Abstract
Breast cancer is the fifth leading cause of cancer death globally. In this retrospective study, we investigated the effects of the diagnosis-to-first-treatment interval (DFTI) and other related factors on cancer-specific survival in patients with breast cancer. We included 49,426 patients newly diagnosed as having breast cancer during 2011–2017. The Cox proportional hazards model was used to analyze the hazard ratio (HR) for mortality with various DFTIs; the HRs of the 31–60-, 61–90-, and ≥ 91-day DFTI groups did not differ significantly compared with the reference group (DFTI ≤ 30 days). After stratifying the patients according to initial tumor stage and age, we found that patients aged 55–64 and ≥ 65 years with stage II breast cancer treated ≥ 91 days after diagnosis had a 3.34- and 2.93-fold higher mortality risk (95% confidence intervals [CIs] 1.29–8.69 and 1.06–8.10, respectively). Patients aged ≥ 65 years with stage IV breast cancer treated within 61–90 or ≥ 91 days after diagnosis had a 7.14- and 34.78-fold higher mortality risk (95% CIs 1.28–39.82 and 3.08–393.32, respectively). In conclusion, DFTI is associated with mortality in patients with stage II and IV breast cancer, especially at an older age.
Similar content being viewed by others
Introduction
In 2020, breast cancer was the fifth leading cause of cancer death globally; it was responsible for 684,996 deaths, accounting for approximately 6.9% of cancer deaths worldwide1. In 2019, nearly 14,856 women were diagnosed as having breast cancer, and 2633 women died of breast cancer in Taiwan, making it the second leading cause of cancer death among Taiwanese women2.
According to the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program data, women diagnosed as having breast cancer between 2011 and 2017 had a 5-year relative survival at localized breast cancer diagnosis was nearly 99.0%3. In women with breast cancer exhibiting regional lymph node metastasis and distant metastasis, the 5-year relative survival rates were approximately 85.8% and 29.0%, respectively3. Taiwan’s Ministry of Health and Welfare provides free mammography to women who meet certain criteria to ensure the early detection and treatment of breast cancer. However, a recent study indicated that delays and refusals of breast cancer treatment remain prevalent in Taiwan4.
Treatment delay influences the overall survival of patients with breast cancer considerably5. After a breast cancer diagnosis, patients are typically concerned about treatment time and its influence on their survival6. Some studies have revealed that a short DFTI could positively influence disease progression, survival rates, and quality of life7,8. However, other studies have indicated that DFTI does not influence patient survival9,10,11. One study revealed that delays in receiving treatment for metastatic breast cancer may be related to adverse survival outcomes5; however, other studies have indicated that the mortality risk increases when treatment is delayed in the early stages6,12. In the current study, we investigated how the DFTI influences survival in patients at different breast cancer stages. The results may aid in determining treatment time and mitigating the risk of poor prognosis due to delayed treatment at different cancer stages and in monitoring other factors associated with survival.
Results
Characteristics of patients with breast cancer for different treatment intervals
In this study, we included 49,426 patients who had been diagnosed with breast cancer between 2011 and 2017. Various factors were associated with the interval between diagnosis and treatment initiation. As listed in Table 1, most of the patients had undergone treatment within 30 days of diagnosis (n = 40,010; 80.95%). Patients who earned a lower monthly salary (NTD20,009–NTD22,800), had stage IV breast cancer or a tumor size of 2–5 cm, had received treatment in a district hospital, and had received treatment in a public hospital had a relatively short DFTI (< 30 days; Ps < 0.001). However, with regard to patient age, CCI score, hormone receptor status (ERA, PRA), HER2 status, and number of involved regional lymph nodes, the differences in DFTIs were found to be nonsignificant (P > 0.05).
Influence of DFTI and other related factors on mortality risk
A Cox proportional hazards model was used to examine the relationship of patient survival with the relevant variables (Table 2). The DFTI, hospital level, and hospital ownership were nonsignificant predictors of mortality. ER, PR, and HER2 status were protective factors for mortality risk (adjusted HRs [95% CIs] 0.64 [0.56–0.72], 0.51 [0.45–0.57], and 0.88 [0.80–0.97], respectively; all Ps < 0.05) after other related factors were controlled for. Tumor size, number of involved lymph nodes, and cancer stage also were positively correlated with mortality risk (P < 0.05).
Mortality risk in patients with delayed treatment according to the different ages and various breast cancer stages
Figure 1 lists the mortality risk after stratifying the patients according to initial tumor stage with different DFTIs. In patients at stages I, III, and IV, a DFTI of > 30 days did not have a significant effect on mortality compared with the reference group (DFTI ≤ 30 days). However, patients at stage II with a ≥ 91-day DFTI showed a 2.84-fold increase in mortality risk compared with the reference group (DFTI ≤ 30 days; 95% CI 1.63–4.98, P < 0.001). As illustrated in the adjusted survival curves (Fig. 2), after stratification according to initial tumor stage, DFTIs exhibited no significant difference compared with the reference group (DFTI ≤ 30 days) in all patients except for those at stage II. The DFTI remained a significant prognosticator in patients at stage II who started treatment ≥ 91 days after diagnosis (Fig. 2). Moreover, we analyzed the impact of DFTIs on mortality risk according to age and cancer stage with multivariate Cox proportional hazard models (Table 3). We determined that compared with the reference group (DFTI ≤ 30 days), stage II patients with a ≥ 91-day DFTI were 3.34 and 2.93 times more likely to die at the ages of 55–64 and ≥ 65, respectively (95% CIs 1.29–8.69, P = 0.013, and 1.06–8.10, P = 0.039, respectively). Moreover, compared with the reference group (DFTI ≤ 30 days), stage IV patients at the age of ≥ 65 with a 61–90 and > 91-day DFTI had a 7.14- and 34.78-fold increased mortality risk (95% CIs 1.28–39.82, P = 0.025, and 3.08–393.32, P = 0.004, respectively).
Influence of DFTI on mortality risk in patients at various breast cancer stages. The related variables (age, marital status, education level, monthly salary, level of urbanization, CCI score, tumor size, number of involved lymph nodes, hormone receptor status, HER2 status, treatment type, hospital level, and hospital ownership) were controlled in each model.
Adjusted survival curves of patients with different breast cancer stages and DFTIs. The related variables (age, marital status, education level, monthly salary, level of urbanization, CCI score, tumor size, number of involved lymph nodes, hormone receptor status, HER2 status, treatment type, hospital level, and hospital ownership) were controlled.
Discussion
In this study, we analyzed 49,426 patients with new-onset breast cancer and found no statistically significant differences between early treatment and survival. However, stratified analysis by age and stage demonstrated that stage II patients aged > 55 years with a ≥ 91-day DFTI and stage IV patients aged ≥ 65 years with a > 60-day DFTI had an elevated mortality risk.
Some studies have explored the impact of delayed treatment on mortality risk in patients with breast cancer, but they have not reported any conclusive results. Yoo et al. demonstrated that a delay in treatment initiation with a cutoff value of 15, 30, 45, and 60 days after biopsy confirmation did not affect disease-free and overall breast cancer survival11. Mujar et al., who also reported that a DFTI of > 2 weeks, 1 month, and 2 months did not affect survival10. However, several studies have demonstrated the opposite. In female patients with breast cancer whose first treatment was surgery, the 5-year survival rate was significantly higher in patients with a < 2-week DFTI compared with those with a > 6-week DFTI (90% vs. 80%; P < 0.05)18. Yun et al. demonstrated that the 5-year survival rate in patients with breast cancer and a DFTI of > 1 month was low (HR 1.59; 95% CI 1.37–1.84)19. According to the National Breast and Cervical Cancer Early Detection Program, the DFTI should not exceed 60 days20.
Studies have reported the impact of delayed treatment on patient survival in various subgroups. McLaughlin et al. found that a ≥ 60-day DFTI substantially increased the mortality risk of patients at a late cancer stage but did not affect the overall survival of patients at an early stage21. Our results revealed that at stage IV, a higher risk existed between treatment delay and breast cancer-related mortality among people aged ≥ 65 years. Nonetheless, the results accord with those of previous studies showed that the overall mortality HR significantly increased for each increasing interval at cancer stages I and II6. Besides, Khorana et al. reported that an increased DFTI is associated with worsened survival in patients with stage I and II breast cancer but that it is associated to a much lower degree with outcomes in patients with stage III breast cancer12. This result suggests that cancer stage is an important prognostic factor for survival response after delayed treatment. Thus, we further evaluated the association between treatment delay and cancer stage at different ages and breast cancer mortality. A study reported that compared with older women with breast cancer, younger women with breast cancer are more likely to have higher-grade, larger tumors with ER/PR negativity and lymph node positivity; moreover, their mortality risk is higher22. That study also revealed that compared with older women, younger women are more likely to die if diagnosed as having stage I or II disease and less likely to die if diagnosed as having stage IV disease22. However, the database used in the aforementioned study did not contain information on comorbidities or other cancer treatments, such as chemotherapy and endocrine therapy. Patients who are younger and have late-stage disease may be undergoing more aggressive treatment than older women at the same stage due to the lack of comorbidities. In the current study, we collected complete data on patient treatments and comorbidities. Women aged 55–64 years exhibited significantly less mortality risk than those aged 20–54 years (HR 0.88; 95% CI 0.79–0.98).
Previous research showed that the lower the income of a patient was, the later the stage at which a patient’s cancer was diagnosed23. A study also revealed that patients at a later cancer stage with a lower socioeconomic status experience an especially longer DFTI and poorer prognosis than do those with a higher socioeconomic status24. Although the quality of medical services has been improved in Taiwan after the introduction of National Health Insurance in recent years, patients with higher incomes still have access to more treatment options than do those with lower incomes. A study indicated that the presence of comorbid diseases at breast cancer diagnosis was an independent adverse prognostic factor for mortality in patients with breast cancer25, which accords with the current results. In breast cancer, immunohistochemistry subtypes, together with grade, tumor size, and nodal status, are related to survival. These factors were previously found to be independent predictors of breast cancer mortality: the mortality risk was 20–40-fold higher in patients with the worst prognosis than in those with tumors having smaller size, lower grade, and ER(+)/PR(+)/HER2(−) status26. Women who initially visited a teaching hospital also had significantly better survival than did those who initially visited a community hospital27.
The strengths of our study, compared with previous studies, are related to the sample size, data source, and breast cancer stage analysis. We used nationwide data from the Taiwan Cancer Registry to prevent any selection bias, which may exist in traditional hospital-based observational studies. Our study also examined the impact of the DFTI on the survival rates of patients at different breast cancer stages and different age subgroups. The databases we included contain complete information on the factors that may also influence breast cancer mortality.
This study, however, has several limitations. We evaluated the effects of immunohistochemistry subtype, tumor size, and nodal spread on breast cancer mortality; however, our collected data did not include Ki67 status, and therefore, we could not analyze the combined effects of the aforementioned factors (i.e., hormone receptor status, HER2 status and Ki67 status) on breast cancer death. Moreover, our results cannot be generalized to patients in other countries, mainly because the National Health Insurance system has improved health care access and cost in Taiwan, enabling Taiwan’s residents to receive breast cancer treatments without a copayment. Finally, in the current study, 8167 patients of 81,906 included patients—roughly 9% of the total study population—did not have cancer stage information, and therefore, they were excluded from our study participants. Nonetheless, because this study included a large nationwide population, we believe that a negligible number of women with an unknown cancer stage were excluded.
Conclusions
The results of this study highlight how the cancer stage and age at diagnosis affect survival among patients with different DFTIs. To increase the timeliness of receiving treatment, we suggest that interventions should especially be targeted at older patients with stage II and IV breast cancer.
Materials and methods
Study design
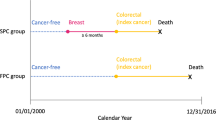
This retrospective cohort study was conducted to evaluate the influence of the DFTI and other related factors on mortality among female patients with breast cancer. Confirmed breast cancer cases diagnosed between 2011 and 2017 were sampled, and their survival was followed until the end of 2018. The start date of follow-up was the date of first diagnosis, and the end date was the date of loss to follow-up, death, or the end of the study period (December 31, 2018), whichever occurred first.
Data sources
To select our sample participants, we accessed the Taiwan Cancer Registry, published by the Health Promotion Administration, Ministry of Health and Welfare. The Taiwan Cancer Registry is a nationwide population-based cancer registry system that was established in 1979. The database includes detailed information on cancer staging, cancer site-specific factors, treatment, and recurrence13. In addition, we connected these data to 2009–2018 data from the National Health Insurance Research Database (NHIRD), the Registry for Catastrophic Illness Patients Database (RCIPD), and the Cause of Death File, Ministry of Health and Welfare. The RCIPD is a subdatabase of the NHIRD, which contains the data of 99.99% of Taiwan’s population14. The RCIPD includes the health-care data of patients diagnosed as having any of the 30 specified catastrophic illnesses (such as malignancies, severe hereditary diseases, immune diseases or disorders)15. People with any catastrophic illness are exempt from consultation, pharmacy, treatment, and hospitalization fees under National Health Insurance. We extended 2 years of data (2009 and 2010) from the NHIRD to evaluate the Charlson comorbidity index (CCI) scores and other catastrophic illnesses of the included patients. This study was approved by the institutional review board of Cheng Ching Hospital (IRB number: HP150004) and was conducted in accordance with the Helsinki Declaration. The informed consent was waived by the Research Ethnics Committee of China Medical University Hospital.
Study participants
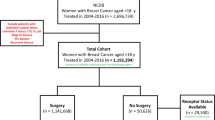
In total, 81,906 patients were newly diagnosed as having breast cancer between 2011 and 2017. Their diagnosis was defined on the basis of International Classification of Diseases for Oncology, Third Edition codes C50.0–C50.9. Of all patients, only 49,426 patients were included for further analysis (Fig. 3) after the following patients were excluded: male patients, patients diagnosed as having any type of cancer before or after breast cancer diagnosis, patients having any type of catastrophic illness before breast cancer, patients aged < 20 years, patients without a known date of initial treatment, patients at an unknown cancer stage, patients with carcinoma in situ, and patients without any immunohistochemistry information.
Variable definitions and descriptions
We defined DFTI as the interval between the date of breast cancer diagnosis based on biopsy and the date on which the first treatment was initiated. Four DFTI groups were used to divide the included patients: ≤ 30, 31–60, 61–90, and ≥ 91 days. Next, we defined age as the age at which a patient received a confirmed breast cancer diagnosis based on pathological findings. We categorized marital status as single, married, divorced, widowed, and missing (people with unknown marital status) and grouped the education levels into six categories. The income of the patients was based on their monthly salary. The environmental factors were based on the urbanization level of the patients’ areas of residence before cancer diagnosis; a total of seven levels—from highly developed urban cities (level 1) to remote districts (level 7)—were employed16.
The degree of comorbidity—a weighted index based on the presence of comorbid conditions within 2 years before cancer diagnosis—was classified into four levels based on CCI scores (Deyo’s CCI)17. Tumor subtypes were classified on the basis of estrogen receptor, progesterone receptor, and human growth factor/neu receptor status, as recorded according to the pathologists’ interpretation of the assays. The largest dimension of the tumor (in centimeters) as determined through pathology examination were considered the tumor size. Regional lymph nodes were defined as the most proximal lymph nodes serving as immediate drainage sites for the tumors, and these included axillary nodes, ipsilateral intramammary nodes, internal mammary nodes, and supraclavicular nodes. Cancer stages were categorized according to the American Joint Committee on Cancer Staging Manual, Eighth Edition. The patients were also classified based on the treatment they received within 6 months after breast cancer diagnosis. Other variables included hospital level and treatment hospital ownership.
Main outcome measurements
The primary outcome was cancer-specific mortality in the patients with breast cancer. Follow-up duration was defined as the duration from the date of diagnosis to the date of death or follow-up endpoint (December 31, 2018). Confirmation of death was made on the basis of the administrative data (Cause of Death File).
Statistical analysis
For descriptive statistics, we included the basic characteristics, income, environmental factors, health status, tumor characteristics, stage, treatment type, primary hospital information, and DFTI distribution.
The log-rank test and Cox proportional hazards model were adopted for inferential statistics. First, a bivariate analysis was performed using the log-rank test to determine significant differences between the survival status by the end of 2018 and the DFTI or other variables. The adjusted Cox proportional hazards model was used to analyze the relative mortality risk in breast cancer patients with different DFTIs, after the related variables were controlled for. Subsequently, we analyzed the influence of the DFTI on the survival of patients at various cancer stages and of different ages. Finally, we estimated survival time according to the adjusted survival curves for all patients with breast cancer; the stratification analyses by tumor stage were performed to investigate the influences of different DFTIs on patient survival.
In this study, SAS (version 9.4; SAS Institute, Cary, NC, USA) was used for data analysis, with the significance level (α) set at 0.05.
Data availability
This study used the National Health Insurance Research Database published by the Ministry of Health and Welfare, Taiwan. Due to legal restrictions imposed by the Taiwan government under the Personal Information Protection Act, the database cannot be made publicly available. All researchers can apply to use the database to conduct their studies. Requests for data can be sent as a formal proposal to the Science Center of the Ministry of Health and Welfare (https://www.mohw.gov.tw/np-108-2.html). Any raw data are not allowed to be brought out from the Science Center. Only the analytic outputs in table or figure format can be printed out. The restrictions prohibit authors from making the minimal data set publicly available.
References
International Agency for Research on Cancer. (2020). W. H. O. Breast. Accessed 30 June 2021 from https://reurl.cc/AKWV73.
Cancer Registry Annual Report, 2019 Taiwan 5 (Health Promotion Administration, Ministry of Health and Welfare, 2021).
Cancer Stat Facts: Female Breast Cancer. Accessed 21 July 2021 from https://reurl.cc/zMR78N.
Chen, S. J., Kung, P. T., Huang, K. H., Wang, Y. H. & Tsai, W. C. Characteristics of the delayed or refusal therapy in breast cancer patients: A longitudinal population-based study in Taiwan. PLoS ONE 10, e0131305. https://doi.org/10.1371/journal.pone.0131305 (2015).
Jung, S. Y. et al. The effect of delays in treatment for breast cancer metastasis on survival. Breast Cancer Res. Treat. 130, 953–964. https://doi.org/10.1007/s10549-011-1662-4 (2011).
Bleicher, R. J. et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2, 330–339. https://doi.org/10.1001/jamaoncol.2015.4508 (2016).
Williams, F. Assessment of breast cancer treatment delay impact on prognosis and survival: A look at the evidence from systematic analysis of the literature. J. Cancer Biol. Res. 3, 1071 (2015).
Konieczny, M., Cipora, E., Roczniak, W., Babuska-Roczniak, M. & Wojtaszek, M. Impact of time to initiation of treatment on the quality of life of women with breast cancer. Int. J. Environ. Res. Public Health 17, 228325. https://doi.org/10.3390/ijerph17228325 (2020).
Brazda, A. et al. Delays in time to treatment and survival impact in breast cancer. Ann. Surg. Oncol. 17(Suppl 3), 291–296. https://doi.org/10.1245/s10434-010-1250-6 (2010).
Mujar, M., Dahlui, M., Yip, C. H. & Taib, N. A. Delays in time to primary treatment after a diagnosis of breast cancer: Does it impact survival? Prev. Med. 56, 222–224. https://doi.org/10.1016/j.ypmed.2012.12.001 (2013).
Yoo, T. K. et al. Delay of treatment initiation does not adversely affect survival outcome in breast cancer. Cancer Res. Treat. 48, 962–969. https://doi.org/10.4143/crt.2015.173 (2016).
Khorana, A. A. et al. Time to initial cancer treatment in the United States and association with survival over time: An observational study. PLoS ONE 14, e0213209. https://doi.org/10.1371/journal.pone.0213209 (2019).
Chiang, C. J., Wang, Y. W. & Lee, W. C. Taiwan’s Nationwide Cancer Registry System of 40 years: Past, present, and future. J. Formos Med. Assoc. 118, 856–858. https://doi.org/10.1016/j.jfma.2019.01.012 (2019).
Taiwan Universal Health Coverage in Taiwan. 2016. Accessed 30 June 2021 from https://reurl.cc/nEakr6.
Registry for Catastrophic Illness Patients. Accessed 25 June 2021 from https://reurl.cc/5Gan5z.
Liu, C.-Y. et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J. Health Manag. 4, 1–22 (2006).
Deyo, R. A., Cherkin, D. C. & Ciol, M. A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 45, 613–619. https://doi.org/10.1016/0895-4356(92)90133-8 (1992).
Smith, E. C., Ziogas, A. & Anton-Culver, H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 148, 516–523. https://doi.org/10.1001/jamasurg.2013.1680 (2013).
Yun, Y. H. et al. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann. Oncol. 23, 2731–2737. https://doi.org/10.1093/annonc/mds101 (2012).
Richardson, L. C. et al. Timeliness of breast cancer diagnosis and initiation of treatment in the National Breast and Cervical Cancer Early Detection Program, 1996–2005. Am. J. Public Health 100, 1769–1776. https://doi.org/10.2105/AJPH.2009.160184 (2010).
McLaughlin, J. M. et al. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J. Clin. Oncol. 30, 4493–4500. https://doi.org/10.1200/JCO.2012.39.7695 (2012).
Gnerlich, J. L. et al. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J. Am. Coll. Surg. 208, 341–347. https://doi.org/10.1016/j.jamcollsurg.2008.12.001 (2009).
Wang, Q. et al. Breast cancer stage at diagnosis and area-based socioeconomic status: A multicenter 10-year retrospective clinical epidemiological study in China. BMC Cancer 12, 122. https://doi.org/10.1186/1471-2407-12-122 (2012).
Aziz, Z., Sana, S., Akram, M. & Saeed, A. Socioeconomic status and breast cancer survival in Pakistani women. J. Pak. Med. Assoc. 54, 448–453 (2004).
Cronin-Fenton, D. P. et al. Comorbidity and survival of Danish breast cancer patients from 1995 to 2005. Br. J. Cancer 96, 1462–1468. https://doi.org/10.1038/sj.bjc.6603717 (2007).
Johansson, A. L. V. et al. In modern times, how important are breast cancer stage, grade and receptor subtype for survival: A population-based cohort study. Breast Cancer Res. 23, 17. https://doi.org/10.1186/s13058-021-01393-z (2021).
Chaudhry, R., Goel, V. & Sawka, C. Breast cancer survival by teaching status of the initial treating hospital. CMAJ 164, 183–188 (2001).
Acknowledgements
The authors are very grateful for the use of the National Health Insurance Research Database, Cause of Death File, and Taiwan Cancer Registry Database provided by the Science Center, Ministry of Health and Welfare, Taiwan. They are also grateful to the Health Data Science Center, China Medical University Hospital, for providing administrative, technical, and funding support.
Funding
This study was supported by the Grants from China Medical University and Asia University, Grant Number CMU110-ASIA-12, and the Ministry of Science and Technology, Taiwan, grant number MOST104-2410-H-039-002). The study sponsors did not have any roles in study design, data collection, analysis, interpretation of data, writing of manuscript, and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
N.-C.S., P.-T.K. and W.-C.T. conceived the study concepts. N.-C.S., P.-T.K., W.-Y.K. and W.-C.T. contributed to the study design. W.-C.T. provided software. N.-C.S. and W.-Y.K. performed the data analysis. P.-T.K. and W.-C.T. obtained funding sources; N.-C.S. and W.-C.T. drafted the manuscript. N.-C.S., P.-T.K. and W.-C.T. revised manuscript. N.-C.S., W.-Y.K. and P.-T.K. conducted project administration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shih, NC., Kung, PT., Kuo, WY. et al. Association of treatment delay and stage with mortality in breast cancer: a nationwide cohort study in Taiwan. Sci Rep 12, 18915 (2022). https://doi.org/10.1038/s41598-022-23683-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23683-y
This article is cited by
-
Five-year survival prognosis of young, middle-aged, and elderly adult female invasive breast cancer patients by clinical and lifestyle characteristics
Breast Cancer Research and Treatment (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.