Abstract
The magnetic nanoparticles coated with carbon quantum dot and copper (I) iodide (Fe3O4@CQD@CuI) were used as eco-friendly heterogeneous Lewis / Brønsted acid sites and Cu (I) nanocatalysts. In the first step, it was applied in the synthesis of kojic acid-based dihydropyrano[3,2-b]pyran derivatives in a three-component reaction and in the second step, as a recyclable catalyst for the synthesis of kojic acid-1,2,3-triazole based dihydropyrano[3,2-b]pyran derivatives in the CuI-catalyzed azide/alkyne cycloaddition (CuAAC) reaction. The catalyst was characterized fully by using the different techniques including fourier transform infrared spectroscopy (FT-IR), elemental mapping analysis, X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), X-ray spectroscopy (EDX), transmission electron microscopy (TEM), thermal gravimetric (TG) and value-stream mapping (VSM) methods. The final synthesized derivatives were identified by 1H- and 13C-NMR spectroscopy.
Similar content being viewed by others
Introduction
Carbon quantum dots (CQDs) are the latest class and one of the usage nanoparticles including carbon and heteroatoms in their structure. The CQDs because of the three-dimensional truncation have more atoms on their surfaces1,2. These materials have a parallel arrangement of carbons with a large number of carboxylic acid groups on their surface that caused to be the good solubility in aqueous media. This type of structure plays a major role for CQDs in various applications such as catalyst3,4, biotechnology5,6, sensors7, and chemiluminescence8, waste water9 and food safety10. CQDs have a wide variety of functional groups on their surface used as catalysts and substrates are used in the preparation of various catalysts3,4,11,12,13,14.
Performing a chemical reaction under ultrasound condition can be explained by a physical phenomenon called cavitation: cavitation is a phenomenon in which a decrease in pressure causes the liquid to evaporate locally and bubbles to form15,16. The bursting of bubbles produces a shock wave with enough energy to break the covalent bond. Sonication can be used to speed dissolution, by breaking intermolecular interactions17. Ultrasonic is used in the synthesis of various biological, pharmaceutical, and chemical compounds in mild or green conditions15,18,19. Ultrasonic provides the possibility of performing various chemical reactions such as coupling20, compaction, nitration21, and click22 in milder conditions, higher efficiency and green and environmentally friendly solvents.
Heterocyclic compounds are a group of organic chemical compounds in which some or all of the atoms of its molecules in the ring consist of an atom of an element other than carbon (C)23. The emergence of heteroatoms in the skeleton of chemical compounds is a reason for the emergence of various biological properties that can change the applications of chemical compounds and be used as drugs, pesticides, and solar cells24,25,26,27,28,29,30,31,32. The heteroatomic polycyclic compounds exhibit broad biological properties compared to simple mono-cyclic compounds33,34,35,36,37. The presence of each ring in the skeleton is a reason for the occurrence of biological and medicinal properties in the structure38,39. In 2001, Club, Finn, and Sharpless introduced click reaction as a group of chemical reactions in the synthesis of heterocycles that have potential advantages over traditional reactions such as ease of execution, easy separation, and inexpensive solvents. The most used “click” reaction that can fulfil these conditions is by far the CuI-catalyzed azide/alkyne cycloaddition (CuAAC)40,41,42,43,44,45,46,47,48.
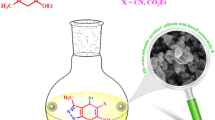
In this paper, we successfully developed a new method for the synthesis of heterocyclic polycyclic compounds using a new heterogeneous nanocatalyst based on CQDs as a nano-catalyst under ultrasonic condition. The novel catalyst Fe3O4@CQD@CuI was used to synthesize kojic acid-based dihydropyrano[3,2-b]pyran derivatives in a multicomponent reaction of kojic acid, malononitrile, and various aldehydes and kojic acid-triazole based dihydropyrano-pyran derivatives via a click reaction, respectively. Subsequently, the newer triazole compounds were synthesized using benzyl halide derivatives and sodium azide (Fig. 1).
Experimental
General
Transmission electron microscopy (TEM) was determined by TEM Philips EM 208S. X-ray photoelectron spectroscopy (XPS) was recorded by BESTEC (EA 10). Vibrating sample magnetometer (VSM) was created by LBKFB model Meghnatis Daghigh Kavir Company. Scanning electron microscope (SEM) was produced by FE-SEM ZEISS Sigma 300. Energy dispersive X-ray (EDX) was performed by Fesem Tescan Mira 2.
Synthesis of Fe3O4 nanoparticles
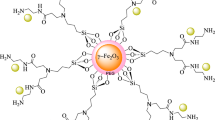
In a 250 mL round-bottomed flask, 10 mmol FeCl3·6H2O and 5 mmol FeCl2·4H2O were well dissolved in 100 mL distilled water and was stirred. Then 10 mL NH4OH drop by drop was added to the mixture until the pH reached to 11. Then, the mixture was stirred under reflux condition for 1 h under N2 atmosphere. Finally, iron oxide nanoparticles were separated with an external magnet and washed several times with distilled water (Fig. 2)49.
Schematic diagram of Fe3O4@CQD@CuI synthesis3.
Synthesis of CQD with glucose
In a 100 mL round-bottomed flask, 5 g of glucose was added to 10 cc of oil, a mixture of oleic acid (65%), linoleic acid (30%) and stearic acid (5%), which had already been heated to 250 °C. Half-burning of glucose in a mixture of the above acids led to the formation of carbon quantum dot. With the browning of glucose, the burning of glucose stopped and after cooling the mixture, by adding 30 mL of water and 30 mL of diethyl ether, carbon dot was separated through the aqueous phase (Fig. 2)13.
Synthesis of Fe3O4@CQD nanocomposite
Iron oxide nanoparticles Fe3O4 (1 g) was dispersed in 50 mL of water for 15 min with ultrasonic, then 0.05 g of carbon dot was added and stirred well for 24 h at room temperature. Finally, it was easily separated by an external magnetic field and washed twice with distilled water (Fig. 2)14.
Synthesis of Fe3O4@CQD@CuI
Loading of copper iodide on the Fe3O4@CQD was done by dispersing 1 g of Fe3O4@CQD in 50 mL of methanol, on the other hand 1 mmol of copper iodide was sonicated in 5 mL of methanol and then two solutions were mixed and stirred for 12 h under reflux condition. Finally, it was separated with a super magnet and washed with methanol (3 × 5) (Fig. 2).
General procedure for the synthesis of kojic acid based dihydropyrano[3,2-b]pyran-derivatives using Fe3O4@CQD@CuI
A mixture of kojic acid (5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one) (1 mmol, 0.142 g), aromatic aldehydes (1 mmol), malononitrile (1.2 mmol, 0.066 g) and nano catalyst Fe3O4@CQD@CuI (5 mg) in a round-bottomed flask were sonicated in an ultrasonic bath in mixture of ethanol and H2O (2:1) as solvent. The progress of the reaction was monitored using TLC (n-hexane:ethyl acetate, 1:3). After the reaction was completed, the insoluble catalyst was easily separated by an external magnet bar. After evaporation of solvent, the precipitate was collected and recrystallized with ethanol (5 mL) to afford the pure product (Fig. 1). The analytic results (melting points, FT-IR, NMR) are shown in the supporting file (Supplementary Information).
General procedure for the synthesis of kojic acid-triazole based dihydropyrano-pyran derivatives using Fe3O4@CQD@CuI
In a 25 mL round-bottomed flask, A mixture of kojic acid based dihydropyrano[3,2-b]pyran-derivatives (4-hydroxy, 3hydroxy and vanillin) (1 mmol), sodium azide (1.2 mmol, 0.078 g), benzyl chloride derivatives (1.2 mmol) and nano catalyst Fe3O4@CQD@CuI (0.01 g) were sonicated in 5 mL water. The progress of the reaction was monitored using TLC (ethyl acetate:MeOH, 8:1). After the reaction was completed, 5 mL ethyl acetate was added and the catalyst was easily separated by an external magnet bar. Then, the product was separated through the organic phase. The residue was purified by plate chromatography (ethyl acetate: methanol, 95:5) to give the desired products (Fig. 1). The analytic results (melting points, FT-IR, NMR) are provided in the supporting file (Supplementary Information).
Result and discussion
The structure of Fe3O4@CQD@CuI as a nano magnetic catalyst coated with carbon quantum dot containing the hydroxyl and carboxyl groups on its surface with copper iodide, was studied and fully characterized by FT-IR, elemental mapping analysis, the scanning electron microscopy (SEM), X-ray spectroscopy (EDX), transmission electron microscopy (TEM), Thermal gravimetric (TG-DTG), X-ray photoelectron spectroscopy (XPS) and value-stream mapping (VSM) methods.
The characterization of Fe3O4@CQD and Fe3O4@CQD@CuI were confirmed and compared by FT-IR spectroscopy in Fig. 3. The broad peak that appeared at 3000–3500 cm−1 was related to OH and CO2H groups of CQD. Also, the absorption bands appeared at 1642 cm−1 and 1441 cm−1 Which are related to stretching modes C=O and C=C bonds, respectively. The peak in the region of 1000 to 1200 cm−1 is related to the C–O stretching modes of CQD. The aromatic ring in the carbon dot skeleton was observed. Furthermore, the peak of Fe–O of Fe3O4 appeared at 642 cm−1.
Using SEM, the morphology of the surface and particle size of Fe3O4@CQD@CuI were investigated. In Fig. 4, SEM images revealed that the shape of particles was spherical and dimensions were in a nanoscale size (approximately 26–55 nm based on images of SEM). TEM images (Fig. 5A,B) showed that the morphology of Fe3O4 nanoparticles was also spherical and the average size was less than 20 nm. TEM images also indicated numerous small particles (CQDs) with sizes about fewer than 10 nm surrounding Fe3O4 nanoparticle, evidencing that CQDs were successfully synthesized on the Fe3O4 nanoparticles11.
EDX and elemental mapping analysis confirmed the presence of iron (Fe), carbon (C), oxygen (O), copper (Cu) and iodine (I) components in the catalyst based on Fig. 6. The results of elemental mapping analysis also revealed that the elements had a uniform distribution in the catalyst structure.
The pattern of thermal gravimetric (TGA-DTG) curve of Fe3O4@CQD@CuI as shown in Fig. 7, revealed three stages of weight loss for Fe3O4@CQD@CuI up to 600 °C. The first weight loss (1–2%) which observed between 25 and 100 °C was related to the removal of moisture from the catalyst structure. The second weight loss (5–6%) appeared at 400 °C, which was attributed to the release of CO2 groups due to C–C bond breaking of the aromatic ring and the carboxylic acid groups. The final stage of weight loss (10–12%) at 600 °C was assigned to the decomposition of the carbon quantum dot coated on the Fe3O4. In addition, The Differential Thermogravimetric (DTG) curve shows endothermic peaks in this region which confirms the successful chemical adsorption of organic complex layers via chemical bonding on the support.
XPS analysis is a powerful surface sensitive technique that has been used to confirm the chemical composition, purity, and oxidation states of element. The C 1s (carbon 1s) peak at 284.60 eV was used as a reference for the calibration of all binding energies. Figure 8a shows the wide scan spectrum XPS (survey spectrum) of the Fe3O4@CQD@CuI nanocatalyst with characteristic peaks of the elements including copper (Cu), oxygen (O), carbon (C), iodine (I), and iron (Fe). Figure 8b–e show the high-resolution spectra of C 1s, O 1s, Cu 2p, and Fe 2p, respectively. In Fig. 8b, two peaks at 284.18 and 288.41 eV can be attributed to the bonds C–C and C=O49. The spectral band of O 1s consists of five peaks including 534.72 eV, 532.84 eV, 530.92 eV, 529.93 eV, and 529.11 eV, which are related to the H–O, C=O, C–O, Cu–O and Fe–O bonds, respectively (Fig. 8c)50. Figure 8d shows the spectrum of the nucleus of a copper atom on the surface of the catalyst. The peak at 932.37 eV and 951.61 eV are related to Cu 2p3/2 and Cu 2p1/2, respectively. Also, the appearance of two satellite peaks at 95.53 eV and 942.43 eV confirms the existence of Cu–O bonds51,52,53. The XPS results of Cu 2p indicate that copper ions exist in two oxidation states. The 931.92 and 951.45 eV bond energy bands are assigned to Cu+1 2p3/2 and Cu+1 2p1/2, respectively, and the peaks of 933.37, 937–946.5 (satellite peaks), and 953.46 eV are corresponded to Cu+2 2p3/2 and Cu+2 2p1/2 (Fig. 8d)54. The two spectral bands at 712.20 eV and 725.47 eV are related to Fe 2p3/2 and Fe 2p1/2 (Fig. 8e). The two weak satellite peaks at 720.04 eV and 734.24 eV indicate the purity and presence of the Fe3O4 phase in the Fe3O4@CQD@CuI catalyst. Also, the presence of Fe+3 and Fe+2 species, which are the characteristics of Fe3O4 nanoparticles, is shown in the Fig. 8e55.
An attempt was made to investigate magnetic measurements of Fe3O4@CQD@CuI at the room temperature using vibrating sample magnetometer (VSM). As shown in Fig. 9, based on magnetization curves, the saturation of the obtained catalyst dropped to 58.11 emu g−1.
Application of Fe3O4@CQD@CuI as magnetic nanoparticle (MNP) catalyst in the synthesis of kojic acid based dihydropyrano[3,2-b]pyran and new kojic acid-triazole hybrid based dihydropyrano[3,2-b]pyran derivatives
After the synthesis and fully characterization of Fe3O4@CQD@CuI, Beginning to investigate its catalytic activity, it was used as a MNP catalyst for the synthesis of kojic acid based dihydropyrano[3,2-b]pyran derivatives in a multi component reaction via a condensation reaction of suitable starting materials. In the following, the new triazole compounds were synthesized via the click reaction using kojic acid based dihydropyrano[3,2-b]pyran derivatives having an acetylene group in the presence of MNP catalysts Fe3O4@CQD@CuI.
In order to optimize the reaction condition, the three-component reaction were performed between kojic acid (1 mmol, 0.142 g), malononitrile (1.1 mmol, 0.072 g) and benzaldehyde (1 mmol, 0.106 g) to synthesis kojic acid based dihydropyrano[3,2-b]pyran derivatives under various conditions including different temperatures, reflux and ultrasonic in water, acetonitrile, ethanol, ethyl acetate and n-hexane (5 mL) as solvents in the presence a catalytic amount of Fe3O4@CQD@CuI. Based on the results shown in Table 1, a mixture of water and ethanol (1:2) as solvent and ultrasonic condition at 50 °C was the best condition of reaction for the synthesis of kojic acid based dihydropyrano[3,2-b]pyrans derivatives (Table 1, entry 9). Any change did not appearance in efficiency by increasing the amount of catalyst and the temperature (Table 1, entry 10 and 11, respectively). At the ultrasonic condition, by decreasing the temperature, a decrease in the reaction efficiency was observed (Table 1, entry 8) whereas by decreasing the amount of catalyst, the reaction efficiency was decreased (Table 1, entry 12). The product was obtained with lower efficiency under longer time when the reaction carried out in non-ultrasonic conditions (Reflux). According to the obtained data, the ultrasonic waves reduce the time and increase the efficiency compared to other conditions in the synthesis of dihydropyrano[3,2-b]pyrans compounds.
After determining the best reaction conditions for synthesis of dihydropyrano[3,2-b]pyrans, a wide range of aromatic aldehydes having the electron-donating and electron-withdrawing groups were synthesized (Fig. 10). As specified in Table 2, the aldehydes with electron-withdrawing groups compared to electron-donating groups resulted in higher efficiencies in this reaction.
This observation can be excused on the basis of the acceptable mechanism suggested for the synthesis of kojic acid based dihydropyrano[3,2-b]pyrans using Fe3O4@CQD@CuI catalyst as shown in Fig. 11. According to the reaction pathway, aldehyde is initially activated by the acidic and hydroxyl sites of the catalyst, then reacts with malononitrile to afford intermediate (I) by removing one water molecule. Then, intermediate (I) as Michael acceptor reacts with 2-hydroxynaphtalen-1,4-dione, 5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one to form intermediate (II). Finally, intermediate (II) to give the desired corresponding dihydropyrano[3,2-b]pyrans will be will be undergone the intramolecular cyclization and tautomerization.
The recovery and reusing capability of the catalyst in a model reaction in the synthesis of kojic acid based dihydropyrano-pyran derivatives was investigated. Kojic acid (1 mmol, 0.142 g), malononitrile (1.1 mmol, 0.072 g) and benzaldehyde (1 mmol, 0.106 g) were used for this purpose. The results showed that the MNP catalyst of Fe3O4@CQD@CuI could be recovered and reused up to 5 times without any noticeable loss of catalytic activity (Fig. 12).
After the high efficiency revelation of this catalyst in the synthesis of kojic acid based dihydropyrano[3,2-b]pyrans different derivatives, a new class of kojic acid-triazole hybrids were investigated using acetylene of dihydropyrano[3,2-b]pyrans derivatives. In order to synthesize triazole derivatives, the reaction between 2-amino-6-(hydroxymethyl)-8-oxo-4-(4-(prop-2-yn-1-yloxy)phenyl)-4,8-dihydropyrano[3,2-b]pyran-3-carbonitrile (1 mmol, 0.350) with benzyl chloride derivatives (1.1 mmol) and sodium azide (1.5 mmol, 0.0975 g) under various conditions including different solvents (water, dimethylformamide (DMF), methanol and tert-Butyl alcohol), temperatures and ultrasonic in the presence of a catalytic amount of Fe3O4@CQD@CuI were tested which its results shown in Table 2. The results displayed that water as solvent and ultrasonic condition at 60 °C was the best reaction condition of choices for the production of kojic acid-triazole based dihydropyrano[3,2-b]pyran in click reaction (Table 2, entry 10). The product was obtained in stirring condition in water with lower efficiency than ultrasonic condition (Table 2, entry 1). Lowering the temperature and the catalyst values led to low efficiency while no increasing efficiency was observed with increasing them (Table 2, entries 11–13).
After determining the optimal condition, it was used to evaluate the efficiency of the catalyst in the synthesis of new triazole compounds using benzyl halide derivatives in reaction with dihydropyrano[3,2-b]pyrans derivatives containing acetylene group. The results revealed that the products had high efficiency and low reaction time (Fig. 13).
To evaluate the performance of Fe3O4@CQD@CuI as catalyst for the synthesis of kojic acid-triazole based dihydropyrano-pyran derivatives, the various homogeneous and heterogeneous catalysts containing copper were used for the click reaction between 2-amino-6-(hydroxymethyl)-8-oxo-4-(4-(prop-2-yn-1-yloxy)phenyl)-4,8-dihydropyrano[3,2-b]pyran-3-carbonitrile (1 mmol, 0.350 g), sodium azide (1.5 mmol, 0.0975 g) and benzyl chloride derivatives (1.1 mmol, 0.138 g) under ultrasonic condition in the water as a solvent at 60 °C temperature (Table 3). The results in Table 3 predicate that Fe3O4@CQD@CuI is the best catalyst for the synthesis of kojic acid-triazole based dihydropyrano-pyran derivatives. Furthermore, the spent catalyst was characterized after the 5th catalytic cycle using SEM and TEM analyses. The morphology and particle size of the Fe3O4@CQD@CuI after the 5th catalytic cycle was not changed based on SEM and TEM images prior and after using in the reaction (Fig. 14).
Conclusion
In this research, we have designed and synthesized the NMP Fe3O4@CQD@CuI having carboxylic acid groups and copper iodide salt as an acid high efficiency catalyst for the synthesis of kojic acid based dihydropyrano-pyran and kojic acid–triazole based triazol-dihydropyrano-pyran compounds that favorably combines the properties Brønsted and Lewis acid and advantages of nanomagnetics catalyst in a three-component and the click reactions. The considerable advantages of this method are easily catalyst removal from the reaction medium using an external magnetic field, its reusing capability and high efficiency in lower time reaction.
References
Lim, S. Y., Shen, W. & Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 44, 362–381 (2015).
Zhao, D. L. & Chung, T.-S. Applications of carbon quantum dots (CQDs) in membrane technologies. Water Res. 147, 43–49 (2018).
Sricharoen, P. et al. Fe3O4/hydroxyapatite/graphene quantum dots as a novel nano-sorbent for preconcentration of copper residue in Thai food ingredients: Optimization of ultrasound-assisted magnetic solid phase extraction. Ultrason. Sonochem. 37, 83–93 (2017).
Mohammadi, M., Rezaei, A., Khazaei, A., Xuwei, S. & Huajun, Z. Targeted development of sustainable green catalysts for oxidation of alcohols via tungstate-decorated multifunctional amphiphilic carbon quantum dots. ACS Appl. Mater. Interfaces 11, 33194–33206 (2019).
Venerando, A. et al. Biotechnological applications of nanostructured hybrids of polyamine carbon quantum dots and iron oxide nanoparticles. Amino Acids 52, 301–311 (2020).
Kuang, Y. et al. A novel nanosystem realizing curcumin delivery based on Fe3O4@ carbon dots nanocomposite for Alzheimer’s disease therapy. Front. Bioeng. biotechnol. 8, 614806 (2020).
Molaei, M. J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 196, 456–478 (2019).
Li, J. et al. Electrogenerated chemiluminescence detection of trace level pentachlorophenol using carbon quantum dots. Analyst 138, 2038–2043 (2013).
Zhang, J. et al. A simple approach for synthesizing of fluorescent carbon quantum dots from tofu wastewater. Nanoscale Res. Lett. 12, 1–7 (2017).
Fan, H., Zhang, M., Bhandari, B. & Yang, C.-H. Food waste as a carbon source in carbon quantum dots technology and their applications in food safety detection. Trends. Food. Sci. Technol. 95, 86–96 (2020).
Juang, R. S. et al. Synthesis of carbon dots on Fe3O4 nanoparticles as recyclable visible-light photocatalysts. IEEE Trans. Magn. 53(11), 1–4 (2017).
Zhang, S. et al. A new SiP QDs/TiO2 NRs composite catalyst with Al2O3 passivation layer for enhanced photoelectrochemical water splitting. Chem. Eng. Sci. 429, 132248 (2022).
Liu, X. et al. Green anhydrous synthesis of hydrophilic carbon dots on large-scale and their application for broad fluorescent pH sensing. Sens. Actuators B Chem. 255, 572–579 (2018).
Prathibha, E., Rangasamy, R., Sridhar, A. & Lakshmi, K. Synthesis and characterization of Fe3O4/carbon dot supported MnO2 nanoparticles for the controlled oxidation of benzyl alcohols. ChemistrySelect 5, 988–993 (2020).
Majhi, S. Applications of ultrasound in total synthesis of bioactive natural products: A promising green tool. Ultrason. Sonochem. 77, 105665 (2021).
Leighton, T. The Acoustic Bubble 234–243 (Academic Press, 1994).
Suslick, K. S., Hammerton, D. A. & Cline, R. E. Sonochemical hot spot. J. Am. Chem. Soc. 108, 5641–5642 (1986).
Azarifar, D., Khatami, S.-M., Zolfigol, M. A. & Nejat-Yami, R. Nano-titania sulfuric acid-promoted synthesis of tetrahydrobenzo [b] pyran and 1, 4-dihydropyrano [2, 3-c] pyrazole derivatives under ultrasound irradiation. J. Iran. Chem. Soc. 11, 1223–1230 (2014).
Mohammadpoor-Baltork, I. et al. Ultrasound-assisted eco-friendly synthesis of triarylmethanes catalyzed by silica sulfuric acid. J. Iran. Chem. Soc. 8, 840–850 (2011).
Rajagopal, R., Jarikote, D. V. & Srinivasan, K. Ultrasound promoted Suzuki cross-coupling reactions in ionic liquid at ambient conditions. Chem. Commun. 6, 616–617 (2002).
Rajagopal, R. & Srinivasan, K. Ultrasound promoted para-selective nitration of phenols in ionic liquid. Ultrason. Sonochem. 10, 41–43 (2003).
Cintas, P., Barge, A., Tagliapietra, S., Boffa, L. & Cravotto, G. Alkyne–azide click reaction catalyzed by metallic copper under ultrasound. Nat. Protoc. 5, 607–616 (2010).
Sharma, P. K., Amin, A. & Kumar, M. A review: Medicinally important nitrogen sulphur containing heterocycles. Open J. Med. Chem. 14(1), 49 (2020).
Mahesh, K., Karpagam, S. & Pandian, K. How to design donor–acceptor based heterocyclic conjugated polymers for applications from organic electronics to sensors. Top. Curr. Chem. 377, 1–39 (2019).
Fascio, M. L., Errea, M. I. & D’accorso, N. B. Imidazothiazole and related heterocyclic systems. Synthesis, chemical and biological properties. Eur. J. Med. Chem. 90, 666–683 (2015).
Najafi, Z. et al. Novel tacrine-1, 2, 3-triazole hybrids: In vitro, in vivo biological evaluation and docking study of cholinesterase inhibitors. Eur. J. Med. Chem. 125, 1200–1212 (2017).
Najafi, Z. et al. Novel tacrine-coumarin hybrids linked to 1, 2, 3-triazole as anti-Alzheimer’s compounds: In vitro and in vivo biological evaluation and docking study. Bioorg. Chem. 83, 303–316 (2019).
Najafi, Z. et al. Design and synthesis of novel anti-Alzheimer’s agents: Acridine-chromenone and quinoline-chromenone hybrids. Bioorg. Chem. 67, 84–94 (2016).
Najafi, Z. et al. 1, 2, 3-Triazole-Isoxazole based acetylcholinesterase inhibitors: Synthesis, biological evaluation and docking study. Lett. Drug. Des. Discov. 14, 58–65 (2017).
Poater, A. et al. Thermodynamics of N-heterocyclic carbene dimerization: The balance of sterics and electronics. Organometallics 27, 2679–2681 (2008).
Esmaili, S., Moosavi-Zare, A. R. & Khazaei, A. Nano-[Fe3O4@SiO2/N-propyl-1-(thiophen-2-yl) ethanimine][ZnCl2] as a nano magnetite Schiff base complex and heterogeneous catalyst for the synthesis of pyrimido [4, 5-b] quinolones. RSC Adv. 12(9), 5386–5394 (2022).
Babaee, S., Zolfigol, M. A., Zarei, M., Abbasi, M. & Najafi, Z. Synthesis of pyridinium-based salts: Catalytic application at the synthesis of six membered O-heterocycles. Mol. Catal. 475, 110403 (2019).
Esmaili, S., Khazaei, A., Ghorbani-Choghamarani, A. & Mohammadi, M. Silica sulfuric acid coated on SnFe2O4 MNPs: Synthesis, characterization and catalytic applications in the synthesis of polyhydroquinolines. RSC Adv. 12(23), 14397–14410 (2022).
Joubert, J. et al. Polycyclic cage structures as lipophilic scaffolds for neuroactive drugs. Chem. Med. Chem. 7(3), 375–384 (2012).
Sun, W. Chemical signatures and new drug targets for gametocytocidal drug development. Sci. Rep. 4, 1–11 (2014).
Gholamhosseini-Nazari, M., Esmati, S., Safa, K. D., Khataee, A. & Teimuri-Mofrad, R. Fe3O4@ SiO2-BenzIm-Fc[Cl]/ZnCl2: A novel and efficient nano-catalyst for the one-pot three-component synthesis of pyran annulated bis-heterocyclic scaffolds under ultrasound irradiation. Res. Chem. Intermed. 45, 1841–1862 (2019).
Saraei, M., Ghasemi, Z., Dehghan, G., Hormati, M. & Ojaghi, K. Synthesis of some novel 1, 2, 3-triazole derivatives containing kojic acid moiety and evaluation for their antioxidant activity. Monatsh. Chem. 148, 917–923 (2017).
Sadafi Kohnehshahri, M. et al. Novel tacrine-based acetylcholinesterase inhibitors as potential agents for the treatment of Alzheimer’s disease: Quinolotacrine hybrids. Mol. Divers. 26, 489–503 (2022).
Arefi, E., Khojastehnezhad, A. & Shiri, A. A magnetic copper organic framework material as an efficient and recyclable catalyst for the synthesis of 1, 2, 3-triazole derivatives. Mol. Divers. 11, 1–14 (2021).
Kolb, H. C., Finn, M. & Sharpless, K. B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Evans, R. A. The rise of azide–alkyne 1, 3-dipolar ‘click’ cycloaddition and its application to polymer science and surface modification. Aust. J. Chem. 60, 384–395 (2007).
Sadeghi, B., Nezhad, P. F. & Hashemian, S. SiO2–OSO3H nanoparticles: An efficient, versatile and new reagent for the one-pot synthesis of 2-amino-8-oxo-4, 8-dihydropyrano [3, 2-b] pyran-3-carbonitrile derivatives in water, a green protocol. J. Chem. Res. 38, 54–57 (2014).
Teimuri-Mofrad, R., Esmati, S., Rabiei, M. & Gholamhosseini-Nazari, M. Ferrocene-containing ionic liquid supported on silica nanospheres (SiO2@Imid-Cl@Fc) as a mild and efficient heterogeneous catalyst for the synthesis of pyrano [3,2-b] pyran derivatives under ultrasound irradiation. J. Chem. Res. 42, 7–12 (2018).
Baghbanian, S. M. Synthesis, characterization, and application of Cu2O and NiO nanoparticles supported on natural nanozeolite clinoptilolite as a heterogeneous catalyst for the synthesis of pyrano [3, 2-b] pyrans and pyrano [3, 2-c] pyridones. RSC Adv. 4, 59397–59404 (2014).
Azarifar, D., Ebrahimiasl, H., Karamian, R. & Ahmadi-Khoei, M. s-Triazinium-based ionic liquid immobilized on silica-coated Fe3O4 magnetic nanoparticles: An efficient and magnetically separable heterogeneous catalyst for synthesis of 2-amino-4, 8-dihydropyrano [3, 2-b] pyran-3-carbonitrile derivatives for antioxidant and antifungal evaluation studies. J. Iran. Chem. Soc. 16, 341–354 (2019).
Ojaghi, A. K., Noroozi Pesyan, N. & Batmani, H. Cu-Kojic acid complex anchored to functionalized silica-MCM-41: A promising regioselective and reusable nanocatalyst for click reaction. ACS Omega 5, 22099–22108 (2020).
Namitharan, K., Kumarraja, M. & Pitchumani, K. CuII–hydrotalcite as an efficient heterogeneous catalyst for Huisgen [3+2] cycloaddition. Chem. Eur. J. 15, 2755–2758 (2009).
Zirak, M. & Jamali Garegeshlagi, E. Picolinimidoamide-Cu (II) complex anchored on Fe3O4@ SiO2 core–shell magnetic nanoparticles: An efficient reusable catalyst for click reaction. J. Coord. Chem. 71, 1168–1179 (2018).
Wei, Y. et al. Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng. 27, 632–637 (2012).
Jiao, C. et al. Synthesis of a poly (amidoxime-hydroxamic acid) cellulose derivative and its application in heavy metal ion removal. RSC Adv. 7, 27787–27795 (2017).
Khan, M. A., Nayan, N., Shadiullah, S., Ahmad, M. K. & Soon, C. F. Surface study of CuO nanopetals by advanced nanocharacterization techniques with enhanced optical and catalytic properties. J. Nanomater. 10, 1298 (2020).
Jin, Z., Liu, C., Qi, K. & Cui, X. Photo-reduced Cu/CuO nanoclusters on TiO2 nanotube arrays as highly efficient and reusable catalyst. Sci. Rep. 7, 39695 (2017).
Swadźba-Kwaśny, M. et al. Facile in situ synthesis of nanofluids based on ionic liquids and copper oxide clusters and nanoparticles. Dalton Trans. 4, 219–227 (2012).
Rafiee, F. & Khavari, P. Preparation of aryl azides of aryl boronic acids and one-pot synthesis of 1,4-diaryl-1,2,3-triazoles by a magnetic cysteine functionalized GO–Cu I/II nanocomposite. Appl. Organomet. Chem. 34, e5789 (2020).
Ai, Q. et al. One-pot co-precipitation synthesis of Fe3O4 nanoparticles embedded in 3D carbonaceous matrix as anode for lithium ion batteries. J. Mater. Sci. 54, 4212–4224 (2019).
Acknowledgements
This work was supported by Vice-chancellor for Research and Technology of Hamadan University of Medical Sciences with project No. 9805153724.
Author information
Authors and Affiliations
Contributions
G.C. contributed to the preparation of the reagents. T.A. contributed to the preparation of some materials. S. B. wrote the manuscript. M.K. analyzed data. B.K. performed the synthesis of compounds. S.E. produced Catalyst. Z.N. designed the experiments and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Najafi, Z., Esmaili, S., Khaleseh, B. et al. Ultrasound-assisted synthesis of kojic acid-1,2,3-triazole based dihydropyrano[3,2-b]pyran derivatives using Fe3O4@CQD@CuI as a novel nanomagnetic catalyst. Sci Rep 12, 19917 (2022). https://doi.org/10.1038/s41598-022-24089-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24089-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.