOX9+ cells were distributed similarly through all layers, although they were more abundant in the mutant neocortex (Fig. 2). A similar increase in SOX9+ cells was observed in the Dyrk1a+/− hippocampus (Supplementary Fig. S3a-c), consistent with the reportedly higher density of GFAP+ cells in the hippocampus of Dyrk1a+/− mutants36. Microglia also influence neural circuit development29 and a dysfunction of these cells has been implicated in ASD30. However, when brain microglia were labelled with the cell marker IBA1 (ionized calcium binding adapter molecule 143), no difference in the number of hippocampal or neocortical IBA1+ microglial cells was seen between control and Dyrk1a+/− mutants, or in their IBA1 staining (Supplementary Figs. S3d,e and S4). Together, these results suggest that microglia do not contribute to the brain phenotype in DYRK1A syndrome and that the abnormal number of astrocytes in the Dyrk1a+/− mice was not the result of pathological gliosis but rather, it reflects a developmental problem.
Increased numbers of astrocytes in the neocortex of adult Dyrk1a+/− mice. (a) Schematic representation of a coronal brain section indicating the corpus callosum in grey and the region of the cerebral cortex for cell quantification (rectangle). (b) Brain sections from 2-month-old (2 M) Dyrk1a+/+ (+/+) and Dyrk1a+/− (+/−) mice stained for SOX9 and with the nuclei labelled by DAPI (blue). The position of the neocortical layers I to VI are indicated. (c) The number of SOX9+ astrocytes (mean ± SEM) in 500 μm wide columns. The values correspond to individual animals obtained from 2 litters: *P < 0.05, Student’s t-test. Scale bar: 100 μm.
The cerebral cortex of Dyrk1a +/− mice is deficient in ventral oligodendrocyte progenitor cells
The OPCs that populate the developing cerebral cortex are produced in temporal waves and in different domains of the VZ. In mice, the first OPCs are generated in the ventral telencephalon and they enter the cortex after E16. At birth, another wave of OPC production commences in the dorsal telencephalon, and these dorsal OPCs spread throughout the cortex and reach similar proportions to ventral OPCs by around P1035,44. Based on the defects in myelination in children with DYRK1A syndrome and the hypoplasia of the CC (the major white matter tract of the brain)16,19, and given the expression of DYRK1A by OPCs in the human (CoDEx database,45) and mouse (Supplementary Fig. S5,46) brain, oligodendroglial development could also be affected in this syndrome. To assess this possibility, we first counted the cells expressing the oligodendroglial marker OLIG2 in the CC midline of control and Dyrk1a+/− mutants at different developmental times (Fig. 3a,b). There was a significantly lower percentage of OLIG2+ cells (OLIG2+ nuclei/total nuclei) in Dyrk1a+/− brain at E17.5 and P0. Moreover, a smaller but significant reduction of OLIG2+ cells was also observed at P7 when dorsal OPCs start populating the CC, although OPC numbers reached normal levels three days later (Fig. 3c). The delayed occupation of the mutant CC by OLIG2+ cells suggests that ventral OPC production may be impaired. Hence, we examined the onset of oligodendrogenesis (E15.5) in the lateral ganglionic eminence (LGE), the main contributor of ventral originated callosal OPCs44. New-born OPCs continue to express OLIG2 as they move from the VZ to the mantle zone and they then begin to express platelet-derived growth factor receptorα (PDGFRα47: Fig. 3a). Consistent with a deficit in OPC production, the mantel zone of the Dyrk1a+/− LGE contained fewer OLIG2+ cells and fewer double labelled OLIG2+;PDGFRα+ cells than the control LGE (Fig. 3d,e).
A deficit in ventral oligodendroglia cells during the development of Dyrk1a+/− mutants. (a) Scheme of progression along the oligodendroglial lineage from a radial glia progenitor (RGC) to a pre-myelinated oligodendrocyte (OL): OPC, oligodendroglial precursor cell. (b) Representative images of the central region of the corpus callosum (CC: defined by dashed lines) of Dyrk1a+/+ (+/+) and Dyrk1a+/− (+/−) E17.5 brains stained for OLIG2 and with the nuclei labelled by DAPI (blue): V, ventricle. (c) Percentage (mean ± SEM) of OLIG2+ cells (OLIG2+ cells/total cells) in this region at the developmental stages indicated. The ventral and dorsal origins of the OLIG2+ cells at these times is indicated. n = 3–9 embryos/animals each condition (1–3 litters each developmental stage). (d) Image of the lateral ganglionic eminence (LGE) of a control (+/+) E15.5 brain stained for OLIG2 and PDGFRα, and with the nuclei labelled by DAPI (blue), and magnifications of the region in the mantle zone (MZ, indicated by a dashed box) in a +/+ and +/− brain. Arrowheads indicate OLIG2+;PDGFRα+ oligodendroglial cells and the asterisks PDGFRα+ blood vessel cells: VZ-SVZ, ventricular and subventricular zones. (e) Mean density (± SEM) of OLIG2+ cells and OLIG2+;PDGFRα+ (PDGFRα+) cells in the MZ region indicated in d (dashed box) of E15.5 +/+ and +/− embryos. (f) Images of the dorsal telencephalon of E17.5 +/+ and +/− brains stained for OLIG2 and PDGFRα, and with the nuclei labelled by DAPI (blue) and magnifications of the OLIG2+;PDGFRα+ cells (arrowheads). (g, h) The mean number (± SEM) of OLIG2+ cells and PDGFRα+ cells quantified in the VZ-SVZ or the intermediate zone (IZ) of 200 μm wide columns at E17.5 (g) and E18.5 (h). Values in e, g and h correspond to individual embryos obtained from 1 to 3 litters: ns, not significant; *P < 0.05; **P < 0.01, Student’s t-test. Scale bar: 100 μm (b, d) and 50 μm (f).
Dorsal OPC production was then evaluated in a similar manner but at E17.5, the time at which the first OLIG2+ cells appear in the dorsal germinal region. The Dyrk1a+/− dorsal telencephalon had fewer OLIG2+ cells and fewer OLIG2+PDGFRα+ OPCs in the germinal region than the controls, as well as fewer OLIG2+ cells in the intermediate zone migrating towards the cortical plate (Fig. 3f,g). However, OPC numbers in these cortical regions reached normal levels 24 h later (Fig. 3h), suggesting that OPC production in the dorsal Dyrk1a+/− embryonic brain is not impaired but slightly delayed.
Altered oligodendroglial differentiation in Dyrk1a +/− mutants
Progression along the oligodendroglia lineage was examined in the Dyrk1a+/− mutant cerebral cortex by analysing the expression of differentiation markers (Fig. 4a). Dyrk1a+/− and control P7 brain sections were initially labelled for OLIG2 and the oligodendrocyte marker CC1 (Fig. 4a), the latter expressed in the lateral part of the CC in control brains and diminishing progressively towards the medial region to become almost undetectable at the midline (Fig. 4b). The same lateral to medial expression gradient was observed in the CC of Dyrk1a+/− mutants but with a smaller proportion of CC1+ cells (CC1 + cells/total cells), and with a smaller population of oligodendroglial cells that expresses CC1+ (CC1+;OLIG2+ cells/total OLIG2+ cells) in the mutants than in the controls (Fig. 4c). Moreover, the area of the CC labelled for MBP, a marker of mature myelinating oligodendrocytes (Fig. 4a), was also more restricted in Dyrk1a+/− mice at P7 (Supplementary Fig. S6). When the same studies were performed later in development, from P10 to P60, there were no differences between the genotypes in the relative CC1+ oligodendrocyte numbers in the centre of the CC (Fig. 4d,e). Hence, oligodendroglial development appears to be delayed in the Dyrk1a+/− cerebral cortex.
Oligodendroglial differentiation is delayed in the cerebral cortex of postnatal Dyrk1a+/− mice. (a) Schematic representation of oligodendroglial lineage progression from a radial glial cell (RGC) to a mature myelinating oligodendrocyte (OL), and a coronal brain section indicating the ventral to dorsal gradient of OL maturation in the corpus callosum (CC): OPC, oligodendrocyte precursor cell; V, ventricle. (b) Representative images of P7 Dyrk1a+/+ (+/+) and Dyrk1a+/− (+ /−) coronal brain sections stained for CC1 and OLIG2, and with the nuclei labelled by DAPI (blue). The CC is defined by the dashed lines. Cell counting was performed in the CC regions indicated by rectangles and the images on the right correspond to magnifications of these regions. Note that CC1+;OLIG2+ cells were only detected in the ventral area (b’ and b’’). (c) Percentage (mean ± SEM) of CC1+ cells (OLIG2+;CC1+ cells/total cells) and CC1+ oligodendroglial cells (OLIG2+;CC1+ cells/OLIG2+ cells) in these areas. The values represent individual animals obtained from 2 litters: *P < 0.05; ***P < 0.001, Student’s t-test. (d, e) Representative images of the central region of the CC in P10 + / + and + /- brains stained for CC1 and OLIG2, and with the nuclei labelled by DAPI (blue, d). Histogram showing the percentages (mean ± SEM) of CC1+ oligodendroglial cells in this area at P10, P15 and P60 (e): n = 4–8 animals each condition (1–3 litters each developmental stage). The differences between genotypes were not significant, two way ANOVA. Scale bars: 500 μm (b) and 50 μm (d and magnifications in b).
Cortical white matter alterations and thinner callosal axons in the Dyrk1a +/− mutant mice
To assess whether the alterations in OPC production and differentiation influence the onset and progression of myelination in Dyrk1a+/− mutants, the ultrastructure of the neocortical Dyrk1a+/− white matter was analysed at P19, at the onset of myelination (myelination of the CC commences in the second postnatal week and continues at high rates until P4548). Electron microscopy showed that there was a similar percentage of myelinated axons in control and Dyrk1a+/− mutants (Dyrk1a+/+ 6.6 ± 0.8%, Dyrk1a+/− 4.7 ± 0.9%; P = 0.1359, Student’s t-test: see Supplementary Fig. S7a), although in the mutants these axons had a smaller calibre and their myelin sheaths were slightly thicker than those around the control axons (smaller g-ratios: axon diameter/fibre diameter: Supplementary Fig. S7b,c). Myelin maturation requires its compaction, and it starts after a few membrane wraps and depends on MBP synthesis49. Therefore, the small g-ratios of Dyrk1a+/− axons (Supplementary Fig. S7c) could result from a delay in oligodendrocyte differentiation and it correlates with weaker MBP expression. The CC of Dyrk1a+/− mice was examined after the rapid phase of myelination, at 2 months of age. At this age, callosal axons in Dyrk1a+/− mutants were abnormally packed (No. of total axons/1,000 μm2 Dyrk1a+/+ 2,578 ± 211, Dyrk1a+/− 3,433 ± 292; P = 0.038, Student’s t-test) and the proportion of myelinated axons in the mutant CC decreased significantly (myelinated axons/total axons Dyrk1a+/+ 53.21 ± 2.27%, Dyrk1a+/− 34.48 ± 1.24%; P = 0.002, Student’s t-test: Fig. 5a). As in P19 animals, myelinated callosal axons were thinner in 2-month-old Dyrk1a+/− mice than in the controls (Fig. 5b,c), although the g-ratio values were normal (Fig. 5c,d). This result indicates that myelin-membrane growth is not affected in Dyrk1a+/− mutants and that compaction of the growing myelin sheaths would appear to be delayed. As axon diameter is a determinant factor for myelination50,51, hypomyelination of the Dyrk1a+/− mutant’s CC could result from dysfunctional axon development.
The corpus callosum of adult Dyrk1a+/− mice is hypomyelinated. (a) Schematic representation of a sagittal brain section indicating the region of the corpus callosum (CC) analysed and representative images of the CC ultrastructure in 2-month-old (2 M) Dyrk1a+/+ (+/+) and Dyrk1a+/− (+ /−) mice. Scale bar: 1 μm. (b) Median value of the diameter (± interquartile range) of myelinated axons from each genotype. (c) Scattered plot of the g-ratios of individual fibres relative to their respective axon diameter. Green dots correspond to +/+ axons and red crosses to +/- axons. The dashed line indicates the median axon diameter in +/+ mice and the numbers on both sides of this line the percentages of axons below (left) or above (right) this value for each genotype. (d) Median value of the g-ratio (± interquartile range) for each genotype. Values in b-d were obtained from 980 (+/+) and 2024 (+/−) axons: n = 7–8 animals each genotype (3 litters); ns = not significant; ***P < 0.001, Mann–Whitney U test.
To further characterize the white matter in the Dyrk1a+/− cerebral cortex the expression of MBP and PLP, two major myelin-associated proteins in the CNS, was analysed in 2-month-old mice. At this age, similar cortical expression of Mbp and Plp1 (the gene encoding PLP) was evident in Dyrk1a+/+ and Dyrk1a+/− mice, along with similar levels of MBP isoforms. However, the cerebral cortex of Dyrk1a+/− mutants accumulated twice as much PLP than the controls (Supplementary Fig. S8, original blots are presented in Supplementary Fig. S10), suggesting alterations in myelin biogenesis and/or PLP trafficking to the oligodendrocyte plasma membrane.
Dyrk1a +/− mutants have abnormal nodes of Ranvier
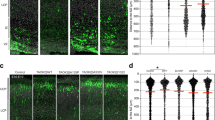
Deficient axon-oligodendroglia communication can affect myelin biogenesis and the formation and maintenance of the nodes of Ranvier, events that are crucial for the rapid propagation of the APs through saltatory conduction50,51. Therefore, the organization of the nodes and the flanking paranodes was examined in the CC of Dyrk1a+/− mice by immunostaining for NAV1.6 (voltage-gated sodium channel Nav1.6), the major sodium channel in the node region52, and for the CASPR (contactin-associated protein) that concentrates in the paranodal region53. No differences in callosal axon node densities were observed between Dyrk1a+/− mutants and control mice (Fig. 6a,b). However, axonal regions immunolabeled for NAV1.6 were shorter in Dyrk1a+/− mice (Fig. 6c,d) and their labelling was significantly weaker (Fig. 6e,f), indicating that mutant nodes may contain fewer NAV1.6 channels that could affect the speed of AP propagation.
Alterations to the nodes of Ranvier in adult Dyrk1a+/− mice. (a) Representative images from the corpus callosum of 2-month-old Dyrk1a+/+ (+/+) and Dyrk1a+/− (+ /−) mice showing the nodes of Ranvier labelled for NAV1.6 (red) and the paranodes for CASPR (green). (b) Density (mean ± SEM) of the nodes of Ranvier. The values correspond to the node density obtained from 16 (+/+) and 13 (+/−) images: ns, not significant, Student’s t-test. (c) Image showing a node of Ranvier and the intensity of CASPR staining in the paranodes. Green dots in the intensity profile represent the center of mass for each paranode as determined by the 3D object reconstruction. (d–f) Scatter dot plots showing the node length (d), intraparanodal length (e) and NAV1.6 labelling index (L.I.) (f) for individual nodes of Ranvier (n = 2–3 animals each genotype, 1 litter): 95 (+/+) and 100 (+/−) nodes in d; 356 (+/+) and 217 (+/−) in (e); 88 (+/+) and 90 (+/−) in (f); *P < 0.05 and **P < 0.01, Mann–Whitney U test; **P < 0.001, Student’s t-test.
Slower conduction velocities in the Dyrk1a +/− corpus callosum
The abnormalities described so far in the CC of Dyrk1a+/− mice indicate possible dysfunctions in AP propagation. To assess this possibility the conduction velocities of compound action potentials (CAPs) evoked by stimulation of callosal axons were measured in young (2-month-old) Dyrk1a+/− and control mice (Fig. 7a). Conduction velocities arising from myelinated and unmyelinated axons were significantly slower in Dyrk1a+/− mutants, although greater differences between the genotypes were evident in continuous rather than in saltatory conduction (slower velocities with respect to controls–myelinated axons 7.76 ± 3.29%, unmyelinated axons 15.63 ± 3.23%: see Fig. 7b,c). Hence, both myelin defects and axon dysfunction may contribute to the abnormal conduction velocities in Dyrk1a+/− mutants.
Slow velocities of action potential propagation in the corpus callosum of Dyrk1a+/− mice in vivo. (a) Scheme of the electrophysiological recordings performed in 2- (2 M) and 12-month-old (12 M) Dyrk1a+/− (+/−) and control (+/+) mice, and representative recording of compound action potentials (CAPs) arising from myelinated (N1) and unmyelinated (N2) axons. (b, c) Conduction velocities (mean ± SEM) for myelinated (b) and unmyelinated (c) axons in animals of the indicated age and genotype. n = 8–12 animals per genotype at 2 months (4 litters) and n = 7–9 animals per genotype at 12 months (3 litters): ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001 and ****P < 0.00, two-way ANOVA and Fisher’s LSD post-hoc test.
Myelination continues at different rates throughout life to adapt brain circuits to diverse physiological needs35. Therefore, the same in vivo recordings were performed in aged (12-month-old) Dyrk1a+/− and control mice, and like young animals continuous and saltatory conduction velocities were slower in older Dyrk1a+/− mutants (myelinated axons 22.43 ± 4.43% and unmyelinated axons 19.27 ± 4.36%: see Fig. 7b,c). Notably, both continuous and saltatory conduction velocities decreased with age in Dyrk1a+/− CCs, whereas only continuous conduction velocities were affected by age in control animals (Fig. 7b,c). This result indicates that defects in myelin homeostasis are enhanced with age in Dyrk1a+/− mutants. Abnormalities in the CC would be expected to alter network synchronicity, thereby affecting higher brain functions54. Thus, the structural CC defects reported here probably contribute to the cognitive, language deficits and autistic traits in DYRK1A syndrome.
Discussion
In this study we show that DYRK1A haploinsufficiency alters the production of cortical astrocytes and OPCs during development, defects that correlate with a delay in OPC differentiation in the CC and dysfunctions in AP propagation along callosal axons in adulthood.
The assembly of functional brain circuits relies on the generation of adequate numbers of neurons and glial cells at specific developmental times33,34. We previously identified alterations to the cortical neurogenic program of Dyrk1a+/− embryos that produced an increase in neurons in all neocortical layers12,27, and here, we provide evidence that astrocyte generation is enhanced in the developing neocortex of Dyrk1a+/− mice. Gliosis has been observed in magnetic resonance images of DYRK1A patients younger than 6-years-old16, suggesting that DYRK1A haploinsufficiency may also enhance glial cell production in humans. There is an increase of astrocytes in the frontal lobe in post-mortem DS foetal brains, indicating that trisomy 21 affects cortical astrogliogenesis55. Indeed, there is an increase in cortical astrocyte generation in the Ts1Cje mouse model of DS39, in which 67 mouse genes homologous to genes on human chromosome 21 are in trisomy, including Dyrk1a56. Notably, the overexpression of DYRK1A in this model upregulates the transcriptional activity of the pro-astrogliogenic factor STAT during the neurogenic-gliogenic switch, thereby promoting the differentiation of VZ progenitors into astrocytes39. In accordance with the role of DYRK1A in regulating STAT transcriptional activity, STAT3 activity was significantly reduced in the dorsal progenitors of Dyrk1a+/− embryos (Supplementary Fig. S9). However, in vivo cell fate experiments showed that cortical Dyrk1a+/− progenitors produce similar numbers of GFAP+ astrocytes to the wild-type mice. Therefore, the higher astrocyte densities in the Dyrk1a+/− cerebral cortex don’t appear to be the result of enhanced neural progenitor differentiation but rather, to the surplus of progenitors at the onset of gliogenesis due to earlier developmental defects.
Evidence indicates that astroglial-derived signals regulate dendritic growth, synaptogenesis, and synapse pruning and maintenance57,58. Therefore, it is plausible that the excess of brain astrocytes contributes to the altered synaptic connectivity attributed to DYRK1A syndrome. In addition, DYRK1A influences neurogenesis, neuronal morphogenesis and glutamate neurotransmission, as it can interacts with key post-synaptic proteins and it modulates the membrane trafficking of synaptic vesicles at the pre-synaptic terminal22,59,60. At the synapse, astrocytes modulate neurotransmitter release by presynaptic terminals and the fidelity of excitatory neurotransmission by clearing glutamate and K+ ions from the synaptic cleft61,62. DYRK1A is expressed by the astrocytes in both human and mouse developing brains46,63. Thus, in addition to the excess of astrocytes, a dysfunction in these glial cells may also contribute to the neuropathology of DYRK1A syndrome, as shown previously in other ID and ASD disorders like Down’s, Rett’s and Fragile X syndromes31,64.
In the mammalian brain the CC is the principal axonal tract for interhemispheric communication. It facilitates higher-order cortical functions including language, intellectual processing, control of social and emotional stimuli, and the coordination of sensory-motor responses65. Alterations to the size of the CC and intracortical functional connectivity are common features of autism66,67. Abnormalities in the CC have been reported in patients with DYRK1A syndrome19 and other syndromic forms of ID/ASD, usually accompanied by language problems, such as in Angelman syndrome68, and FOXG169 and CHD8-related syndromes70. The origin of white matter connectivity defects in ASD is not yet understood, although transcriptome data from animal models and post-mortem human tissue has recently pointed to dysfunctions in oligodendroglial cells and myelin-associated pathways32. In line with this, the chromatin remodelling factor CDH8 encoded by the gene mutated in CHD8-related syndrome is essential for oligodendrocyte development and repair70. Moreover, an oligodendrocyte targeted mutation in one Chd8 allele causes similar deficits in CC myelination, AP propagation and social behaviour as that seen in the heterozygous Chd8+/− mouse model71. The generation of the OPCs that first populate the developing CC is impaired in Dyrk1a+/− mice, a defect correlated with a delay in the arrival of OPCs to the developing CC and in their differentiation into mature oligodendrocytes. OPC differentiation depends on a cell-intrinsic timer that defines the number of divisions before the cells exit the cell cycle and undergo differentiation72. Recent evidence indicates that this OPC timer is controlled by the interplay of several intrinsic factors with extracellular signalling molecules, and by neuronal activity50,73. Thus, the delay in oligodendroglial development in the Dyrk1a+/− mouse is likely to be the result of the early deficit in OPC production as well as changes to the extrinsic cues provided by neurons and astrocytes.
The high density of axons (axons with smaller diameters) and the low number of myelinated axons are the most salient ultrastructural abnormalities to the CC in young Dyrk1a+/− mice. The insolation provided by myelin is the main determinant of the speed of electrical signal propagation, the second being the diameter of the axon51. After the first synapse is formed, the cross-sectional area of long-range axons increases rapidly (radial axon growth)74. Thus, impaired radial axon growth is likely to be the basis of callosal hypomyelination and slow AP propagation in Dyrk1a haploinsufficient mice. There is significant evidence that the neuronal cytoskeleton is essential for axon development and function75. During the rapid phase of radial growth, neurofilaments (NFs) formed by the assembly of NF proteins (NFL, NFM and NFH) accumulate and they become the most abundant cytoskeletal component of the axon. Notably, during the first postnatal week there is a significantly reduction in the expression of the genes encoding NFL (Nefl) and NFM (Nefm) in the cerebral cortex of Dyrk1a+/− mice12. NFL and NFM selectively regulate the axonal transport of neurofilament proteins and other cytoskeleton elements that promote radial axon growth76. Thus, changes in cytoskeleton composition during axonal development due to the abnormal levels of neurofilament proteins, as well as alterations to the neuronal actin and microtubule cytoskeleton as a result of the changes to the phosphorylation status of the DYRK1A substrates N-WASP, MAPB1 and β-Tubulin22, could underlie the formation of the small calibre callosal axons in the Dyrk1a+/− mutant brains.
The correct function of saltatory conduction requires clustering of Na+ and K+ channels at the node of Ranvier. The recruitment and clustering of these ion channels relies on the precise organization of cytoskeleton scaffolding proteins77,78. Thus, alterations to the axonal cytoskeleton might also cause a shortening and a decrease in the NAV6.1 channel density at the nodes of Ranvier in Dyrk1a+/− mutant brains. Once an axon is myelinated, the patterning of myelin changes in response to neuronal activity, adding new myelin sheaths and remodelling the existing ones35,73. In Dyrk1a+/− neocortices, there is an increase in excitatory neurons and synapses and a decrease in proteins related to the GABAergic system12,79. Thus, disturbances in neuronal activity may also influence myelination in Dyrk1a haploinsufficient mice. Defects in myelination have been observed in other ID syndromes80,81, and recent evidence shows that myelination and myelin remodelling can be modulated by social experience and activity-dependent processes, which critically influences learning, memory and behaviour54,82. Therefore, it is likely that defects in both developmental myelination and adaptive experience-dependent myelination contribute to the neurological dysfunction evident in DYRK1A syndrome.
In addition to structural white matter abnormalities, there is an increase in PLP in the cerebral cortex of Dyrk1a+/− mutants, the accumulation of which could result from defects in the membrane trafficking of PLP or to the neuronal signals that orchestrate this trafficking50. The accumulation of PLP in humans and mice caused by duplication of the PLP1 gene leads to progressive demyelination83. Thus, the elevated PLP in Dyrk1a+/− mice might also affect myelin integrity in the long-term, thereby contributing to the deficit in AP propagation along myelinated axons with age.
Myelination increases rapidly in the human cerebral cortex from birth until around 10 years of age, although it continues at a lower rate until the third decade of life84. Thus, therapies aim at improving myelination and axon conduction velocities during development and in adulthood could help ameliorate or revert the cognitive, social and language deficits in DYRK1A syndrome.
Methods
Mice
Dyrk1a+/− embryos, postnatal and adult mice, and their control Dyrk1a+/+ littermates were generated from crosses between C57BL/6;129S2 Dyrk1a+/− males and wild-type females and genotyped as described elsewhere27. These crosses produced an average of 8 littermates. The day of the vaginal plug was defined as E0.5 and the day of birth was defined as P0. After weaning, mice from the same litter and gender were housed in groups, with food and water supplied ad libitum. Studies in embryos and postnatal animals were performed in both sexes. In adult animals, immunofluorescence was performed on female tissue, electron microscopy on male tissue, and in vivo electrophysiology on both males and female mice.
Tissue preparation and immunostaining
Embryos were recovered at the times indicated and their heads were fixed overnight at 4 °C by immersion in 4% paraformaldehyde (PFA). Postnatal and adult mice were anesthetized deeply in a CO2 chamber and perfused transcardially with PFA at room temperature (RT). The brain of each embryo or animal was removed, post-fixed and embedded in agarose to obtain serial free-floating vibratome Sects. (50 μm: Leica VT1000S). For immunofluorescence, the sections were incubated for 1 h at RT in phosphate buffered saline (PBS, pH 7.4) containing 0.2% Triton X-100 and 10% foetal bovine serum (FBS), and probed for 48 to 72 h at 4 °C with the primary antibodies diluted in antibody buffer (PBS with 0.2% Triton X-100 and 5% FBS). The primary antibodies used were: goat anti-SOX2 (1:250, Cat# sc-17320, RRID AB_2286684: Santa Cruz Biotechnology); mouse anti-CASPR (1:200, Cat# 75–001, RRID AB_2083496: NeuroMab), anti-APC (1:200, clone CC-1, Cat# OP80, RRID AB_2057371: Millipore) and anti-DYRK1A (1:250, Cat# H00001859-M01, RRID AB_534844: Abnova); rabbit anti-GFAP (1:500, Cat# Z0334, RRID AB_10013382: DAKO), anti-IBA1 (1:600, Cat# 019–19,741, RRID AB_839504: Wako), anti-NAV1.6 (1:200, Cat# ASC009, RRID AB_2040202: Alomone Labs), anti-OLIG2 (1:500, Cat# ab9610, RRID AB_570666: Millipore) and anti-SOX9 (1:500, a kind gift from Dr Wegner, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany); rat anti-MBP (1:500, Cat# MAB386, RRID AB_94975: Millipore) and anti-PDGFRα (1:100, Cat# 558,774, RRID AB_397117: BD Biosciences). The sections were then washed and the primary antibodies were detected using Alexa-565, Alexa-488 or Alexa-633 conjugated secondary antibodies (1:1000: Life Technologies). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI: Sigma-Aldrich). The specificity of the immunoreactions was assessed by omitting the primary antibody.
Image acquisition and cell counts
Images from coronal brain sections were acquired on: a Leica AF7000 motorized wide-field microscope using a 5 × or 10 × objective and a digital CCD camera; on a SP5 confocal microscope using an HCX PL APO 40x/1.25–0.75 OIL CS objective; or on a Zeiss LSM-780 confocal microscope using a 25 × multi immersion objective. Fiji software was used to obtain the maximal projections from the z-stack images and for image merging, resizing and cell counting. The counting of OLIG2+ and PDGFRα+ cells in embryos was performed in a 200 μm wide column of the dorso-lateral cortical wall, or in a 150 × 300 μm rectangle of the LGE. GFAP+ and SOX9+ cells were counted in 500 μm or 1000 μm wide columns of the somatosensory barrel cortex of postnatal and adult brains. Unless otherwise stated, OLIG2+ and CC1+ (Adenomatous polyposis-APC) cells were quantified in the centre of the CC. The total cells were quantified based on DAPI staining. Cell counts in the adult CC and somatosensory cortex were estimated using Fiji software from serial sections spanning from Bregma + 1.10 mm to − 1.82 mm85. All counts were carried out on 2 to 8 images obtained from 3 to 17 embryos/animals per experimental condition.
Density and length of the Nodes of Ranvier
Coronal sections at the level of the CC were stained for NAV1.6 and CASPR, and images were acquired with a Zeiss LSM-780 microscope. Only nodes with the NAV1.6 labelled domain flanked by CASPR labelled domains were considered in the analysis. To estimate the node densities, we used a 40 × oil DIC objective, a 3 × optical zoom and 15 μm thick z-stacks, processing the images were with the Fiji software. A 63 × oil DIC objective and 45 μm thick z-stacks, with 0.15 μm z-steps, were used to estimate node and intraparanodal lengths. Deconvolution of the images was performed with the Huygens Professional Deconvolution Software (https://svi.nl/Huygens-Software) and the deconvoluted stacks were then processed with the 3D Object Counter Plug-in of Fiji. The intraparanodal length was calculated as the Euclidean distance between two CASPR labelled paranodes separated by a NAV1.6 labelled node according to the following equation:
where (x, y, z) were the three-dimensional coordinates of the centre of masses of a paranode. We selected labelled nodes with the two labelled paranodes in at least eight z-steps to calculate the node length. The maximum intensity projection of all the slices was generated and the intensity profile plotted using the Plot Profile Command in Fiji. The nodes and node lengths were quantified in images from 4–5 animals, and from 2 to 3 animals per genotype, respectively.
Electron microscopy
Anesthetized P19 and P60 mice were perfused transcardially with 4% PFA and 2% glutaraldehyde in PBS at RT. Their brains were removed and post-fixed in the same fixative at 4 ℃ for 24 h, and then segmented in a medial sagittal plane and trimmed into 2–3 mm thick segments. Sagittal slices were washed in PBS, stained with 1% OsO4, dehydrated in graded concentrations of ethanol and embedded in Epon 812 (Electron Microscopy Science). Serial 0.5 μm semi-thin sections were stained with toluidine blue and examined on a Leica DMB microscope. Thin Sects. (60–80 nm) were obtained with a Reichert Ultracut ultramicrotome equipped with a diamond knife, stained with uranyl acetate and lead citrate, and examined on a JEOL JEM-1001 electron microscope. Digital images were acquired with a MegaViewIII camera, and the image fields were analysed using the Fiji software and with a modified version of software designed to study axonal morphometry86. For the morphometric evaluation of axons, fibres were examined at 11,000 × magnification. Only fibres with microtubules and neurofilaments sectioned perpendicular to their longitudinal axes were selected for analysis. For small axons and thinly myelinated fibres, the boundary between the nerve fibres and the surrounding background was defined by a semi-automatic method. The axonal contour and the external contour of the myelin sheath (fibre contour) were traced manually on enlarged images. A set of custom macros allowed the length of a line, the cross-sectional area, and the lengths of the major and minor axes of the best fitting ellipse to be calculated. Fibre and axonal diameters were deduced from the fibre and axonal perimeters, assuming a cylindrical shape of the axons. These basic data were used to derive the g-ratio and myelin sheath thickness86. Electron microscopy images obtained from a minimum of three animals per experimental conditions were included in the analysis.
Electrophysiology
Electrophysiological recordings were obtained from Dyrk1a+/− and Dyrk1a+/+ mice at two different ages: 2- and 12-months. Surgical anaesthesia was induced by intraperitoneal injection of a cocktail of ketamine (80 mg/Kg) and xylazine (10 mg/Kg). The depth of anaesthesia was evaluated by continuously monitoring the response to paw pinching and through electrocorticogram recordings, and a supplementary dose of ketamine (40 mg/Kg) was administrated if necessary. The animal’s body temperature was maintained using a heating pad (Kent Scientific, Torrington, CT, USA) during the surgical procedure. Mice were placed in a stereotaxic device and two craniotomies were drilled to access the CC, and to allow insertion of the recording and stimulation electrodes on each side of the midline (Bregma positions: 0.98–1.1–1.5 in Dyrk1a+/+ mice and 0.8–1.0–1.4 in Dyrk1a+/− mice). Ten consecutive electrical rectangular pulses (100 µs length and 800 µA intensity) were delivered at 1 s intervals through bipolar stainless steel electrodes, and the neural activity of the callosal CAPs generated in response to the stimulation were recorded through tungsten microelectrodes with a 12 MΩ resistance (AM Systems, Sequim, WA). The evoked callosal CAPs were amplified and filtered at a frequency band-pass of 0.1–1000 Hz (AM-systems Differential AC amplifier 1700) and at an additional 50 Hz specific filter (Hum Bug, Digitimer, Welwyn Garden City, UK), digitized through an analogue-to-digital interface (ADInstruments, PowerLab 4/25 T, Dunedin, New Zealand), and the Scope v3.7.3 (ADInstruments, PowerLab) software was used to acquire and average 10 consecutive CAP traces in response to stimulation. LabChart Reader v.8 software was used to analyse the neuronal activity offline. Latencies to the maximal amplitude of 1st (N1) and 2nd (N2) negative CAP peaks were measured, and conduction velocities were assessed according to the distance between the stimulation and recording electrodes.
Statistical analysis
Statistical analyses were performed with GraphPad Prism v5.0a (GraphPad Software). The normal distribution of the values was assessed by the D’Agostino-Pearson omnibus normality test. Significance was assessed with a 2-tailed unpaired Student’s t-test, a two-way ANOVA and a Fisher’s LSD post-hoc test for normally distributed data, or with a 2-tailed unpaired Mann–Whitney U test for non-normally distributed data. Differences were considered significant at P < 0.05. The data are represented as the mean ± standard error of the mean (SEM), unless indicated in the figure legend.
Ethics approval
Experiments with mice were carried out in accordance with the European Union guidelines (Directive 2010/63/EU), and all the protocols were approved by the Ethics Committees of the CSIC, Parc Científic de Barcelona (PCB) and University Miguel Hernandez, and by the appropriate local government bodies. This study is reported in accordance with ARRIVE guidelines.
Data availability
The datasets obtained and analysed in the current study are available from the corresponding authors on reasonable request.
References
Lord, C. et al. Autism spectrum disorder. Lancet 392(10146), 508–520 (2018).
Iossifov, I. et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515(7526), 216–221 (2014).
Yuen, R. et al. Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nat. Neurosci. 20(4), 602–611 (2017).
Stessman, H. A. et al. Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat. Genet. 49(4), 515–526 (2017).
Satterstrom, F. K. et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 180(3), 568-584 e23 (2020).
Willsey, A. J. et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 155(5), 997–1007 (2013).
Parikshak, N. N. et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 155(5), 1008–1021 (2013).
De Rubeis, S. et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515(7526), 209–215 (2014).
O’Roak, B. J. et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338(6114), 1619–1622 (2012).
van Bon, B. W. et al. Disruptive de novo mutations of DYRK1A lead to a syndromic form of autism and ID. Mol. Psychiatry 21(1), 126–132 (2016).
Evers, J. M. et al. Structural analysis of pathogenic mutations in the DYRK1A gene in patients with developmental disorders. Hum. Mol. Genet. 26(3), 519–526 (2017).
Arranz, J. et al. Impaired development of neocortical circuits contributes to the neurological alterations in DYRK1A haploinsufficiency syndrome. Neurobiol. Dis. 127, 210–222 (2019).
Moller, R. S. et al. Truncation of the Down syndrome candidate gene DYRK1A in two unrelated patients with microcephaly. Am. J. Hum. Genet. 82(5), 1165–1170 (2008).
van Bon, B. W. et al. Intragenic deletion in DYRK1A leads to mental retardation and primary microcephaly. Clin. Genet. 79(3), 296–299 (2011).
Courcet, J. B. et al. The DYRK1A gene is a cause of syndromic intellectual disability with severe microcephaly and epilepsy. J. Med. Genet. 49(12), 731–736 (2012).
Ji, J. et al. DYRK1A haploinsufficiency causes a new recognizable syndrome with microcephaly, intellectual disability, speech impairment, and distinct facies. Eur. J. Hum. Genet. 23(11), 1473–1481 (2015).
Earl, R. K. et al. Clinical phenotype of ASD-associated DYRK1A haploinsufficiency. Mol. Autism 8, 54 (2017).
Fenster, R. et al. Characterization of phenotypic range in DYRK1A haploinsufficiency syndrome using standardized behavioral measures. Am. J. Med. Genet. A 5, 22 (2022).
Hanly, C. et al. Description of neurodevelopmental phenotypes associated with 10 genetic neurodevelopmental disorders: A scoping review. Clin. Genet. 99(3), 335–346 (2021).
Becker, W. & Sippl, W. Activation, regulation, and inhibition of DYRK1A. FEBS J. 278(2), 246–256 (2011).
Aranda, S., Laguna, A. & de la Luna, S. DYRK family of protein kinases: Evolutionary relationships, biochemical properties, and functional roles. FASEB J. 25(2), 449–462 (2011).
Arbones, M. L. et al. DYRK1A and cognition: A lifelong relationship. Pharmacol. Ther. 194, 199–221 (2019).
Tejedor, F. J. & Hammerle, B. MNB/DYRK1A as a multiple regulator of neuronal development. FEBS J. 278(2), 223–235 (2011).
Fotaki, V. et al. Dyrk1A haploinsufficiency affects viability and causes developmental delay and abnormal brain morphology in mice. Mol. Cell Biol. 22(18), 6636–6647 (2002).
Arque, G. et al. Impaired spatial learning strategies and novel object recognition in mice haploinsufficient for the dual specificity tyrosine-regulated kinase-1A (Dyrk1A). PLoS ONE 3(7), e2575 (2008).
Raveau, M. et al. DYRK1A-haploinsufficiency in mice causes autistic-like features and febrile seizures. Neurobiol. Dis. 110, 180–191 (2018).
Najas, S. et al. DYRK1A-mediated cyclin D1 degradation in neural stem cells contributes to the neurogenic cortical defects in down syndrome. EBioMedicine 2(2), 120–134 (2015).
Benavides-Piccione, R. et al. Alterations in the phenotype of neocortical pyramidal cells in the Dyrk1A+/− mouse. Neurobiol. Dis. 20(1), 115–122 (2005).
Allen, N. J. & Lyons, D. A. Glia as architects of central nervous system formation and function. Science 362(6411), 181–185 (2018).
Zeidan-Chulia, F. et al. The glial perspective of autism spectrum disorders. Neurosci. Biobehav. Rev. 38, 160–172 (2014).
Sloan, S. A. & Barres, B. A. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr. Opin. Neurobiol. 27, 75–81 (2014).
Phan, B. N. et al. A myelin-related transcriptomic profile is shared by Pitt-Hopkins syndrome models and human autism spectrum disorder. Nat. Neurosci. 23(3), 375–385 (2020).
Miller, F. D. & Gauthier, A. S. Timing is everything: Making neurons versus glia in the developing cortex. Neuron 54(3), 357–369 (2007).
Kriegstein, A. & Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149–184 (2009).
Bergles, D. E. & Richardson, W. D. Oligodendrocyte Development and Plasticity. Cold Spring Harb Perspect. Biol. 8(2), a020453 (2015).
Guedj, F. et al. DYRK1A: A master regulatory protein controlling brain growth. Neurobiol. Dis. 46(1), 190–203 (2012).
Ge, W. P. et al. Local generation of glia is a major astrocyte source in postnatal cortex. Nature 484(7394), 376–380 (2012).
Fernandez-Martinez, J. et al. Attenuation of Notch signalling by the Down-syndrome-associated kinase DYRK1A. J. Cell Sci. 122(Pt 10), 1574–1583 (2009).
Kurabayashi, N., Nguyen, M. D. & Sanada, K. DYRK1A overexpression enhances STAT activity and astrogliogenesis in a Down syndrome mouse model. EMBO Rep. 16(11), 1548–1562 (2015).
Hutton, S. R. & Pevny, L. H. SOX2 expression levels distinguish between neural progenitor populations of the developing dorsal telencephalon. Dev. Biol. 352(1), 40–47 (2011).
Sofroniew, M. V. & Vinters, H. V. Astrocytes: biology and pathology. Acta Neuropathol 119(1), 7–35 (2010).
Sun, W. et al. SOX9 is an astrocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J. Neurosci. 37(17), 4493–4507 (2017).
Ito, D. et al. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. Mol. Brain Res. 57(1), 1–9 (1998).
Kessaris, N. et al. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 9(2), 173–179 (2006).
Polioudakis, D. et al. A single-cell transcriptomic atlas of human neocortical development during mid-gestation. Neuron 103(5), 785-801 e8 (2019).
Zhang, Y. et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89(1), 37–53 (2016).
Pringle, N. P. & Richardson, W. D. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development 117(2), 525–533 (1993).
Sturrock, R. R. Myelination of the mouse corpus callosum. Neuropathol. Appl. Neurobiol. 6(6), 415–420 (1980).
Snaidero, N. & Simons, M. Myelination at a glance. J. Cell Sci. 127(Pt 14), 2999–3004 (2014).
Simons, M. & Trajkovic, K. Neuron-glia communication in the control of oligodendrocyte function and myelin biogenesis. J. Cell Sci. 119(Pt 21), 4381–4389 (2006).
Stassart, R. M. et al. The axon-myelin unit in development and degenerative disease. Front. Neurosci. 12, 467 (2018).
Caldwell, J. H. et al. Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc. Natl. Acad. Sci. U S A 97(10), 5616–20 (2000).
Einheber, S. et al. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J. Cell Biol. 139(6), 1495–1506 (1997).
Xin, W. & Chan, J. R. Myelin plasticity: Sculpting circuits in learning and memory. Nat. Rev. Neurosci. 21(12), 682–694 (2020).
Zdaniuk, G. et al. Astroglia disturbances during development of the central nervous system in fetuses with Down’s syndrome. Folia Neuropathol. 49(2), 109–114 (2011).
Duchon, A. et al. Identification of the translocation breakpoints in the Ts65Dn and Ts1Cje mouse lines: Relevance for modeling Down syndrome. Mamm. Genome 22(11–12), 674–684 (2011).
Perez-Catalan, N. A., Doe, C. Q. & Ackerman, S. D. The role of astrocyte-mediated plasticity in neural circuit development and function. Neural Dev. 16(1), 1 (2021).
Baldwin, K. T. & Eroglu, C. Molecular mechanisms of astrocyte-induced synaptogenesis. Curr. Opin. Neurobiol. 45, 113–120 (2017).
Brault, V. et al. Dyrk1a gene dosage in glutamatergic neurons has key effects in cognitive deficits observed in mouse models of MRD7 and Down syndrome. PLoS Genet. 17(9), e1009777 (2021).
Grau, C. et al. DYRK1A-mediated phosphorylation of GluN2A at Ser(1048) regulates the surface expression and channel activity of GluN1/GluN2A receptors. Front. Cell Neurosci. 8, 331 (2014).
Araque, A. & Perea, G. Glial modulation of synaptic transmission in culture. Glia 47(3), 241–248 (2004).
Dallerac, G., Zapata, J. & Rouach, N. Versatile control of synaptic circuits by astrocytes: where, when and how?. Nat. Rev. Neurosci. 19(12), 729–743 (2018).
Marti, E. et al. Dyrk1A expression pattern supports specific roles of this kinase in the adult central nervous system. Brain Res. 964(2), 250–263 (2003).
Blanco-Suarez, E., Caldwell, A. L. & Allen, N. J. Role of astrocyte-synapse interactions in CNS disorders. J. Physiol. 595(6), 1903–1916 (2017).
De Leon, R. N. S., Bragg-Gonzalo, L. & Nieto, M. Development and plasticity of the corpus callosum. Development 147(18), 555 (2020).
Just, M. A. et al. Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex 17(4), 951–961 (2007).
Fenlon, L. R. & Richards, L. J. Contralateral targeting of the corpus callosum in normal and pathological brain function. Trends Neurosci. 38(5), 264–272 (2015).
Harting, I. et al. Abnormal myelination in Angelman syndrome. Eur. J. Paediatr. Neurol. 13(3), 271–276 (2009).
Kortum, F. et al. The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis. J. Med. Genet. 48(6), 396–406 (2011).
Zhao, C. et al. Dual requirement of CHD8 for chromatin landscape establishment and histone methyltransferase recruitment to promote CNS myelination and repair. Dev. Cell 45(6), 753-768 e8 (2018).
Kawamura, A. et al. Oligodendrocyte dysfunction due to Chd8 mutation gives rise to behavioral deficits in mice. Hum. Mol. Genet. 29(8), 1274–1291 (2020).
Durand, B. & Raff, M. A cell-intrinsic timer that operates during oligodendrocyte development. BioEssays 22(1), 64–71 (2000).
Tomassy, G. S., Dershowitz, L. B. & Arlotta, P. Diversity matters: A revised guide to myelination. Trends Cell Biol. 26(2), 135–147 (2016).
Cleveland, D. W. Neuronal growth and death: Order and disorder in the axoplasm. Cell 84(5), 663–666 (1996).
Kevenaar, J. T. & Hoogenraad, C. C. The axonal cytoskeleton: From organization to function. Front. Mol. Neurosci. 8, 44 (2015).
Kirkcaldie, M. T. K. & Dwyer, S. T. The third wave: Intermediate filaments in the maturing nervous system. Mol. Cell Neurosci. 84, 68–76 (2017).
Nelson, A. D. & Jenkins, P. M. Axonal membranes and their domains: Assembly and function of the axon initial segment and node of Ranvier. Front. Cell Neurosci. 11, 136 (2017).
Rasband, M. N. & Peles, E. Mechanisms of node of Ranvier assembly. Nat. Rev. Neurosci. 22, 7–20 (2020).
Souchet, B. et al. Excitation/inhibition balance and learning are modified by Dyrk1a gene dosage. Neurobiol. Dis. 69, 65–75 (2014).
Olmos-Serrano, J. L. et al. Down syndrome developmental brain transcriptome reveals defective oligodendrocyte differentiation and myelination. Neuron 89(6), 1208–1222 (2016).
Barak, B. et al. Neuronal deletion of Gtf2i, associated with Williams syndrome, causes behavioral and myelin alterations rescuable by a remyelinating drug. Nat. Neurosci. 22(5), 700–708 (2019).
Kato, D. & Wake, H. Myelin plasticity modulates neural circuitry required for learning and behavior. Neurosci. Res. 167, 11–16 (2021).
Inoue, K. Pelizaeus-merzbacher disease: Molecular and cellular pathologies and associated phenotypes. Adv. Exp. Med. Biol. 1190, 201–216 (2019).
Miller, D. J. et al. Prolonged myelination in human neocortical evolution. Proc. Natl. Acad. Sci. U S A 109(41), 16480–16485 (2012).
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates 2nd edn. (Elsevier, 2008).
Fernandez, E., Cuenca, N. & De Juan, J. A useful programme in BASIC for axonal morphometry with introduction of new cytoskeletal parameters. J. Neurosci. Methods. 39(3), 271–289 (1991).
Acknowledgements
We are grateful to E. Rebollo and the IBMB Molecular Imaging platform, as well as to Eva Prats (CID Animal Facility) for the outstanding support and assistance, and to Mark Sefton for English editorial work. We also thank F. Miller (Hospital for Sick Children, Toronto, Canada) for providing the pCAG-PBase and pPB-CAG-GFP plasmids, K. Nakashima (Kyushu University, Fukuoka, Japan) for providing the pGF1L-pGL3 and pGFL1-S-pGL3 plasmids, and M. Wegner (Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany) for providing the SOX9 antibody.
Funding
This work was supported by Grants from the Spanish Government (MCIN/AEI/10.13039/501100011033, Grants SAF2016-77971-R, PID2019-105902RB-I00 and RED2018-102553-T to M.L.A. and RTI2018-098969-B-100 and PROMETEO/2019/119 to E.F.) and grants from the European Union’s Horizon 2020 programme under Grant agreement No. 899287 to E.F. and from The Jérôme Lejeune Foundation to M.J.B. I.P. received a PhD fellowship from the Spanish Ministry of Economy and Competitivity (MINECO, BES2014-069217) and E.B. received a PhD fellowship from the Spanish Ministry of Education and Science (AP2006-04190). M.J.B. was supported by the CIBERER, an initiative of the ISCIII.
Author information
Authors and Affiliations
Contributions
M.L.A. and M.J.B. conceived the project and interpreted the data. I.P., E.B., C.S.S. and M.J.B. performed the experiments. I.P., C.S.S. and E.F. analysed the data. M.L.A. wrote the manuscript, and M.J.B. and I.P. prepared the illustrations.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pijuan, I., Balducci, E., Soto-Sánchez, C. et al. Impaired macroglial development and axonal conductivity contributes to the neuropathology of DYRK1A-related intellectual disability syndrome. Sci Rep 12, 19912 (2022). https://doi.org/10.1038/s41598-022-24284-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24284-5
This article is cited by
-
The role of TSC1 and TSC2 proteins in neuronal axons
Molecular Psychiatry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.