Abstract
To reveal the mechanism of desorption of methane in coal seams by inert gas N2, the desorption behavior of CH4 after N2 injection was studied by using Giant Canonical ensemble Monte Carlo (GCMC) and Molecular Dynamics (MD) methods with wiser bituminous coal as the research object. The results show that the adsorption isotherms of CH4 and N2 in the molecular structure model of bituminous coal are in good agreement with the Langmuir adsorption isotherm model. The adsorption capacity of the two gases in the bituminous coal structure model is CH4 > N2. The higher the N2 injection pressure, the higher the temperature, and the more methane desorption. N2 can replace some adsorbed CH4 through competitive adsorption with CH4. Compared with injecting high-temperature nitrogen to desorb methane in coal seams, in high-pressure nitrogen, the diffusion effect of CH4 flowing in coal is more significant. The higher the nitrogen injection pressure, the better the effect of N2 promoting CH4 desorption. The relative concentration of CH4 in the vacuum layer gradually increases with the increase of water content. This indicates that the water in coal promotes the desorption of CH4. The mechanism of N2 injection and CH4 desorption in coal seams mainly includes gas displacement and gas dilution and diffusion. This study provides theoretical support for methane extraction technology in goaf.
Similar content being viewed by others
Introduction
Gas injection mining is an important stimulation technology for CBM development. For coal seams with poor gas permeability, low permeability, and rapid borehole gas flow decay, traditional negative pressure drainage takes a long time and achieves slow results, which can no longer meet the needs of mining replacement and underground safety production1,2,3. Coal seam gas injection not only increases the internal pressure of the coal body and increases the gas seepage velocity, but also reduces the effective partial pressure of gas and promotes the desorption of adsorbed gas4,5. Therefore, it is of great significance to study CBM gas injection stimulation technology and improve the permeability of CBM wells.
Many scholars have carried out relevant studies on nitrogen injection to promote emission/gas extraction. Clarkson et al.6 believed that injecting non-gas gas into coal seam can reduce the partial pressure of the gas, promote the desorption of adsorbed gas, and increase the gas recovery rate. Yang conducted a field test of low-pressure nitrogen injection in coal seam to promote gas extraction/drainage in the underground coal mine of Yangquan Mining area7, and found that injecting nitrogen into coal seam could significantly promote gas extraction/drainage. Katayama8 believed that the mechanism of CO2 replacing CH4 was due to the stronger adsorption capacity of CO2, while N2 replacing CH4 was due to the reduction of the partial pressure of CH4 by N2 injection. Wu et al.9 carried out an experimental study on the effect of nitrogen injection based on the theory of diffusion seepage and multi-component adsorption equilibrium and discussed the stimulation mechanism of gas displaced by nitrogen injection. Most of the above studies are carried out through laboratory experiments and field tests. However, coal is composed of inorganic and organic matter, and its inner surface has complex structural characteristics, so the gas injection displacement mechanism should be explained from the molecular level10,11,12. Molecular simulation method can be used to study the microscopic mechanism of interaction between adsorbent and adsorbent at the molecular level13,14.
Scholars at home and abroad have done a lot of research on molecular simulation of the adsorption characteristics of CO2, N2, and other mixed gases of coalbed methane15,16. Wu et al.17 used the grand canonical integrated Monte Carlo method to analyze the ability and competitive difference of coal to adsorb CO2, O2, and N2 according to the fire prevention and storage practice of flue gas injected into the goaf of power plant. Lou et al.18 established a physical adsorption model for different gas molecules such as O2, CO2, and N2 on the surface of coal macromolecules to explore the gas competition difference of mixed gas molecules on the surface of coal macromolecules. Cui et al.19 studied the adsorption of CH4, N2, and CO2 gases by coal, and the results showed that the adsorption capacity of coal for the three gases was CO2 > CH4 > N2. Song et al.20 used GCMC and DFT methods to study the influence of oxygen-containing functional groups and electrostatic interaction on the competitive adsorption of CO2, CH4, and N2 in the low-rank coal molecular model, and found that the strong quadrupole moment and polarization ability of CO2 led to the stronger selective adsorption of oxygen-containing functional groups than CH4 and N2.
In terms of nitrogen injection to promote methane extraction, the adsorption/desorption mechanism of different gases in coal is studied mainly through laboratory experiments, field tests, and numerical simulation. In fact, coal itself contains adsorbed methane, and different temperatures, types of injected gas, and injection pressure of coal seam have a great influence on the desorption of coal seam methane. Therefore, the interaction mechanism between injected gas molecules and methane-containing coal in the process of gas injection is not clear at present. Coal contains methane, and the molecular dynamics mechanism of gas injection under different conditions to promote methane desorption in coal needs to be further studied. Although some scholars have studied the mechanism of methane adsorption by coal from a microscopic point of view, there is still a lack of systematic simulation of the influence of water on methane desorption by coal. Therefore, the mechanism of nitrogen injection promoting gas desorption by water in coal has not been fully grasped. Taking Wiser bituminous coal as an example, the molecular simulation was used to study the desorption behavior of CH4 in methane-containing coal structure after N2 injection at different temperatures, pressures, and moisture content, to clarify the mechanism of promoting CH4 desorption in coal by N2 injection. It provides theoretical support for methane extraction technology in goaf.
Calculation methods
Coal molecular structure model
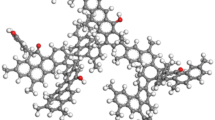
Theoretical coal models are generally composed of coal molecules consisting of a large number of atoms, and these idealized coal models are applied to solve practical problems21,22. In this study, a typical bituminous coal Wiser molecular substrate (C192H165O20N5S8) was selected, as shown in Fig. 1. The lignite substrate model is composed of an aromatic skeleton amorphous molecular structure composed of 390 atoms, including carbon, hydrogen, oxygen, nitrogen, and sulfur elements. This molecular model has been successfully applied in the study of gas competitive adsorption on bituminous coal surface23. Table 1 shows the structural parameters of Wiser bituminous coal macromolecular model.
Molecular dynamics simulation
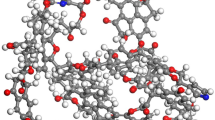
The molecular dynamics simulation was carried out on Materials Studio software, and the COMPASSII force field was used in the calculation process. In this paper, the surface model of bituminous coal was constructed through the Amorphous Cell module, and the initial surface model of bituminous coal was obtained by filling the 16 optimized bituminous coal molecular models into the 3D periodic boundary Cell. Then Anneal is annealed through Anneal task in Forcite module, the precision is set as Fine, the temperature range is 300–500 K, and the total simulation time is 1 ns. Finally, the structure was further optimized with Fine precision and the Ewald summation method was used for van der Waals interaction with A truncation radius of 15.0 Å to obtain the final bituminous coal surface model (Fig. 2).
The density of the model was 1.20 g/cm3. The cell was expanded to construct a 1 × 2 × 2 supercell, and 50 Å vacuum layer was added, as shown in Fig. 3.
CH4 and N2 molecules were adsorbed in the coal surface model to form the CH4-N2 system, and the structure of the system was optimized by repeating the above structural optimization method. The Dynamics properties are calculated under the Dynamics task in the Forcite module. The NVT canonical ensemble was used to study the dynamics of the system with 40 ps of simulated data. The setting of the calculation method is consistent with that in the structural optimization of the model mentioned above.
Mean Square Displacement (MSD) is the statistical average of particle trajectories, which refers to the measurement of the average distance of other particles around a given particle24. MSD calculation formula is as follows:
where \({\text{r}}_{{\text{i}}} ({\text{t}})\) and \({\text{r}}_{{\text{i}}} (0)\) are the instantaneous and initial position vectors of the ith particle, respectively. Moreover, N is the total number of particles in the system.
The diffusion coefficient of gas can be obtained from the following expression:
where D is the diffusion coefficient of the gas, N is the number of particles in the system, and KMSD is the slope of the MSD curve.
Monte Carlo simulation
In the Sorption module, the injection pressure is 10–1000 kpa, and the temperature is 293–333 K; The number of simulated loading steps to the equilibrium state is 1 × 108 steps, and the total number of steps is 2 × 108 steps. The Metropolis method was chosen for configuration calculation, and the charge balance (QEq) method was used for charge calculation. The Settings of force field, van der Waals force, hydrogen bond force, and Coulomb force calculation methods were consistent with those in the structural optimization of the previous model.
Figure 4 shows the initial model of nitrogen injection. Regions 0–57 Å and 107–164 Å are the molecular layers of coal, and region 57–107 Å is the vacuum layer. The amount of N2 injected under different pressures is consistent with the adsorption amount of N2 in Table 1. The quantity of N2 injected at different temperatures was 256. The simulation calculation of desorption CH4 adopts the Focite module. The task is set as Dynamics and the temperature is set as 293–333 K. The temperature control method, ensemble and force field are consistent with the Dynamics parameters in “Molecular dynamics simulation” section.
Results and analysis
Distribution characteristics of the gas in the model
Figure 5 shows the adsorption isotherms of CH4 and N2 in the molecular structure model of bituminous coal when the temperature is 293 K, 303 K, 313 K, 323 K, and 333 K, and the pressure range is 0.01–10 MPa. The nonlinear form of the Langmuir adsorption isotherm model was used to fit the adsorption equilibrium data of CH4 and N2, and the fitting curves are shown in Fig. 5. The results show that when the adsorption temperature is 293 K, 303 K, 313 K, 323 K, 333 K, the square R2 of the correlation coefficient of CH4 adsorption isotherm fitting curve is 0.9963, 0.9974, 0.9966, 0.9971, 0.9972, respectively. The R2 of N2 was 0.9979, 0.9992, 0.9974, 0.9994, 0.9991, respectively. It can be seen that the Langmuir adsorption model can well describe the adsorption of the two gases by the molecular structure model of bituminous coal.
As can be seen from Fig. 5, the adsorption capacity of CH4 is significantly higher than that of N2 under the pressure of 1 MPa. When the pressure increased from 0.01 mpa to 1 MPa, the adsorption capacity of the two gases increased rapidly, and CH4 increased the fastest. In the process of pressure increasing from 1 to 8 MPa, the increased rate of adsorption capacity of the two gases showed an obvious downward trend. When the pressure increased to 8 MPa, the adsorption capacity gradually tended to be flat. When the pressure is constant, the adsorption capacity of the two gases decreases gradually with the increase of temperature. The increase in temperature is not conducive to the adsorption of gas, so the adsorption process of gas is exothermic. Under isothermal and isobaric conditions, the adsorption capacity of the two gases in bituminous coal is CH4 > N2. This is because the amount of gas adsorbed decreases with the increase of molecular dynamics diameter, and the molecular dynamics diameter of N2 is larger than that of CH4, which makes the adsorption of N2 by coal weaker than that of CH4.
Effect of temperature on methane desorption
In this paper, the effect of N2 at different temperatures on the determination of CH4 in coal is studied from the microscopic point of view. The average relative concentration of the two systems after kinetic optimization is analyzed by the Forcite module. After N2 injection, the average relative concentration distribution of the two types of gases in coal molecules is shown in Fig. 6. Regions 0–57 Å and 107–164 Å are the molecular layers of coal, and region 57–107 Å is the vacuum layer. As can be seen from Fig. 6, both types of gases diffuse into the vacuum layer. The increase of CH4 molecules in the vacuum layer is the most, and the increase of N2 molecules is the least. In the range of 293–333 K, the mean relative concentration of CH4 increased by 59.94% after N2 injection. At the same temperature, the concentration of CH4 in the vacuum layer is 62.15%(293 K), 60.97%(303 K), 53.62%(313 K), 61.08%(323 K), 50.43% (333 K) higher than that of N2.
As shown in Fig. 7, the root means the square displacement of gas in the CH4-N2 system increases with the increase of simulation time. At the same time, the MSD of gas in the system increases with the increase of temperature. After N2 is injected into the coal, the CH4 adsorbed on the coal molecules under the influence of pressure gradient is desorbed out. On the contrary, due to a large number of free N2 molecules in the vacuum layer, the concentration of N2 in the vacuum layer increases beyond that in the coal molecular layer, and part of N2 will diffuse to the coal molecular layer and be adsorbed by the coal molecules. Therefore, what actually happens in coal is that N2 resolves CH4 by diffusion. At the same temperature, the overall relationship between the root mean square displacement of the two gases is CH4 > N2.
As shown in Fig. 8, the diffusion coefficients of CH4 increased with the increase of N2 injection temperature, indicating that the molecular activity in the system increased with the increase of temperature. In the range of 293–333 K, the diffusion coefficient of CH4 increases from 0.06 to 0.11 nm2/ps. The diffusion coefficient of N2 increases from 0.03 to 0.08 nm2/ps. At the same temperature, the diffusion coefficient of CH4 is always greater than that of N2. It can be seen that the CH4 molecule is more active in the N2-CH4 system.
Effect of pressure on methane desorption
The average relative concentration distribution of the two types of gases in coal molecules after N2 injection at different pressures is shown in Fig. 9. As can be seen from Fig. 9, both types of gases diffuse into the vacuum layer. The increase of CH4 molecules in vacuum layer is the most, and the increase of N2 molecules is the least. The concentration in the vacuum layer increases with the increase of pressure. In the range of 1–5 MPa, the mean relative concentration of CH4 increased by 71.82% after N2 injection. Under the same pressure, the concentration of CH4 in the vacuum layer is 62.15%(1 MPa), 48.88%(2 MPa), 49.65%(3 MPa), 50.18%(4 MPa), and 47.23% (5 MPa) higher than that of N2.
Figure 10 shows the root means square displacements of CH4 and N2 at different pressures. In the CH4-N2 system, the root means square displacements of gases increased with the increase of the simulated pressure. Under the same pressure, the overall relationship between the root means square displacement of the two gases is CH4 > N2.
Figure 11 shows the relationship between the CH4 diffusion coefficient and N2 injection pressure. It can be seen from the figure that the diffusion coefficient of both gases increases with the increase of pressure, indicating that the molecular activity in the system increases with the increase of pressure. In the range of 1–5 MPa, the diffusion coefficient of CH4 increases from 0.06 to 0.16 nm2/ps. The diffusion coefficient of N2 increases from 0.03 to 0.09 nm2/ps. The diffusion coefficient of CH4 is always greater than that of N2 under the same pressure. It can be seen that the CH4 molecule is more active than N2 in the N2-CH4 system. Since the diffusion coefficient of CH4 is higher than that of N2, the concentration of CH4 diffusing into the vacuum layer is also higher than that of N2.
Effect of water content on methane desorption
As shown in Fig. 12, the relative concentration of CH4 in the vacuum layer gradually increases with the increase of water content. This indicates that the water in coal promotes the desorption of CH4. This is because the force between coal and methane molecules is greater than that between water molecules, but water molecules are polar molecules, and there is hydrogen bonding between coal and water molecules, so the estimated force between coal and water molecules is far greater than that between coal and methane molecules. Therefore, from the perspective of intermolecular forces, H2O is in a dominant competitive position. Because the interaction between coal and water molecules is far greater than that between coal and methane molecules, when the three molecules coexist, water molecules and methane molecules compete for adsorption on the surface of coal, and water molecules will replace some of them to adsorb methane.
Discussion on the action mechanism of gas molecules
By comparing the effects of injection pressure and temperature on methane resolution, it can be seen that the average relative concentration of CH4 increases by 59.94% at 1 MPa and 293–333 K. The diffusion coefficient increased from 0.06 to 0.11 nm2/ps. The average relative concentration of CH4 at 1–5 MPa and 293 K increased by 71.82% after N2 injection. The diffusion line increased by 0.10 nm2/ps. It shows that the influence of injection pressure on methane desorption is greater than that of temperature in the simulated range. Compared with the injection of high-temperature nitrogen to desorption coal seam methane, the diffusion effect of high-pressure nitrogen flowing in the coal body is more significant. The higher the injection pressure of nitrogen, the better the effect of N2 promoting CH4 desorption.
According to the above simulation results, the concentration of desorbed CH4 changes with the pressure and temperature of N2 injection. With the injection of N2, the pore pressure in the coal increases, and the diffusion velocity of CH4 in the coal accelerates accordingly. The adsorbed CH4 in the coal also begins to desorption and flow out with N2 airflow. The increase of gas injection pressure accelerates the seepage rate, improves the pore pressure in the coal body, and promotes the increase of adsorbed CH4 desorption amount. Therefore, the desorbed CH4 rises with the increase of gas injection pressure. Within the same temperature, the higher the pressure is, the greater the CH4 analytical amount is, and the more obvious the promoting effect is. After the injected N2 enters the coal, the pressure in the coal increases, and the partial pressure of N2 also shows an upward trend. When CH4 does not desorption, the partial pressure of CH4 remains unchanged. However, after N2 injection, there is an additional gas and partial pressure in the system, so the adsorbed CH4 will desorption and become a free state. In this simulation, CH4 in the coal body is resolved with the continuous injection of N2, and the partial pressure of CH4 decreases, which intensifies the desorption of adsorbed CH4 in the coal body. When the partial pressure of N2 promotes the desorption of CH4, a part of N2 will also be adsorbed into the coal, which shows that N2 is adsorbed by the coal while CH4 is replaced. It can be seen that the higher the N2 injection pressure and temperature, the more methane desorption, and N2 can replace some adsorbed CH4 through competitive adsorption with CH4. The simulation results are consistent with Zhou’s experimental results26.
Due to the decrease of partial pressure, CH4 in coal will desorption from the pores of the matrix. After desorption of CH4 molecules adsorbed in coal, its main form of movement is diffusion movement under the action of the concentration gradient. In this simulation, after N2 injection, CH4 in the vacuum layer was diluted, which resulted in a concentration difference between the coal molecular layer and the vacuum layer, and directional gas diffusion continued. Not only does CH4 diffuse from the coal molecular layer to the vacuum layer, but also N2 diffuses into the vacuum layer under the effect of concentration difference. N2 promotes the desorption of CH4 by diffusion. This is also one of the mechanisms by which N2 injection promotes CH4 desorption.
Conclusions
In this paper, the desorption of CH4 by N2 injection was studied by using GCMC and MD methods. The results show that:
-
1.
The adsorption isotherms of CH4 and N2 in the molecular structure model of bituminous coal are in good agreement with the Langmuir adsorption isotherm model. The adsorption capacity of the two gases in the bituminous coal structure model is CH4 > N2. Temperature is negatively correlated with the adsorption amount.
-
2.
In the range of 293–333 K, the average relative concentration of CH4 in the vacuum layer increased by 59.94% after N2 injection. In the range of 1–5 MPa, the average relative concentration of CH4 increased by 71.82% after N2 injection. The higher the N2 injection pressure and temperature, the more methane desorption, and N2 can replace some adsorbed CH4 through competitive adsorption with CH4. The activity of the CH4 molecule is higher in N2–CH4 system.
-
3.
The relative concentration of CH4 in the vacuum layer gradually increases with the increase of water content. This indicates that the water in coal promotes the desorption of CH4. The results of this article provide theoretical support for methane extraction technology in goaf. Since this study only carried out a molecular simulation on the bituminous coal model, the research scope will be expanded in the future to further improve the research results.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Wang, K., Du, F. & Wang, G. D. Investigation of gas pressure and temperature effects on the permeability and steady-state time of Chinese anthracite coal: an experimental study. J. Nat. Gas Sci. Eng. 40, 179–188 (2017).
Xin, Y., Wang, G. D., Du, F., Jin, L. Z. & Gong, H. R. N2 injection to enhance coal seam gas drainage (N2-ECGD): insights from underground field trial investigation. Energy 239, 122247 (2022).
You, J. et al. Adsorption behavior of carbon dioxide and methane in bituminous coal: a molecular simulation study. Chin. J. Chem. Eng. 24(9), 1275–1282 (2016).
Gao, D. M., Lin, H., Wang, J. R. & Zheng, D. Molecular simulation of gas adsorption characteristics and diffusion in micropore of lignite. Fuel 269, 117443 (2020).
Gensterblum, Y., Merkel, A., Busch, A. & Krooss, B. M. High-pressure CH4 and CO2 sorption isotherms as a function of coal maturity and the influence of moisture. Int. J. Coal Geol. 118, 45–57 (2013).
Clarkso, C. R. & Bustin, R. M. Binary gas adsorption/desorption isotherms: effect of moisture and coal composition upon carbon dioxide selectivity over methane. Int. J. Coal Geol. 42(4), 241–272 (2000).
Katayama, Y. Study of coal bed methane in Japan. In Proceedings of United Nations International Conference on Coalbed Methane Development and Utilization 238–243 (1995).
Yang, H. M. Study on Mechanism and Characteristics Laws of Displacement Coal Bed Methane by Underground Gas Injection (Henan Polytechnic University, 2010).
Wu, S. Y. & Guo, Y. Y. Research on the mechanism of increasing production of coalbed methane by gas injection. J. China Coal Soc. 26(2), 199–203 (2001).
Moshe, K., He, J. J. & Liu, Y. Y. Molecular simulation of methane adsorption in micro- and mesoporous carbons with applications to coal and gas shale systems. Int. J. Coal Geol. 109(110), 36–44 (2013).
Li, S. G., Bai, Y. & Lin, H. F. Molecular simulation of adsorption of gas in coal slit model under the action of liquid nitrogen. Fuel 255, 115775 (2019).
Li, S. G., Bai, Y. & Lin, H. F. Molecular simulation of adsorption thermodynamics of multicomponent gas in coal. J. China Coal Soc. 43(9), 2476–2483 (2018).
Li, Z. X., Ding, C., Wang, W. Q., Lu, B. & Gao, D. M. Simulation study on the adsorption characteristics of CO2 and CH4 by oxygen-containing functional groups on coal surface. Energy Sources Part A Recover. Util. Environ. Effects 44(2), 3709–3719 (2022).
Liu, X. Q. et al. Molecular simulation of CH4, CO2, H2O and N2 molecules adsorption on heterogeneous surface models of coal. Appl. Surf. Sci. 389, 894–905 (2016).
Zhang, J., Clennell, M. B., Dewhurst, D. N. & Liu, K. Combined Monte Carlo and molecular dynamics simulation of methane adsorption on dry and moist coal. Fuel 122, 186–197 (2014).
Chen, J. Density functional calculation of the adsorption of different amine/ammonium cations on the surface of kaolinite (001). J. China Coal Soc. 41(12), 3115–3121 (2016).
Wu, S. Y., Jin, Z. X. & Deng, C. B. Molecular simulation of coal-fired plant flue gas competitive adsorption and diffusion on coal. Fuel 239, 87–96 (2019).
Lou, H. Z. & Jia, T. G. Competitive adsorption differences during coal spontaneous combustion process in noble gas atmosphere. China Safety Sci. J. 30(4), 60–67 (2020).
Cui, Y. J., Zhang, Q. & Zhang, H. Adsorption of single component gas CH4, N2, and CO2 on various rank coals. Nat. Gas Ind. 25(1), 61–65 (2005).
Song, Y., Jiang, B. & Lan, F. J. Competitive adsorption of CO2/N2/CH4 onto coal vitrinite macromolecular: effects of electrostatic interactions and oxygen functionalities. Fuel 235, 23–38 (2019).
Xia, Y. C. Role of molecular simulation in understanding the mechanism of low-rank coal flotation: a review. Fuel 262, 116535 (2020).
Meng, J. Q. Insight on adsorption mechanism of coal molecules at different ranks. Fuel 267(1), 117234 (2020).
Wiser, W. H., Hill, G. R. & Kertamus, N. J. Kinetic study of pyrolysis of high volatile bituminous coal. Ind. Eng. Chem. Process Des. Dev. 6(1), 133–138 (1967).
Wang, B. J., Zhang, L. N. & Ling, L. X. Effects of coal molecular structure on adsorption and diffusion behaviors of coalbed methane. CIESC J. 67(6), 2548–2557 (2016).
Lu, X. et al. Molecular dynamics simulation of gas diffusion in amorphous polyisoprene. J. Phys. Chem. 32(10), 2523–2530 (2016).
Zhou, H. Study on the characteristics of enhanced coalbed methane extraction by nitrogen injection and gas displacement in low permeability coal seams (China University of Mining and Technology, 2021).
Acknowledgements
Thanks to all the reviewers and editors for their work.
Author information
Authors and Affiliations
Contributions
X.F. wrote the whole article.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fang, X. Simulation study on dynamic characteristics of gas diffusion in coal under nitrogen injection. Sci Rep 12, 18865 (2022). https://doi.org/10.1038/s41598-022-23778-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23778-6
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.