Abstract
The advancements in electrochemical capacitors have noticed a remarkable enhancement in the performance for smart electronic device applications, which has led to the invention of novel and low-cost electroactive materials. Herein, we synthesized nanostructured Al2O3 and Al2O3-reduced graphene oxide (Al2O3-rGO) hybrid through hydrothermal and post-hydrothermal calcination processes. The synthesized materials were subject to standard characterisation processes to verify their morphological and structural details. The electrochemical performances of nanostructured Al2O3 and Al2O3- rGO hybrid were evaluated through computational and experimental analyses. Due to the superior electrical conductivity of reduced graphene oxide and the synergistic effect of both EDLC and pseudocapacitive behaviour, the Al2O3- rGO hybrid shows much improved electrochemical performance (~ 15-fold) as compared to bare Al2O3. Further, a symmetric supercapacitor device (SSD) was designed using the Al2O3- rGO hybrid electrodes, and detailed electrochemical performance was evaluated. The fabricated Al2O3- rGO hybrid-based SSD showed 98.56% capacity retention when subjected to ~ 10,000 charge–discharge cycles. Both the systems (Al2O3 and its rGO hybrid) have been analysed extensively with the help of Density Functional Theory simulation technique to provide detailed structural and electronic properties. With the introduction of reduced graphene oxide, the available electronic states near the Fermi level are greatly enhanced, imparting a significant increment in the conductivity of the hybrid system. The lower diffusion energy barrier for electrolyte ions and higher quantum capacitance for the hybrid structure compared to pristine Al2O3 justify improvement in charge storage performance for the hybrid structure, supporting our experimental findings.
Similar content being viewed by others
Introduction
The conventional resources utilized to provide the world's energy requirements, such as coal, oil, natural gas, and petroleum products, are facing rapid exhaustion. On the other hand, the combustion of these fossil fuels creates severe environmental issues. Therefore, it is crucial to find alternative energy resources that are sustainable, clean, and abundant. In the current scenario, electrochemical energy storage devices are crucial in overcoming fossil fuel depletion and global warming1,2,3. Among miscellaneous electrical energy storage devices, rechargeable batteries and electrochemical capacitors have drawn significant interest, due to their widespread application in industries and our daily lives, for the last few years, because of their impressive electrochemical profile, characterised by excellent cyclic stability, high rate of charge/discharge, balanced specific energy, and power density values4,5. Supercapacitors (both electric double layer capacitors (EDLCs) and pseudocapacitors) have the advantage of releasing energy in a shorter time by using their fast surface or near surface electrochemical reactions (surface adsorption/diffusion processes)4,6. In the case of both EDLC and pseudocapacitive types of supercapacitors, the most frequently used materials (for electrode fabrication), for academia and industries, including both the carbon-based (for EDLC) and transition-metal-based compounds (for pseudocapacitor)7,8. Among various transition metal compounds, transition metal oxides usually have lower electrical conductivity than carbon-based materials. Likewise, in the case of carbon-based materials, we often observe uneven morphologies and wide pore size distributions. Compared to materials mentioned earlier, oxides of transition group metals are a prominent group of materials for supercapacitors6,9. Metal oxides have unique chemical, physical and electrochemical properties, such as mechanical, thermal stability, and high capacity. They can be easily synthesized and are quite cheaper than metal hydroxides, carbon-based active materials, and conducting polymers10,11.
Aluminum (Al) has the highest abundance (amongst metals) in the earth's crust and is used in various research fields or day-to-day lives due to its low cost and lightweight nature. Previously, Huang et al. used commercial Al foil as the current collector for supercapacitor application. The laser-treated Al current collector shows better electrochemical performance (higher capacitance and cyclic stability) than the pristine-Al current collector due to lower internal resistances12. Aluminum oxide (Al2O3) has many distinctive and attractive behaviours, such as good thermal conductivity, inertness to most acids and alkalis, high mechanical strength, high adsorption capacity, good thermal stability, non-toxicity, and cost-effectiveness13,14,15. These fascinating properties compel Al2O3 nanostructures to be a potential material for catalysis, sensors, batteries, and supercapacitors. To use Al2O3 nanostructures as electrode material for supercapacitors, Dia et al.16 synthesized an Al2O3-ZnO composite using the solvent precipitation method for supercapacitor application. Even though the electrochemical behaviour of electroactive material Al2O3 nanostructures has been found suitable for energy storage applications, electrochemical performance in aspects of gravimetric capacitance, specific energy, and specific power, still needs further improvement. The combination of an EDLC material with a pseudocapacitive material can impart a balance between energy density and power density. The EDLC material typical has high power density with low energy density, while a pseudocapacitive material has high energy density with moderate power density. When integrated into composite or hybrids, EDLC and pseudocapacitive materials will provide significant improvement which is otherwise could not be obtained individually17,18. Reduced graphene oxide can enhance the electrical conductivity, mechanical stability, and surface area of electrochemically active metal oxides for supercapacitor application, the use of reduced graphene oxide hybrid is one of the most effective routes19,20.
In the current work, we describe a low-cost, environmentally benign, and easily scalable method to prepare an Al2O3-reduced graphene oxide hybrid for supercapacitor application. We performed detailed electrochemical characterisation of pristine Al2O3 and Al2O3-reduced graphene oxide hybrid, along with computational analyses. Interestingly, the Al2O3-reduced graphene oxide hybrid shows higher storage performance (~ 15-fold higher) than pristine Al2O3, which can be attributed to the synergistic effect of both EDLC and pseudocapacitive behaviour. To support our experimental data as well as to get theoretical insights regarding interactions between Al2O3 and reduced graphene oxide, we have done Density Functional Theory simulations for Al2O3 and Al2O3-reduced graphene oxide hybrid.
Experimental section
Materials
Aluminium nitrate nonahydrate [Al(NO3)3.9H2O], glucose anhydrous [C6H12O6], ammonia (NH3.H2O), and sodium sulfate (Na2SO4) were purchased from Merck, India. Activated carbon (AC) and PVDF were bought from Alfa Aesar (India). The commercial grade stainless steel (grade-304, thickness: 1.0 mm) obtained from Amazon (India) was used as the substrate for the electrochemical study. All stainless-steel substrates were thoroughly cleaned with ethanol and DI water many times by ultra-sonication process and polished using sandpapers. Then they were dried in an electric hot-air oven at 60 ℃ for one hour and afterward used as the substrate for electroactive material coating.
Preparation of Al2O3 nanostructures
Nanostructured Al2O3 was prepared through a standard hydrothermal reaction protocol, followed by a high-temperature calcination step. In a typical synthesis protocol, 3.75 g of Al(NO3)3.9H2O and 2.5 g of C6H12O6 were mixed in 60 mL of Millipore water using an ultrasonication mixer. Afterward, 10 mL of NH3·H2O solution was added to the above precursor solution, and the final mixture solution was subject to ultra-sonication for ~ 1.5 h. The mixture was then carefully transferred to a Teflon-lined stainless steel autoclave of 100 mL capacity and was kept for 24 h at 200 °C in a hot air oven. Precipitates were collected once the hydrothermal reaction was complete, repeatedly washed via several centrifugation steps (using absolute ethanol), and dried at 70 °C overnight. After drying, the as-synthesized sample was thermally treated at 1100 ºC for five hours to obtain the product.
Synthesis of Al2O3-reduced graphene oxide hybrid
The commercial grade graphene oxide (GO) powder (purity > 99%) was purchased from ULTRANANOTECH (India). In a typical approach, GO was dispersed in 50 mL of de-ionized water to form a homogeneous GO dispersion by ultra-sonication for about two hours. Then the prepared (aforementioned) Al2O3 powder was added to the GO dispersion solution, which was subsequently kept under ultra-sonication for one hour. Finally, the mixture solution was transferred to a 100 mL autoclave (Teflon lined stainless steel) and kept in a hot air oven at 180 °C for 15 h. The precipitated Al2O3-reduced graphene oxide hybrid was centrifuged in absolute ethanol and kept inside a hot air oven to dry at 60 °C overnight. The details schematic representation of the synthesis process for both the Al2O3 and Al2O3-reduced graphene oxide hybrid has been provided in Fig. 1.
Physical characterization
The XRD analysis of the Al2O3 and Al2O3-reduced graphene oxide hybrid was obtained using a Bruker D8 Advance X-ray diffractometer instrument at 40 kV and 40 mA with Cu-Kα radiation. The surface morphology of the as-prepared Al2O3 and Al2O3-reduced graphene oxide hybrid nanostructure was analyzed using field-emission scanning electron microscopy (FE-SEM; MERLIN Compact with Gemini-I electron column) under different magnifications and energy dispersive X-ray spectroscopy (EDS) was carried out on Oxford instruments. The BET surface area analysis was done using Quanta chrome (Model: Quadrasorbevo 3), USA. The oxidation states of the constituent elements in the Al2O3-reduced graphene oxide hybrid were confirmed with the help of X-ray Photoelectron Spectroscopy (ULVAC PHI (Physical Electronics), USA (Model: PHI 5000 VERSA PROBE III)). High-Resolution Transmission Electron Microscopy (HR-TEM- JEOL JEM 2100 PLUS) analyses were performed for the Al2O3-reduced graphene oxide hybrid to find morphological, crystallographic, and compositional information.
Electrochemical characterization
As reported previously, the fabrication of the working electrode was done with the help of the slurry coating method on stainless-steel substrates21. The process was carried out by preparing a slurry, where the electroactive material (Al2O3 and Al2O3-reduced graphene oxide hybrid), AC, and PVDF (mixture weight ratio 85:10:5) were mixed and ground together with a viable proportion of N-methyl pyrrolidone (NMP) as the dispersing medium, using an agate-mortar. In the next step, the prepared slurry was coated on the surface of a pre-cleaned stainless-steel substrate of an area of 1 × 1 cm2 and was kept for drying in an electric oven. The mass loading in the case of both Al2O3 and Al2O3-reduced graphene oxide hybrid electrodes was ~ 1 mg each, which was calculated by noting the difference in the weights of the bare and the coated stainless-steel electrode, with the help of a precision weighing balance (AUW-220D, SHIMADZU).
For three electrode measurements, Al2O3 and Al2O3-reduced graphene oxide hybrid coated stainless steel served as the working electrode, while Ag/AgCl and platinum were used as the reference and counter electrodes, respectively. The Al2O3-reduced graphene oxide hybrid based 2-electrode symmetric device (SSD) was assembled by using a Whatman filter paper, which electrically isolates the two Al2O3-reduced graphene oxide hybrid electrodes. The electrochemical performances of the Al2O3 electrode, Al2O3-reduced graphene oxide hybrid electrode, and Al2O3-reduced graphene oxide hybrid symmetric cell device (SSD) were evaluated through potentiodynamic cyclic voltammetry (CV), galvanostatic charge–discharge (CD), and electrochemical impedance spectroscopy (EIS) techniques, using a multichannel Biologic electrochemical workstation (model no: VSP-300), by taking 1 M aqueous solution of Na2SO4 as the electrolyte. The gravimetric capacitance (Csp), specific energy (Es), and specific power (Ps) of the Al2O3 electrode, Al2O3-reduced graphene oxide hybrid electrode, and Al2O3-reduced graphene oxide hybrid SSD were evaluated via the following Eqs.21:
Here, “Csp” denotes the gravimetric capacitance (F g−1), “I” represents the applied current (A), “∆V” is the electrochemically stable potential range, “s” is the sweep rate (mV s−1), “∆t” is the discharge duration (s), and “m” is the mass-loading in gram.
Computational details
For electronic structure computations, we have implemented plane wave DFT package VASP(VIENNA ab initio simulation package) code22,23. The pseudopotentials employed for the present system were based on the projector-augmented wave (PAW) functional24. The GGA exchange-correlational functional was used to undertake ion–electron interactions25,26. For optimization and electronic calculations, the convergence criteria were set as 0.01 eV/Å and 10–5 eV for force and energy, respectively. For a plane-wave basis, kinetic energy cut-off was taken at 500 eV. The Brillouin zones were sampled using a K points mesh with the Monkhorst–Pack grid of 5 × 5 × 3 and 5 × 5 × 1 for bulk and surface optimization while a 7 × 7 × 3 and 7 × 7 × 1 for electronic energy calculations, respectively27.
Results and discussion
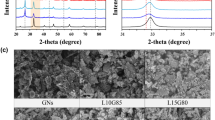
Figure 2a represents the Al2O3 and Al2O3-reduced graphene oxide hybrid XRD comparison chart. The X-ray diffraction pattern for reduced graphene oxide has been provided in Fig. S1. In the Al2O3 XRD pattern, the peaks at 25.77°, 35.36°, 38.06°, 43.65°, 52.75°, 57.64°, 61.54°, 66.73°, 68.33°, and 77.23°, are ascribed to (012), (104), (110), (113), (024), (116), (018), (214), (300), and (119) reflections of rhombohedral Al2O3 (PDF:43-1484), respectively, demonstrating the successful formation of the Al2O3 nanostructure28. In the case of the Al2O3-reduced graphene oxide hybrid, all the peaks are well matched with rhombohedral Al2O3 with an additional broad peak at 2θ = 23°. The peak at 2θ = 23° indicates the (002) plane of reduced graphene oxide sheets via the hydrothermal reduction method. The XRD pattern suggests the formation of high-purity and high-crystalline Al2O3 and Al2O3-reduced graphene oxide hybrid. X-ray photoelectron spectroscopy (XPS) was employed to examine the bonding situation and electronic states in the Al2O3-reduced graphene oxide hybrid29. Figure 2b represents the high-resolution XPS spectrum for Al 2p, showing a peak at 74.02 eV, which is ascribed to Al3+ in Al2O3. The peak observed (in Fig. 2c) at 531.5 eV is attributed to O 1 s, which is associated with bonds of different types, due to the presence of additional functional groups such as C=O (at ~ 531 eV), (CO*)OH (at ~ 532 eV), -OH (at ~ 533 eV)30. Likewise, the C 1 s high-resolution XPS spectrum (in Fig. 2d) is deconvoluted into two peaks corresponding to C–C (284.6 eV) and C–OH (286.5 eV). All these XPS spectra are in good agreement with previously reported work31,32. The BET isotherms of bare Al2O3 and Al2O3-reduced graphene oxide hybrid are illustrated in Fig. 2e,f. The BET isotherms exhibit the typical type (IV) adsorption–desorption isotherms, which is characteristic of mesoporous materials33,34. The BET surface areas of Al2O3-reduced graphene oxide hybrid and pristine Al2O3, respectively, are about 15.100 m2/g and 9.724 m2/g. The BET results indicate that the surface area of the Al2O3-reduced graphene oxide hybrid is 1.5 times higher than that of pristine Al2O3.
(a) Comparative XRD pattern of nanostructured Al2O3 and Al2O3-reduced graphene oxide hybrid. High-resolution XPS spectra for (b) Al 2p, (c) O 1 s, and (d) C 1 s in Al2O3-reduced graphene oxide hybrid. The Brunauer–Emmett–Teller (BET) analysis of pristine Al2O3 and Al2O3-reduced graphene oxide hybrid. The BET isotherms of (e) bare Al2O3 and (f) Al2O3-reduced graphene oxide hybrid.
The micro-scale surface investigation of the samples was done with the help of FE-SEM, which is depicted in Fig. 3. Figure 3a–c shows the FE-SEM micrograph of Al2O3, which reveals the formation of nanoparticles with a typical size in the range of 30–40 nm, evident through their respective high and low magnification micrographs. The FE-SEM micrographs for the Al2O3-reduced graphene oxide hybrid are provided in Fig. 3d–f and Fig. S2, indicating that Al2O3 nanoparticles are well decorated on the surface of the reduced graphene oxide nanosheet. The hybridisation of Al2O3, with reduced graphene oxide as a substrate, provides a highly conductive platform, enhancing the charge transfer kinetics during the electrochemical processes. The charge transfer occurs from highly conductive reduced graphene oxide to the Al2O3 nanoparticles, enhancing their pseudocapacitive performance, as evidenced by the obtained results35. The energy dispersive X-ray spectroscopy (EDX) and elemental mapping of Al2O3 and Al2O3-reduced graphene oxide hybrid have been illustrated in Figs. S3 and S4, respectively. The EDX spectroscopy indicates the presence of elements such as Al, O, and Al, O, C in Al2O3 and Al2O3-reduced graphene oxide hybrid nanostructure. The elemental mapping reveals the homogeneous distribution of elements such as Al, O (Fig. S3b–d) and Al, O, C (Fig. S4b–e) in the Al2O3 and Al2O3-reduced graphene oxide hybrid. Figure 4a–f represents the high-resolution transmission electron microscopic (HR-TEM) images (at various magnifications) and the elemental mapping obtained during the acquisition of the HR-TEM measurement of the Al2O3-reduced graphene oxide hybrid. The HR-TEM micrographs reveal that Al2O3 nanoparticles are decorated on reduced graphene oxide sheets, which agrees with the FE-SEM micrograph of the Al2O3-reduced graphene oxide hybrid. The inset of Fig. 4e shows the SAED pattern of the Al2O3-reduced graphene oxide hybrid. The existence of rings in the SAED pattern acknowledges that the Al2O3-reduced graphene oxide hybrid is polycrystalline36. The elemental mapping (Fig. 4f) indicates the uniform distribution of aluminium, oxygen, and carbon elements in the Al2O3-reduced graphene oxide hybrid.
HR-TEM micrographs of Al2O3-reduced graphene oxide hybrid taken at various magnifications (500 nm (a), 200 nm (b), 100 nm (c), 50 nm (d) and 10 nm (e)). The inset in (e) contains the SAED pattern of Al2O3-reduced graphene oxide hybrid. (f) Elemental mapping of Al2O3-reduced graphene oxide hybrid during acquisition of the HR-TEM measurment (shows the presence of aluminium, oxygen and carbon element).
Electrochemical characteristics of bare Al2O3 and Al2O3-reduced graphene oxide hybrid electrodes were compared in a standard three-electrode electrochemical set-up, using one molar aqueous solution of Na2SO4 as the electrolyte. Figure 5a represents the comparative CV profile of Al2O3 and Al2O3-reduced graphene oxide hybrid at a scan rate of 100 mV s−1, within a potential range of − 0.8 to 0.4 V (vs. Ag/AgCl). It is intriguing to note that the Al2O3-reduced graphene oxide hybrid electrode can work at a much higher current range than the Al2O3 electrode, demonstrating the improved electrochemical performance of the Al2O3-reduced graphene oxide hybrid electrode. The improvement in the electrochemical performance of the Al2O3-reduced graphene oxide hybrid electrode compared to the bare Al2O3 electrode is due to the additional charge adsorption that occurs in the presence of an EDLC component like reduced graphene oxide content besides the pseudocapacitive contribution from the Al2O3. Also, the Al2O3-reduced graphene oxide hybrid electrode possesses a better electrode and electrolyte interface37,38. Figure 5b shows the CV profile of the Al2O3-reduced graphene oxide hybrid electrode at various sweep rates (5–100 mV s−1) in the potential range of − 0.8 to 0.4 V (vs. Ag/AgCl). The CV profile of the Al2O3-reduced graphene oxide hybrid electrode takes a quasi-rectangular shape, indicating the effective intralayer charge transfer and a nearly capacitive behaviour39. The effect of scan rate on the specific capacitance of the Al2O3-reduced graphene oxide hybrid electrode is shown in Fig. 5c. The Al2O3-reduced graphene oxide hybrid electrode exhibits the highest gravimetric capacitance, 157.29 F g−1, at 5 mV s−1, which is ~ 15-fold higher than the bare Al2O3 electrode (11.06 F g−1 at 5 mV s−1). The specific capacitance of the Al2O3-reduced graphene oxide hybrid electrode reduces at faster potential sweep rates, which can be attributed to the slower reaction kinetics and sluggish ion transportation inside the electrolytic medium40,41. The Al2O3-reduced graphene oxide hybrid retains about 62.76% of its initial capacitance, even when the potential sweep rate is increased 20-fold (100 mV s−1), indicating better rate performance of the Al2O3-reduced graphene oxide hybrid electrode.
Electrochemical characterization of as synthesized Al2O3 and Al2O3-reduced graphene oxide hybrid. (a) Comparative cyclic voltammetric profiles of Al2O3 and Al2O3-reduced graphene oxide hybrid electrode recorded at a sweep rate of 100 mV s−1, (b) CV plots of Al2O3-reduced graphene oxide hybrid electrode recorded at different sweep rates, (c) effect of sweep rates on gravimetric capacitances of Al2O3-reduced graphene oxide hybrid electrode, (d) comparative charge–discharge profile of Al2O3 and Al2O3-reduced graphene oxide hybrid electrode recorded at applied current of 1 mA, (e) CD profile of Al2O3-reduced graphene oxide hybrid electrode recorded at various applied currents, (f) effect of applied currents on specific capacitances of Al2O3-reduced graphene oxide hybrid electrode, (g) comparative Nyquist plot Al2O3 and Al2O3-reduced graphene oxide hybrid electrode with inset shows the enlarged portion of the Nyquist plot of the Al2O3-reduced graphene oxide hybrid electrode, (h) Bode phase angle plot of Al2O3-reduced graphene oxide hybrid electrode, and (i) the cyclic stability performance of Al2O3-reduced graphene oxide hybrid electrode over 5000 cycles.
Moreover, Al2O3 and Al2O3-reduced graphene oxide hybrid electrodes' electrochemical behaviour was also quantitatively determined from the CD curves. Figure 5d represents the comparative CD profiles of Al2O3 and Al2O3-reduced graphene oxide hybrid electrodes, which indicate that Al2O3-reduced graphene oxide hybrid electrodes need higher charge and discharge times than Al2O3 electrodes, thus suggesting improved electrochemical performance. The CD characteristics of the Al2O3-reduced graphene oxide hybrid electrode at various applied currents (1–10 mA) are recorded and shown in Fig. 5e. The CD curves exhibit nearly symmetric behaviour, signifying excellent reversibility. The charge storage phenomenon is a combination of EDLC and pseudocapacitive behaviour of the Al2O3-reduced graphene oxide hybrid electrode, which agrees with the CV profile42. Figure 5f represents the effect of different applied currents on the specific capacitance. The Al2O3-reduced graphene oxide hybrid electrode obtained a maximum specific capacitance of about 159.28 F g−1 at an applied current of 1 mA, which is quite high compared to the bare Al2O3 electrode (10.38 F g−1). The electrochemical performance of the Al2O3-reduced graphene oxide hybrid electrode is found to be higher than various electroactive materials listed in Table S1.
EIS measurements were performed using Nyquist and Bode plots in the frequency-dependent mode to verify the superior electrochemical behaviour of the Al2O3-reduced graphene oxide hybrid electrode over the bare Al2O3 electrode43. Figure 5g shows the Nyquist plots for Al2O3 and Al2O3-reduced graphene oxide hybrid electrodes, recorded in the frequency range of 0.01 Hz to 100 kHz. It is visible that the Al2O3-reduced graphene oxide hybrid electrode has a lower Warburg region (the straight line at low frequency) compared to the bare Al2O3 electrode, with solution resistance (Rs) and charge transfer resistance (Rct) values, 1.33 Ω and 16.67 Ω, respectively. The Al2O3-reduced graphene oxide hybrid electrode obtained a short diffusion path length for ion transfer, which could be noticed from the low resistance of the capacitive part on the Nyquist plot44. The Nyquist plot reveals that the Al2O3-reduced graphene oxide hybrid electrode has a higher electrical conductivity than the bare Al2O3 electrode due to the inclusion of reduced graphene oxide, which greatly enhanced the overall electron transfer rate45. The Bode phase angle plot of the Al2O3-reduced graphene oxide hybrid electrode is shown in Fig. 5h. The Bode phase angle of the Al2O3-reduced graphene oxide hybrid electrode tail at the low-frequency region is about − 72.29°, which suggests that the charge storage occurs in terms of both EDLC and pseudocapacitive activities in the hybrid46. The electrode was then subject to continuous CD measurement for over 5000 cycles at a constant charge–discharge current of 7.5 mA, which is shown in Fig. 5i. The Al2O3-reduced graphene oxide hybrid electrode retained ~ 89% of the initial specific capacitance after 5000 CD cycles. To investigate the specific capacitance decay in the Al2O3-reduced graphene oxide hybrid electrode, we executed EIS measurement using the Nyquist plot before and after cyclic stability and presented in Fig. S5. There is a slight variation in the value of Rs (1.33 to 1.43) and Rct (16.67 to 23.07) values before and after the cyclic stability test, which may be the reason behind the observed capacitance decay. Detailed CV and CD tests were carried out on the bare Al2O3 electrode at various scan rates and normalised current values, as shown in Fig. S6. The variation of specific capacity to the applied current of bare Al2O3 electrode and Al2O3-reduced graphene oxide hybrid electrode is provided in Fig. S747,48. To further identify the charge storage or capacitance contribution by EDLC and pseudocapacitance process, the Trasatti plot of the bare Al2O3 electrode and Al2O3-reduced graphene oxide hybrid electrode has been provided in Fig. S849,50. The overall capacitance of the electroactive material can be ascribed to the combined effect of the surface adsorbed electric double-layer process and the diffusion-controlled pseudocapacitive process. In Fig. S8a,b, the y-intercept of the linear fit of 1/Csp vs. v1/2 at v = 0 indicates the total charge stored in the bare Al2O3 electrode and Al2O3-reduced graphene oxide hybrid electrode, respectively. Similarly, in Fig. S8c,d, the y-intercept of the linear fit of Ccsp vs. v-1/2 at v = ∞ illustrates the amount of charge stored due to the electric double-layer process in the bare Al2O3 electrode and Al2O3-reduced graphene oxide hybrid electrode, respectively. The pseudocapacitive contribution can be estimated by subtracting the electric double-layer capacitance from the overall capacitance. According to the Trasatti method (as shown in Fig. S8e), the pseudocapacitive contributions of the bare Al2O3 electrode and Al2O3-reduced graphene oxide hybrid electrode were found to be 80.25% and 50.74%.
In contrast, the electric double-layer contributions are 19.75% and 49.26%, respectively. The higher electrochemical performance of the Al2O3-reduced graphene oxide hybrid electrode, compared to the bare Al2O3 electrode, can be attributed to the constructive synergistic effects caused by the attachment of Al2O3 nanoparticles to the reduced graphene oxide nanosheets. In the Al2O3-reduced graphene oxide hybrid electrode, the open space inside the reduced graphene oxide nanosheets for Al2O3 nanoparticles offers an easy path for electrolyte diffusion during the electrochemical analysis, which leads to higher electronic and ionic conduction, resulting in higher electrochemical performance compared to bare Al2O3 electrode51,52.
A supercapacitor device was fabricated (in symmetric configuration), using Al2O3-reduced graphene oxide hybrid electrode as both the negative and positive electrodes to examine the potential of the Al2O3-reduced graphene oxide hybrid electrode for real-world applications. The device was tested in 1 M aqueous Na2SO4 electrolyte within the potential range of 1 V. The detailed electrochemical performances of the assembled symmetric device have been illustrated in Fig. 6. Figure 6a,b illustrates the CV profiles of Al2O3-reduced graphene oxide hybrid SSD, recorded at various sweep rates (5–500 mV s−1) in the potential range of 0.0–1.0 V. The CV profiles of Al2O3-reduced graphene oxide hybrid SSD demonstrate typical rectangular behaviour, which remains almost the same with the increase in the sweep rate from 5 to 500 mV s−1, indicating the capacitive characteristic53. The specific capacitance values of Al2O3-reduced graphene oxide hybrid SSD at various scan rates are shown in Fig. 6c. The highest specific capacitance of Al2O3-reduced graphene oxide hybrid SSD has been calculated to be about 9.34 F g−1 at the scan rate of 5 mV s−1. The CD curves of the Al2O3-reduced graphene oxide hybrid SSD are shown in Fig. 6d,e. Figure 6d and e represent the CD profiles of Al2O3-reduced graphene oxide hybrid SSD at a constant current of 1 mA and various applied currents from 0.25 to 2.50 mA. The CD profiles reveal that the charging and discharging profiles of CD curves possess good symmetry. The graph between gravimetric capacitance and different applied current values for Al2O3-reduced graphene oxide hybrid SSD is shown in Fig. 6f. It was observed that the highest specific capacitance of about 10.73 F g−1 can be obtained at an applied current of 0.25 mA. To further understand the charge transfer kinetics in the case of Al2O3-reduced graphene oxide hybrid SSD, EIS measurement was performed in the frequency range of 0.01–100 kHz, using the Nyquist and Bode plot. Figure 6g represents the Nyquist plot for Al2O3-reduced graphene oxide hybrid SSD, with an inset showing the Nyquist plot's enlarged view at the low-frequency region. The Nyquist analysis shows that the values of Rs and Rct are about 0.81 Ω and 23.55 Ω, respectively, indicating good electrical conductivity of the Al2O3-reduced graphene oxide hybrid SSD. The Bode plot (phase angle as a function of frequency) of Al2O3-reduced graphene oxide hybrid SSD is shown in Fig. S9. The Bode plot reveals that the phase angle of Al2O3-reduced graphene oxide hybrid SSD is about -67.90°, which signifies both EDLC and pseudocapacitive contributions46. To describe the specific energy and specific power characteristics of the Al2O3-reduced graphene oxide hybrid SSD, Fig. 6h represents the Ragone plot for the as-fabricated SSD. The Al2O3-reduced graphene oxide hybrid SSD obtained a maximum energy density of about 1.49 Wh kg−1 with a corresponding power density of 78.125 W kg−1. The power density of SSD increases up to 1.562 kW kg−1 with an increased applied current of 5 mA. The Ragone plot clearly indicates higher specific energy of Al2O3-reduced graphene oxide hybrid SSD compared to Ni2P (0.24 Wh kg−1)54, Cu3SbS4 (0.64 Wh kg−1), Cu3SbS3 (0.7 Wh kg−1), Cu12Sb4S13 (0.85 Wh kg−1)55, Mxene (0.089 Wh kg−1)56. Figure 6i represents the cyclic stability plot for Al2O3-reduced graphene oxide hybrid SSD, obtained at a constant current of 1 mA for over 10,000 cycles. The Al2O3-reduced graphene oxide hybrid SSD demonstrates excellent cyclic stability with a retention of 98.56%. The obtained capacitance retention (98.56%) of Al2O3-reduced graphene oxide hybrid SSD is higher compared to the previously reported SSD such as rGO-PMo12 (89–95%)57, Co3O4-VAGN (86.3%)58, MXene-reduced graphene oxide (90%)59, cobalt hexacyanoferrate-reduced graphene oxide (83%)60 and famatinite-reduced graphene oxide (95.5%)61. To support the excellent cyclic stability of Al2O3-reduced graphene oxide hybrid SSD, we performed EIS analyses before and after 10,000 cycles and found no such variation in Rs and Rct values after the cyclic stability test, as shown in Fig. S10. To further clarify the excellent cyclic stability, we have provided the first and last ten cycles of 10,000 cycles (as shown in Fig. S11), which reveals no such changes in the time duration of charging and discharging profiles of Al2O3-reduced graphene oxide hybrid SSD. To demonstrate the real-time application of the Al2O3-reduced graphene oxide hybrid, we have fabricated Al2O3-reduced graphene oxide hybrid based coin cell SSD and successfully powered a red LED by series connection (as shown in Fig. S12).
Electrochemical characterization of Al2O3-reduced graphene oxide hybrid symmetric cell SSD. Cyclic voltammetric profiles Al2O3-reduced graphene oxide hybrid symmetric cell SSD at different sweep rates (a) 150–500 mV s−1, (b) 5–100 mV s−1, (c) effect of sweep rates on gravimetric capacitances of Al2O3-reduced graphene oxide hybrid symmetric cell SSD of Al2O3-reduced graphene oxide hybrid symmetric cell SSD, (d) charge–discharge profile of Al2O3-reduced graphene oxide hybrid symmetric cell SSD recorded at applied current of 1 mA, (e) CD plots of Al2O3-reduced graphene oxide hybrid symmetric cell SSD recorded at various currents, (f) effect of applied currents on specific capacitances of Al2O3-reduced graphene oxide hybrid symmetric cell SSD, (g) Nyquist plot of Al2O3-reduced graphene oxide hybrid symmetric cell SSD with inset shows the enlarged view, (h) Ragone plot of Al2O3-reduced graphene oxide hybrid symmetric cell SSD with other reported SSD, and (i) cyclic stability performance of Al2O3-reduced graphene oxide hybrid symmetric cell SSD over 10,000 cycles.
Structure and electronic properties
We have considered Al2O3 having space group R-3C with the hexagonal unit cell containing 12 six-coordinated Al atoms and 18 four-coordinated O atoms. The relaxed bulk structure is displayed in Fig. 7a,b. DFT optimized lattice parameters are a = b = 4.80304 Ẳ, c = 13.11089 Ẳ, α = β = 90, γ = 120 degree. Computed lattice parameters are in good agreement with the experimental data from the literature, a = b = 4.77 Ẳ, c = 13.01 Ẳ62. Figure 7c presents the density of states for bulk Al2O3. The symmetry between up-spin and down-spin channels indicates a non-magnetic signature where a large gap at the Fermi level indicates that Al2O3 is a wide band gap insulator. The computed band gap employing GGA exchange–correlation functional is 5.95 eV, matching well with PBE-based theoretical data of 6.045 eV from literature63. But, it is lower than the experimental value of 8.8 eV as DFT underestimates the band gap and more sophisticated methods like hybrid functional calculations are needed to match experimental data64. Using the relaxed structure of bulk Al2O3, we have generated (113) surface of Al2O3 since it has the most intense XRD peak. Further, we allowed the (113) surface of Al2O3 to relax for minimum energy configuration, and Fig. 8a depicts the relaxed surface of Al2O3. From the relaxed (113) surface of the Al2O3 and reduced graphene oxide layer, we have generated a hybrid layer of Al2O3-reduced graphene oxide so that the lattice mismatch is negligible. The geometry-optimized hybrid layer of Al2O3-reduced graphene oxide is displayed in Fig. 8b. We have plotted the total electronic density of states for (113) plane Al2O3 and the hybrid layer of Al2O3-reduced graphene oxide in Fig. 8c. We can observe that the hybrid layer becomes metallic, and there is an enhancement in electronic states near the Fermi level, which indicates enhanced conductivity of the system.
(a) DFT optimized structure for generated (113) surface of Al2O3, (b) DFT optimized structure for hybrid layer Al2O3-reduced graphene oxide, (c) Total Density of States for (113) surface of Al2O3(upper panel) and for hybrid layer Al2O3-reduced graphene oxide (lower panel). Fermi level is at 0 eV and indicated by dotted line.
Orbital interaction and charge transfer
To find the orbital interactions between Al2O3 and reduced graphene oxide, in Fig. 9a–c, we have presented the partial density of states for the O 2p orbital of Al2O3 and C 2p orbital of reduced graphene oxide for the hybrid system and corresponding pristine cases. We can notice that for the O 2p orbital of Al2O3, there is an enhancement in the electronic states in the hybrid system compared to pure Al2O3. Similarly, for the C 2p orbital of reduced graphene oxide, there is a reduction in the electronic states near the Fermi level in the hybrid system compared to pristine reduced graphene oxide. This enhancement in states for O 2p orbital and reduction in states for C 2p orbital of reduced graphene oxide indicate that the interaction is due to charge transfer from reduced graphene oxide to Al2O3. To find the quantitative charge transfer, we have performed Bader charge analysis. According to Bader charge partitioning, Al2O3 has a gain of 0.38e charge in the supercell. The difference in the charge density between Al2O3-reduced graphene oxide and Al2O3 has been plotted in Fig. 9c to visualize the spatial variation of electronic charge. The charge-gaining region corresponds to Al2O3 and is denoted by red colour.
(a) Partial Density of States for O 2p orbital of Al2O3 for bare Al2O3 and hybrid Al2O3-reduced graphene oxide, (b) Partial Density of States for C 2p orbital of reduced graphene oxide for pristine reduced graphene oxide and hybrid Al2O3-reduced graphene oxide; Fermi level is at 0 eV and indicated by dotted line, (c) charge density plot for the charge density difference between Al2O3-reduced graphene oxide and Al2O3, (d) diffusion energy barrier of electrolytic ions in bare Al2O3 and hybrid Al2O3-reduced graphene oxide, (e) The change in the quantum capacitance with the variation in the electrode potential for in bare Al2O3 and Al2O3-reduced graphene oxide hybrid.
Diffusion energy barrier of electrolytic ions
We have also computed the diffusion energy barrier of the electrolytic ion Na+ in Al2O3 and in hybrid Al2O3-reduced graphene oxide. The lower the diffusion energy barrier, the higher the mobility and the better the charge transfer capability. We can see from Fig. 9d that the diffusion energy barrier for Na+ ions is lower for the hybrid Al2O3-reduced graphene oxide system, justifying the superior charge storage performance in the hybrid system compared to Al2O3.
Computation of quantum capacitance
Employing the electronic Density of States, we have calculated the quantum capacitance using the following relationship65.
In the above equation, D(E) denotes the density of states, \(\phi_{G}\) indicates electrode potential and the function, FT (E), is the thermal broadening which can be written as;
Figure 9e describes the variation between electrode potential and quantum capacitance. We can observe that for most of the electrode potential, the quantum capacitance is higher for the hybrid system Al2O3-reduced graphene oxide. Quantum capacitance is dominant for low dimensional systems, and the total capacitance is related to quantum capacitance through the relation66:
CEDL indicates electric double-layer capacitance, which depends on the electrode–electrolyte interfacial interaction. The increased quantum capacitance value in the hybrid material suggests higher charge storage performance for the Al2O3-reduced graphene oxide system than bare Al2O3.
Conclusions
In summary, nanostructured Al2O3 and Al2O3-reduced graphene oxide hybrid were successfully synthesized through a two-step method comprising hydrothermal and post-hydrothermal calcination processes. Al2O3 and Al2O3-reduced graphene oxide hybrid electrodes' supercapacitor performances were evaluated through both theoretical and experimental methods for the first time. Due to the synergistic effects of both EDLC and pseudocapacitance, the Al2O3-reduced graphene oxide hybrid obtained higher electrochemical performance than the bare Al2O3 electrode. Remarkably, the Al2O3-reduced graphene oxide hybrid electrodes-based SSD manifests state-of-the-art capacitance retention of 98.56%, even after 10,000 cycles. Furthermore, we have presented the electronic properties, diffusion energy barrier, and quantum capacitance for Al2O3 and Al2O3-reduced graphene oxide hybrid using Density Functional Theory simulations. The interaction between Al2O3 and reduced graphene oxide is due to the charge transfer from reduced graphene oxide to Al2O3. The hybrid structure exhibits higher quantum capacitance and lower diffusion energy barrier for the electrolytic ions, which contribute to improved charge storage performance for the hybrid Al2O3-reduced graphene oxide system supporting our experimental observation. Therefore, this research might give some insights into exploring low-cost advanced electroactive materials for promising applications in energy storage devices with state-of-the-art performance metrics.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Kumar, S. et al. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 403, 126352 (2021).
Patra, A. et al. Understanding the charge storage mechanism of supercapacitors: In situ/operando spectroscopic approaches and theoretical investigations. J. Mater. Chem. A 9, 25852–25891 (2021).
Kandasamy, M., Sahoo, S., Nayak, S. K., Chakraborty, B. & Rout, C. S. Recent advances in engineered metal oxide nanostructures for supercapacitor applications: Experimental and theoretical aspects. J. Mater. Chem. A 9, 17643–17700 (2021).
Zhao, J. & Burke, A. F. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 59, 276–291 (2021).
Sahoo, S., Ratha, S., Rout, C. S. & Nayak, S. K. Self-charging supercapacitors for smart electronic devices: A concise review on the recent trends and future sustainability. J. Mater. Sci. 2, 1–42 (2022).
Xu, Y., Lu, W., Xu, G. & Chou, T.-W. Structural supercapacitor composites: A review. Compos. Sci. Technol. 204, 108636 (2021).
Hussain, I. et al. Binder-free trimetallic phosphate nanosheets as an electrode: Theoretical and experimental investigation. J. Power Sources 513, 230556 (2021).
Hussain, I. et al. An oriented Ni–Co-MOF anchored on solution-free 1D CuO: Ap–n heterojunction for supercapacitive energy storage. J. Mater. Chem. A 9, 17790–17800 (2021).
Ramesh, S., Khandelwal, S., Rhee, K. Y. & Hui, D. Synergistic effect of reduced graphene oxide, CNT and metal oxides on cellulose matrix for supercapacitor applications. Compos. Part B Eng. 138, 45–54 (2018).
Krishnan, S. G., Arulraj, A., Khalid, M., Reddy, M. V. & Zhang, R. Energy storage in metal cobaltite electrodes: Opportunities & challenges in magnesium cobalt oxide. Renew. Sustain. Energy Rev. 141, 110798 (2021).
Yar, A., Krishnan, S. G., Dennis, J. O., Khalid, M. & Jose, R. Template-assisted electrodeposited cupric oxide nanotubes and hierarchical nanospikes for tailoring electrode-electrolyte interfacial charge transfer. Ceram. Int. 47, 34732–34739 (2021).
Huang, Y. et al. Hierarchically mesostructured aluminum current collector for enhancing the performance of supercapacitors. ACS Appl. Mater. Interfaces 10, 16572–16580 (2018).
Hong, S., Bae, J., Koo, B. & Kim, Y.-B. High-performance ultra-thin film solid oxide fuel cell using anodized-aluminum-oxide supporting structure. Electrochem. commun. 47, 1–4 (2014).
Kedzierski, M. A., Brignoli, R., Quine, K. T. & Brown, J. S. Viscosity, density, and thermal conductivity of aluminum oxide and zinc oxide nanolubricants. Int. J. Refrig. 74, 3–11 (2017).
Wei, N., Hu, J., Zhang, M., He, J. & Ni, P. Cross-linked porous polymer separator using vinyl-modified aluminum oxide nanoparticles as cross-linker for lithium-ion batteries. Electrochim. Acta 307, 495–502 (2019).
Di, S., Gong, L. & Zhou, B. Precipitated synthesis of Al2O3-ZnO nanorod for high-performance symmetrical supercapacitors. Mater. Chem. Phys. 253, 123289 (2020).
Maharana, B. et al. High charge-storage performance of morphologically modified anatase TiO2: Experimental and theoretical insight. Phys. Rev. Appl. 15, 34013 (2021).
Sahoo, S., Sahoo, G., Jeong, S. M. & Rout, C. S. A review on supercapacitors based on plasma enhanced chemical vapor deposited vertical graphene arrays. J. Energy Storage 53, 105212 (2022).
Zhao, B. et al. A high-energy, long cycle-life hybrid supercapacitor based on graphene composite electrodes. Energy Storage Mater. 7, 32–39 (2017).
Xiong, C. et al. The recent progress on three-dimensional porous graphene-based hybrid structure for supercapacitor. Compos. Part B Eng. 165, 10–46 (2019).
Sahoo, S. et al. Hydrothermally synthesized chalcopyrite platelets as electrode material for symmetric supercapacitors. Inorg. Chem. Front. 7, 1492–1502 (2020).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. b 59, 1758 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46, 6671 (1992).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188 (1976).
Li, Z., Wu, K., Cao, J. & Wang, Y. Controlled synthesis of α-Al2O3 via the hydrothermal-pyrolysis method. in IOP Conference Series: Materials Science and Engineering vol. 207 12004 (IOP Publishing, 2017).
Madito, M. J. Correlation of the graphene fermi-level shift and the enhanced electrochemical performance of graphene-manganese phosphate for hybrid supercapacitors: Raman spectroscopy analysis. ACS Appl. Mater. Interfaces 13, 37014–37026 (2021).
Kwan, Y. C. G., Ng, G. M. & Huan, C. H. A. Identification of functional groups and determination of carboxyl formation temperature in graphene oxide using the XPS O 1s spectrum. Thin Solid Films 590, 40–48 (2015).
Jiang, Z., Wang, J., Meng, L., Huang, Y. & Liu, L. A highly efficient chemical sensor material for ethanol: Al2O3/Graphene nanocomposites fabricated from graphene oxide. Chem. Commun. 47, 6350–6352 (2011).
Jankovský, O. et al. Towards highly electrically conductive and thermally insulating graphene nanocomposites: Al2O3–graphene. RSC Adv. 4, 7418–7424 (2014).
Deng, L., Liu, J., Ma, Z., Fan, G. & Liu, Z. Free-standing graphene/bismuth vanadate monolith composite as a binder-free electrode for symmetrical supercapacitors. RSC Adv. 8, 24796–24804 (2018).
Rameshbabu, R., Vinoth, R., Navaneethan, M., Hayakawa, Y. & Neppolian, B. Fabrication of Cu2MoS4 hollow nanotubes with rGO sheets for enhanced visible light photocatalytic performance. CrystEngComm 19, 2475–2486 (2017).
Sahoo, S., Krishnamoorthy, K., Pazhamalai, P., Mariappan, V. K. & Kim, S.-J. Copper molybdenum sulfide nanoparticles embedded on graphene sheets as advanced electrodes for wide temperature-tolerant supercapacitors. Inorg. Chem. Front. 6, 1775–1784 (2019).
Mariappan, V. K. et al. Two dimensional famatinite sheets decorated on reduced graphene oxide: A novel electrode for high performance supercapacitors. J. Power Sources 433, 555 (2019).
He, W. et al. Ultrathin and porous Ni3S2/CoNi2S4 3D-network structure for superhigh energy density asymmetric supercapacitors. Adv. Energy Mater. 7, 1700983 (2017).
Stoller, M. D. & Ruoff, R. S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 3, 1294–1301 (2010).
Peng, L. et al. Ultrathin two-dimensional MnO2/graphene hybrid nanostructures for high-performance, flexible planar supercapacitors. Nano Lett. 13, 2151–2157 (2013).
Vinoth, S., Subramani, K., Ong, W.-J., Sathish, M. & Pandikumar, A. CoS2 engulfed ultra-thin S-doped g-C3N4 and its enhanced electrochemical performance in hybrid asymmetric supercapacitor. J. Colloid Interface Sci. 584, 204–215 (2021).
Dubal, D. P., Abdel-Azeim, S., Chodankar, N. R. & Han, Y.-K. Molybdenum nitride nanocrystals anchored on phosphorus-incorporated carbon fabric as a negative electrode for high-performance asymmetric pseudocapacitor. iScience 16, 50–62 (2019).
Liu, Y., Jiang, S. P. & Shao, Z. Intercalation pseudocapacitance in electrochemical energy storage: Recent advances in fundamental understanding and materials development. Mater. Today Adv. 7, 100072 (2020).
Navalpotro, P., Anderson, M., Marcilla, R. & Palma, J. Insights into the energy storage mechanism of hybrid supercapacitors with redox electrolytes by electrochemical impedance spectroscopy. Electrochim. Acta 263, 110–117 (2018).
Baig, M. M., Gul, I. H., Baig, S. M. & Shahzad, F. The complementary advanced characterization and electrochemical techniques for electrode materials for supercapacitors. J. Energy Storage 44, 103370 (2021).
Singh, K. P., Bhattacharjya, D., Razmjooei, F. & Yu, J.-S. Effect of pristine graphene incorporation on charge storage mechanism of three-dimensional graphene oxide: superior energy and power density retention. Sci. Rep. 6, 31555 (2016).
Qi, J. L. et al. Vertically oriented few-layer graphene-nanocup hybrid structured electrodes for high-performance supercapacitors. J. Mater. Chem. A 3, 12396–12403 (2015).
Hussain, I., Hussain, T., Lamiel, C. & Zhang, K. Turning indium oxide into high-performing electrode materials via cation substitution strategy: Preserving single crystalline cubic structure of 2D nanoflakes towards energy storage devices. J. Power Sources 480, 228873 (2020).
Hussain, I. et al. Integration of CuO nanosheets to Zn-Ni-Co oxide nanowire arrays for energy storage applications. Chem. Eng. J. 413, 127570 (2021).
Sathiya, M., Prakash, A. S., Ramesha, K., Tarascon, J. M. & Shukla, A. K. V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J. Am. Chem. Soc. 133, 16291–16299 (2011).
Sankar, K. V., Selvan, R. K. & Meyrick, D. Electrochemical performances of CoFe2O4 nanoparticles and a rGO based asymmetric supercapacitor. RSC Adv. 5, 99959–99967 (2015).
Ma, W. et al. Hierarchical MnO2 nanowire/graphene hybrid fibers with excellent electrochemical performance for flexible solid-state supercapacitors. J. Power Sources 306, 481–488 (2016).
Naderi, H. R., Norouzi, P. & Ganjali, M. R. Electrochemical study of a novel high performance supercapacitor based on MnO2/nitrogen-doped graphene nanocomposite. Appl. Surf. Sci. 366, 552–560 (2016).
Zhang, S. & Pan, N. Supercapacitors performance evaluation. Adv. Energy Mater. 5, n/a-n/a (2015).
Du, W. et al. New asymmetric and symmetric supercapacitor cells based on nickel phosphide nanoparticles. Mater. Chem. Phys. 165, 207–214 (2015).
Ramasamy, K. et al. Layered ternary sulfide CuSbS2 nanoplates for flexible solid-state supercapacitors. J. Mater. Chem. A 3, 13263–13274 (2015).
Rakhi, R. B., Ahmed, B., Hedhili, M. N., Anjum, D. H. & Alshareef, H. N. Effect of postetch annealing gas composition on the structural and electrochemical properties of Ti2CT x MXene electrodes for supercapacitor applications. Chem. Mater. 27, 5314–5323 (2015).
Dubal, D. P., Suarez-Guevara, J., Tonti, D., Enciso, E. & Gomez-Romero, P. A high voltage solid state symmetric supercapacitor based on graphene–polyoxometalate hybrid electrodes with a hydroquinone doped hybrid gel-electrolyte. J. Mater. Chem. A 3, 23483–23492 (2015).
Liao, Q., Li, N., Jin, S., Yang, G. & Wang, C. All-solid-state symmetric supercapacitor based on Co3O4 nanoparticles on vertically aligned graphene. ACS Nano 9, 5310–5317 (2015).
Yang, Q. et al. MXene/graphene hybrid fibers for high performance flexible supercapacitors. J. Mater. Chem. A 5, 22113–22119 (2017).
Zhang, X. et al. A flexible and high voltage symmetric supercapacitor based on hybrid configuration of cobalt hexacyanoferrate/reduced graphene oxide hydrogels. Chem. Eng. J. 335, 321–329 (2018).
Mariappan, V. K. et al. Two dimensional famatinite sheets decorated on reduced graphene oxide: A novel electrode for high performance supercapacitors. J. Power Sources 433, 126648 (2019).
d’Amour, H., Schiferl, D., Denner, W., Schulz, H. & Holzapfel, W. B. High-pressure single-crystal structure determinations for ruby up to 90 kbar using an automatic diffractometer. J. Appl. Phys. 49, 4411–4416 (1978).
Santos, R. C. R., Longhinotti, E., Freire, V. N., Reimberg, R. B. & Caetano, E. W. S. Elucidating the high-k insulator α-Al2O3 direct/indirect energy band gap type through density functional theory computations. Chem. Phys. Lett. 637, 172–176 (2015).
Bortz, M. L. & French, R. H. Optical reflectivity measurements using a laser plasma light source. Appl. Phys. Lett. 55, 1955–1957 (1989).
Yang, G. M., Zhang, H. Z., Fan, X. F. & Zheng, W. T. Density functional theory calculations for the quantum capacitance performance of graphene-based electrode material. J. Phys. Chem. C 119, 6464–6470 (2015).
Zhan, C., Neal, J., Wu, J. & Jiang, D. Quantum effects on the capacitance of graphene-based electrodes. J. Phys. Chem. C 119, 22297–22303 (2015).
Acknowledgements
This work has been financially supported by the National Aluminium Company Limited (NALCO) via grant no. RP-274. Surjit Sahoo acknowledges the DST-SERB for the National Post-Doctoral Fellowship (Grant No. PDF/2020/000620). BC thanks Dr. Nandini Garg, Dr. T. Sakuntala, Dr. S.M. Yusuf, and Dr. A K Mohanty for their support and encouragement. BC also acknowledges the BARC computer center supercomputing facility and its staff for continuous help and support.
Author information
Authors and Affiliations
Contributions
S.R.: conceptualization, writing the original draft and editing; S.S.: conceptualization, editing the draft; P.M.: computational investigation and editing; B.P.: conceptualization and editing; B.S.: technical discussions; B.C.: conceptualization, project administration, supervision; S.K.N.: conceptualization, project administration, funding acquisition, supervision. All the authors have reviewed the manuscript prior to submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ratha, S., Sahoo, S., Mane, P. et al. Experimental and computational investigation on the charge storage performance of a novel Al2O3-reduced graphene oxide hybrid electrode. Sci Rep 13, 5283 (2023). https://doi.org/10.1038/s41598-022-23574-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23574-2
This article is cited by
-
Spectroscopic, Hartree–Fock and DFT study of the molecular structure and electronic properties of functionalized chitosan and chitosan-graphene oxide for electronic applications
Optical and Quantum Electronics (2024)
-
Flexible and sewable electrode based on Ni-Co@PANI-salphen composite-coated on textiles for wearable supercapacitor
Scientific Reports (2023)
-
Silver sulfide nanosheets: a proficient electrode material for energy storage
Ionics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.