Abstract
The physical properties of Mg-carbonate at high temperature and pressure are crucial for understanding the deep carbon cycle. Here, we use first-principles calculations to study the physical properties of MgCO3-C2/m and Mg2CO4-P21/c under high pressure. The research shows that the structure and equation of state of MgCO3-C2/m are in good agreement with the experimental results, and the phase transition pressure of Mg2CO4 from pnma to P21/c structure is 44.66 GPa. By comparing the elastic properties, seismic properties and anisotropy of MgCO3-C2/m and Mg2CO4-P21/c, it is found that the elastic modulus and sound velocity of Mg2CO4-P21/c are smaller than those of MgCO3-C2/m, while the anisotropy is larger than that of MgCO3-C2/m. These results indicate that Mg2CO4-P21/c exists in the deep mantle and may be the main reason why carbonate cannot be detected. The minimum thermal conductivity of MgCO3-C2/m and Mg2CO4-P21/c is the largest in the [010] direction and the smallest in the [001] direction. The thermodynamic properties of MgCO3-C2/m and Mg2CO4-P21/c are predicted using the quasi-harmonic approximation (QHA) method.
Similar content being viewed by others
Introduction
Magnesite (space group \(R\overline{3} c\)) is subducted into the deep mantle as the primary carbon carrier, and its high-temperature and high-pressure physical properties are critical for understanding the deep carbon cycle1,2. Previous studies have mainly focused on the structural phase transition of MgCO3-\(R\overline{3} c\) under high-temperature and high-pressure conditions, transforming to monoclinic MgCO3 (space group C2/m) at around 80 GPa. Oganov et al. used the USPEX method to predict for the first time that MgCO3-C2/m is most stable in the lower mantle greater than 82.4 GPa3. MgCO3-C2/m has 30 atoms, in which adjacent oxygen atoms form tetrahedra around carbon atoms, and Mg atoms are in octet and tenfold coordination. Subsequently, the structure of MgCO3-C2/m was verified experimentally4,5,6 and theoretically7,8,9,10,11,12,13. Recently, Gavryushkin et al. used USPEX and AIRSS methods to find that MgCO3 reacts with MgO to form Mg2CO4, which has two structures, orthorhombic (space group Pnma) and monoclinic (space group P21/c), and its structure is P21/c when the pressure is higher than 50 GPa14. Their experiments later confirmed the existence of Mg2CO4-P21/c at the temperature and pressure of the Earth's lower mantle15. Mg2CO4-P21/c has 28 atoms, it is isostructural to β-Ca2SiO416, and the atoms at the two positions Mg(1) and Mg(2) are six-coordinated, with octahedral coordination polyhedra. Earlier reports17,18,19,20, although the structure of Mg2CO4 was not determined, believed that it is stable at high pressure.
To understand the carbon cycle in the deep earth, it is crucial to study the structure, phase transition, equations of state, elasticity, thermodynamics, and thermal conductivity of MgCO3-C2/m and Mg2CO4-P21/c under high pressure. Recently, Maeda et al. measured the equation of state of MgCO3-C2/m at high pressure6. Since it is extremely difficult to measure the elastic constants, thermodynamic parameters, and thermal conductivity of minerals experimentally, the properties of MgCO3-C2/m and Mg2CO4-P21/c have not been reported experimentally. Even the elastic constant of MgCO3-\(R\overline{3} c\) can only be measured to 13.7 GPa21, and its thermodynamic properties are only at low pressure, and the results at high pressure are extrapolated22,23,24.
In the present work, the structures, phase transitions, equations of state, elastic properties, seismic properties, and minimum thermal conductivity of MgCO3-C2/m and Mg2CO4-P21/c at high pressure are investigated using first-principles calculations and compared with the available experimental and theoretical results. The QHA method is adopted to research the thermodynamic properties of MgCO3-C2/m and Mg2CO4-P21/c.
Methods
First-principles calculations are used to investigate the high-pressure physical properties of MgCO3-C2/m and Mg2CO4-P21/c using projector-augmented wave (PAW)25 as implemented in VASP26,27. The electronic configurations: 2p63s2 for Mg, 2s22p2 for C and 2s22p4 for O are considered for the valence electrons. The Perdew–Burke–Ernzerhof modified solid (PBEsol) in the generalized gradient approximation (GGA)28 describes the exchange and correlation potentials. The generation of k-point mesh and the calculation of elastic properties utilize the vaspkit program29. The cutoff energy for the plane wave is set to 900 eV. The k-points mesh of MgCO3-C2/m and Mg2CO4-P21/c are set to 4 × 5 × 5 and 13 × 9 × 7 using the Monkhorst–Pack scheme30, respectively. The thermodynamic properties are calculated by the QHA method31.
Results and discussion
Structural parameters, phase transition and equation of state
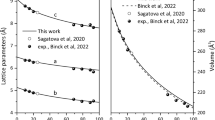
The crystal structures of MgCO3-C2/m and Mg2CO4-P21/c in the unit cell are shown in Fig. 1. The optimized lattice parameters are summarized in Table 1 and compared with available experimental and previously calculated results. At 110 GPa, the calculated results of MgCO3-C2/m are consistent with the experimental results5. The results for Mg2CO4-P21/c at 100 GPa agree well with the previous calculations14.
Crystal structures of MgCO3-C2/m (a) and Mg2CO4-P21/c (b) in unit cell. The crystal structures are drawn by VESTA32.
As shown in Fig. 2, the present calculated transition pressure from Mg2CO4-Pnma to Mg2CO4-P21/c is 44.66 GPa, while Gavryushkin et al. calculated 52 GPa14. This error may be caused by the use of different exchange correction functions, PBEsol is used in the present work, while PBE was used by Gavryushkin et al.14. The accuracy of using PBEsol to calculate the properties of Mg-carbonate has been examined in the previous studies13. In the previous study, MgCO3-C2/m was stable in the lower mantle above 80 GPa3,5,6,7,8,9,10,11,12,13,33. Therefore, in order to facilitate comparison, the high-pressure properties of MgCO3-C2/m and Mg2CO4-P21/c in the pressure range of 50–140 GPa are investigated in this work.
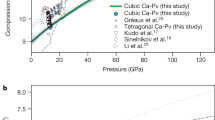
The equations of state for MgCO3-C2/m and Mg2CO4-P21/c at 50 to 140 GPa are shown in Fig. 3. It is found that the equation of state of MgCO3-C2/m is in good agreement with available experimental data6. The equation of state of Mg2CO4-P21/c is smaller than that of MgCO3-C2/m, and is almost parallel. The formula unit volume of MgCO3-C2/m is smaller than that of Mg2CO4-P21/c, which is in line with their molecular formula composition relationship.
Elastic properties
For monoclinic MgCO3-C2/m and Mg2CO4-P21/c, there are 13 independent elastic constants (\(c_{11}\), \(c_{12}\), \(c_{13}\), \(c_{15}\), \(c_{22}\), \(c_{23}\), \(c_{23}\), \(c_{25}\), \(c_{33}\), \(c_{35}\), \(c_{44}\), \(c_{46}\), \(c_{55}\) and \(c_{66}\)). The elastic constants are calculated using the stress–strain method29. In this work, all calculated elastic stiffness constants cij are checked using the mechanical stability criteria34 and find that they all meet the mechanical stability criteria in the studied pressure range, thus they are mechanically stable.
The elastic constants of MgCO3-C2/m and Mg2CO4-P21/c are plotted in Figs. 4 and 5, respectively. It is found from Fig. 4 that at 50–110 GPa, \(c_{22} > c_{33} > c_{11}\), indicating that the a-axis of MgCO3-C2/m is the most easily compressed, and the b-axis is the least compressed. At > 110 GPa, \(c_{33} > c_{22} > c_{11}\), the c-axis is least likely to be compressed. From Fig. 5, it can be seen that \(c_{22} > c_{11} > c_{33}\) in the studied pressure range, indicating that the c-axis of Mg2CO4-P21/c is the most easily compressed, and the b-axis is the least compressible. In the previous study13, the elastic constants of MgCO3-\(R\overline{3} c\) are consistent with the experimental results21. Therefore, the predicted elastic constants of MgCO3-C2/m and Mg2CO4-P21/c in this work should be correct, but it needs to be further verified by experiments.
Based on the calculated elastic constants, the bulk modulus B and shear modulus G of MgCO3-C2/m and Mg2CO4-P21/c are calculated using the Voigt-Reuss-Hill method35,36,37. As shown in Fig. 6, the bulk modulus B and shear modulus G of MgCO3-C2/m and Mg2CO4-P21/c increase with increasing pressure, and the bulk modulus B and shear modulus G of MgCO3-C2/m are larger than those of Mg2CO4-P21/c at 50–140 GPa.
Seismic properties
Based on the calculated bulk and shear moduli and density, the compression (VP) and shear (VS) velocities of MgCO3-C2/m and Mg2CO4-P21/c are given by the following expressions38:
As shown in Fig. 7, the density of Mg2CO4-P21/c is larger than that of MgCO3-C2/m, and the difference between their bulk modulus and shear modulus is smaller, respectively. Therefore, the VP and VS of MgCO3-C2/m are larger than those of Mg2CO4-P21/c in the studied pressure range, and their VP and VS tend to be parallel, respectively (See Fig. 8).
The VP and VS of MgCO3-C2/m and Mg2CO4-P21/c along different directions can be obtained by solving the Christoffel equation39 \(\left| {C_{ijkl} n_{j} n_{l} - \rho V^{2} \delta_{ik} } \right| = 0\). In order to visualize the propagation wave velocities of MgCO3-C2/m and Mg2CO4-P21/c along different directions, the AWESoMe program40,41 is used to plot their 3D representations of VP and VS and shear wave splitting and polarization vectors at various pressures (Figs. 9 and 10).
The anisotropy AP of the VP for MgCO3-C2/m and Mg2CO4-P21/c is defined as42:
The polarization anisotropy AS of the VS is defined as
The seismic anisotropies of MgCO3-C2/m and Mg2CO4-P21/c are shown in Fig. 11. The seismic anisotropy of MgCO3-C2/m at 75 GPa is found to be in good agreement with the previous theoretical calculations10. The anisotropy of Mg2CO4-P21/c is obviously larger than that of MgCO3-C2/m. The seismic anisotropy of MgCO3-C2/m and Mg2CO4-P21/c showed obvious nonlinear dependence on pressure. This is mainly due to the nonlinear pressure of wave velocity caused by the nonlinear pressure dependence of the elastic moduli of MgCO3-C2/m and Mg2CO4-P21/c, especially their shear moduli.
Although the previous experimental4,5,6 and theoretical7,8,9,10,11,12,13 studies obtained MgCO3-C2/m at high temperature and pressure, they did not consider the reaction with MgO, the main mineral of the Earth's lower mantle. The theoretical14 and experimental15 results of Gavryushkin et al. show that MgCO3 reacts with MgO to form Mg2CO4-P21/c orthocarbonate under the temperature and pressure conditions of the lower mantle. By comparing the high-pressure physical properties of MgCO3-C2/m and Mg2CO4-P21/c, it is found that their seismic anisotropy is quite different, while the equation of state, elastic modulus, density and wave velocity have similar relationship with pressure. The low wave velocities of Mg2CO4-P21/c are more suitable to explain the existence of low-velocity zone near the subducting slab. Therefore, we believe that Mg2CO4-P21/c may exist in the deep mantle, providing strong evidence for carbon storage in carbonate minerals, which may be the main reason why carbonate rocks cannot be detected in the lower mantle.
Minimum thermal conductivity
The thermal conductivity of minerals is critical to understanding the Earth's thermal balance and history43. The minimum thermal conductivity of MgCO3-C2/m and Mg2CO4-P21/c are calculated using Cahill’s model:
The anisotropy of the minimum thermal conductivity can be calculated by changing Eq. (5) into the following form:
where kB is Boltzmann’s constant, n is the atomic number density per unit volume. The minimum thermal conductivities of MgCO3-C2/m and Mg2CO4-P21/c are shown in Fig. 12, and it is found that their minimum thermal conductivities increase with the increase of pressure, and that of MgCO3-C2/m is larger than that of Mg2CO4-P21/c. In the studied pressure range, Kmin[010] > Kmin[100] > Kmin[001], indicating that the thermal conductivity in the [010] direction is the largest and the thermal conductivity in the [001] direction is the smallest.
Thermodynamic properties
Thermodynamic parameters are the preconditions for deriving the thermal state of the Earth's interior. Therefore, the thermodynamic properties of MgCO3-C2/m and Mg2CO4-P21/c are crucial for studying the thermal state of the lower mantle. The constant volume heat capacity (CV) and the thermal expansion coefficient (\(\alpha\)) of MgCO3-C2/m and Mg2CO4-P21/c at various pressures are depicted in Figs. 13 and 14, respectively. The CV and \(\alpha\) of MgCO3-C2/m are larger than those of Mg2CO4-P21/c under the same pressure.
Conclusions
On the basis of verifying the structure and equation of state of MgCO3-C2/m, the phase transition pressure of Mg2CO4-P21/c is determined. The high-pressure physical properties of MgCO3-C2/m and Mg2CO4-P21/c at 50–140 GPa are investigated by first-principles calculations. By comparison, it is found that the elastic modulus and wave velocity of Mg2CO4-P21/c are smaller than those of MgCO3-C2/m, and the density and seismic anisotropy are larger than those of MgCO3-C2/m. The low wave velocity of Mg2CO4-P21/c may be more suitable to explain the existence of the low-velocity zone near the subducting slab. Therefore, it is believed that Mg2CO4-P21/c may exist in the deep mantle, providing strong evidence for carbon storage in carbonates and the reason why it cannot be detected in the lower mantle. The minimum thermal conductivity of MgCO3-C2/m is greater than that of Mg2CO4-P21/c, and their minimum thermal conductivity is the largest in the [010] direction and the smallest in the [001] direction. The constant volume heat capacity CV and thermal expansion coefficient \(\alpha\) of MgCO3-C2/m are larger than those of Mg2CO4-P21/c. Unfortunately, there are no experimental data on the elastic constants, thermodynamic parameters, and minimum thermal conductivity of MgCO3-C2/m and Mg2CO4-P21/c, so further verification is required.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Isshiki, M. et al. Stability of magnesite and its high-pressure form in the lowermost mantle. Nature 427, 60–63 (2004).
Plank, T. & Manning, C. E. Subducting carbon. Nature 574, 343–352 (2019).
Oganov, A. R., Ono, S., Ma, Y., Glass, C. W. & Garcia, A. Novel high-pressure structures of MgCO3, CaCO3 and CO2 and their role in Earth’s lower mantle. Earth Planet. Sci. Lett. 273, 38–47 (2008).
Boulard, E. et al. New host for carbon in the deep Earth. Proc. Natl. Acad. Sci. USA. 108, 5184–5187 (2011).
Binck, J. et al. Phase stabilities of MgCO3 and MgCO3-II studied by Raman spectroscopy, X-ray diffraction, and density functional theory calculations. Phys. Rev. Mater. 4, 055001 (2020).
Maeda, F. et al. Diamond formation in the deep lower mantle: A high-pressure reaction of MgCO3 and SiO2. Sci. Rep. 7, 40602 (2017).
Pickard, C. J. & Needs, R. J. Structures and stability of calcium and magnesium carbonates at mantle pressures. Phys. Rev. B 91, 104101 (2015).
Marcondes, M. L., Justo, J. F. & Assali, L. V. C. Carbonates at high pressures: Possible carriers for deep carbon reservoirs in the Earth’s lower mantle. Phys. Rev. B 94, 104112 (2016).
Santos, S. S. M., Marcondes, M. L., Justo, J. F. & Assali, L. V. C. Stability of calcium and magnesium carbonates at Earth’s lower mantle thermodynamic conditions. Earth Planet. Sci. Lett. 506, 1–7 (2019).
Li, Z. & Stackhouse, S. Iron-rich carbonates stabilized by magnetic entropy at lower mantle conditions. Earth Planet. Sci. Lett. 531, 115959 (2020).
Tsuchiya, J., Nishida, R. & Tsuchiya, T. First Principles calculation of the stability of Iron bearing carbonates at high pressure conditions. Minerals 10, 54 (2020).
Sagatova, D. N., Shatskiy, A. F., Gavryushkin, P. N., Sagatov, N. E. & Litasov, K. D. Stability of Ca2CO4-Pnma against the main mantle minerals from ab initio computations. ACS Earth Space Chem. 5, 1709–1715 (2021).
Liu, Z. J. et al. First-principles calculations of high-pressure physical properties anisotropy for magnesite. Sci. Rep. 12, 3691 (2022).
Gavryushkin, P. N., Sagatova, D. N., Sagatov, N. & Litasov, K. D. Formation of Mg-orthocarbonate through the reaction MgCO3+MgO=Mg2CO4 at Earth’s lower mantle P-T Conditions. Cryst. Growth Des. 21, 2986–2992 (2021).
Gavryushkin, P. N. et al. High-pressure synthesis and ambient-pressure TEM investigation of Mg-orthocarbonate. Lithos. https://doi.org/10.2139/ssrn.3966096 (2021).
Yamnova, N. A., Zubkova, N. V., Eremin, N. N., Zadov, A. E. & Gazeev, V. M. Crystal structure of larnite β-Ca2SiO4 and specific features of polymorphic transitions in dicalcium orthosilicate. Crystallogr. Rep. 56, 210–220 (2011).
Fyfe, W. S. Lattice energies, phase transformations and volatiles in the mantle. Phys. Earth Planet. Inter. 3, 196–200 (1970).
Irving, A. J. & Wyllie, P. J. Melting relationships in CaO-CO2 and MgO-CO2 to 36 kilobars with comments on CO2 in the mantle. Earth Planet. Sci. Lett. 20, 220–225 (1973).
Newton, R. C. & Sharp, W. E. Stability of forsterite+CO2 and its bearing on the role of CO2 in the mantle. Earth Planet. Sci. Lett. 26, 239–244 (1975).
Katsura, T. et al. Stability of magnesite under the lower mantle conditions. Proc. Japan Acad. Ser. B. 67, 57–60 (1991).
Yang, J., Mao, Z., Lin, J. F. & Prakapenka, V. B. Single-crystal elasticity of the deep-mantle magnesite at high pressure and temperature. Earth Planet. Sci. Lett. 392, 292–299 (2014).
Litasov, K. D., Fei, Y., Ohtani, E., Kuribayashi, T. & Funakoshi, K. Thermal equation of state of magnesite to 32 GPa and 2073 K. Phys. Earth Planet. Int. 168, 191–203 (2008).
Matas, J., Gillet, P., Ricard, Y. & Martinez, I. Thermodynamic properties of carbonates at high pressures from vibrational modelling. Eur. J. Mineral. 12, 703–720 (2000).
Zhang, J., Martinez, I., Guyot, F., Gillet, P. & Saxena, S. K. X-ray diffraction study of magnesite at high-pressure and high-temperature. Phys. Chem. Miner. 24, 122–130 (1997).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total enery calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P. et al. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 100, 136406 (2008).
Wang, V., Xu, N., Liu, J. C., Tang, G. & Geng, W. T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033 (2021).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Otero-de-la-Roza, A., Abbasi-Pérez, D. & Luaña, V. Gibbs2: A new version of the quasiharmonic model code. II. Models for solid-state thermodynamics, features and implementation. Comput. Phys. Commun. 182, 2232–2248 (2011).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Zhao, C. S., Lv, C. J., Xu, L. X., Liang, L. & Liu, J. Raman signatures of the distortion and stability of MgCO3 to 75 GPa. Am. Mineral. 106, 367–373 (2021).
Wu, Z.-J. et al. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys. Rev. B 76, 054115 (2007).
Hill, R. The elastic behavior of a crystalline aggregate. Proc. Phys. Soc. Lond. 65A, 349–354 (1952).
Voigt, W. Lehrbuch der Kristallphysik-mit Ausschluß der Kristalloptik (Vieweg+Teubner Verlag, 1966).
Reuss, A. Calulation of yielding mixed crystals plasticity condition for single crystals. Z. Angew. Math. Mech. 9, 49–58 (1929).
Anderson, O. L. A simplified method for calculating the debye temperature from elastic constants. J. Phys. Chem. Solids 24, 909–917 (1963).
Anderson, D. L. Theory of the Earth (Blackwell Scientific Publications, 1989).
Muñoz-Santiburcio, D., Hernández-Laguna, A. & Soto, J. I. AWESoMe: A code for the calculation of phase and group velocities of acoustic waves in homogeneous solids. Comput. Phys. Commun. 192, 272–277 (2015).
Muñoz-Santiburcio, D. & Hernández-Laguna, A. AWESoMe 1.1: A code for the calculation of phase and group velocities of acoustic waves in homogeneous solids. Comput. Phys. Commun. 217, 212–214 (2017).
Kiefer, B., Stixrude, L., Hafner, J. & Kresse, G. Structure and elasticity of wadsleyite at high pressures. Am. Mineral. 86, 1387–1395 (2001).
Zhou, Y., Dong, Z. Y., Hsieh, W. P., Goncharov, A. F. & Chen, X. J. Thermal conductivity of materials under pressure. Nat. Rev. Phys. 4, 319–335 (2022).
Acknowledgements
This work is supported by the Industrial Support and Guidance Project of Colleges and Universities of Gansu Province (No. 2022CYZC-37), the Key Natural Science Foundation of Gansu Province (No. 20JR5RA211) and the Talent Innovation and Entrepreneurship Project of Lanzhou City (No. 2020-RC-18).
Author information
Authors and Affiliations
Contributions
Z.-J.L. designed the calculations and wrote the manuscript. X.-W.S. and C.-R.Z. analyzed the results. J.-Q.J. and T.L. performed partial calculations. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, ZJ., Li, T., Sun, XW. et al. Comparative study on high-pressure physical properties of monoclinic MgCO3 and Mg2CO4. Sci Rep 12, 19485 (2022). https://doi.org/10.1038/s41598-022-24033-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24033-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.