Abstract
The clinical relevance of pulmonary non-tuberculous mycobacteria (PNTM) in The Gambia is unknown. The aim of this study was to estimate the prevalence of non-tuberculous mycobacteria (NTM) in colonisation, and the burden of clinically relevant pulmonary NTM (PNTM) disease in The Gambia. This was a cross-sectional study of the prevalence of NTM in participants aged ≥ 15 years, in a nationwide tuberculosis (TB) prevalence survey between December 2011 and January 2013. We enrolled 903 participants with suspected NTM and NTM cultures were confirmed by 16S rRNA gene sequencing analyses. We applied the American Thoracic Society/Infectious Disease Society of America (ATS/IDSA) diagnostic criteria to determine clinical relevance of NTM. A total of 575 participants had acid-fast bacilli (AFB) positive Mycobacterial Growth Indicator Tube (MGIT) cultures and 229 (39.8%) were NTM. M. avium complex was by far the most isolated NTM (71.0%), followed by M. fortuitum (9.5%) and M. nonchromogenicum (2.9%). Older participants (> 24 years old) were four times more likely to have NTM in their sputa. Only 20.5% (9/44) NTM cases met the ATS/IDSA criteria for NTM disease. This study provides important data on the prevalence of NTM in pulmonary samples of suspected TB cases with AFB positive cultures from a nationally representative population in The Gambia. Enhanced PNTM surveillance is recommended to better understand the contribution of NTM to pulmonary disease.
Similar content being viewed by others
Introduction
A very large number of potentially pathogenic and non-pathogenic mycobacterial species other than the better-known Mycobacterium tuberculosis complex (MTBC) and Mycobacterium leprae are designated NTM1. They are ubiquitous in nature and diverse in their virulence, pathogenicity, and growth patterns2,3,4. Over 175 species of NTM have been described and the majority are very rarely implicated in clinical disease. NTM disease in humans is typically pulmonary (80–90% of all NTM disease) but other manifestations occur such as lymphadenitis in children, cutaneous disease and other extra-pulmonary or disseminated infections5.
The most common group of NTM in colonisation and disease is the Mycobacterium avium complex (M. intracellulare and M. avium)6. The Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS) epidemic brought about great interest in the contributions of NTM opportunistic infections to morbidity in the immunocompromised host.
Most of the clinical and epidemiological data on the contribution of NTM to the aetiology of TB-like pulmonary disease are from high-income, low-tuberculosis burden settings5,7,8. In contrast, misdiagnosis and inappropriate treatment of PNTM disease as active pulmonary tuberculosis (PTB) may be a common challenge in many TB endemic countries9, including The Gambia. Despite increasingly emerging data, the epidemiology of prevalent NTM in low and middle-income countries (LMICs), also endemic for MTBC disease, is less known because of the limited availability of diagnostic modalities. In contrast to PTB, PNTM cannot be readily diagnosed using conventional laboratory tools. The culture and molecular identification techniques required for NTM diagnosis are also not readily available in resource-poor health systems where priority is given to expanding access to diagnosis and treatment of PTB4,10.
The American Thoracic Society/Infectious Disease Society of America (ATS/IDSA) has developed clinical, microbiologic, and radiologic criteria for identifying clinically relevant disease when NTM are isolated from pulmonary samples5. However, accurate assessment of true PNTM disease prevalence in many LMICs is constrained by the difficulty in obtaining complete microbiology, radiographic and clinical data.
In a recent review, the prevalence of NTM in pulmonary samples in sub-Saharan Africa was 7.5% (95% CI 7.2–7.8%) and M. avium complex was the most common NTM9. The prevalence of M. avium complex was five times higher in persons previously treated for PTB and/or living with HIV, while M. kansasii was mostly responsible for confirmed PNTM disease. NTM colonisation in presumptive TB cases, persons previously treated for TB or with chronic lung disease, may result in over diagnosis of PTB especially when smear microscopy is the only available diagnostic modality11,12,13.
Using the platform provided by Gambia’s first nationwide TB prevalence survey11 we investigated the prevalence and clinical relevance of NTM in pulmonary samples from a nationally representative population sample and describe the molecular epidemiology of NTM in the Gambia.
Results
Participant characteristics
Of the 903 participants with suspected NTM in the parent study, samples from 575 (63.7%) participants had positive MGIT cultures and 229 (39.8%) of these yielded NTM as shown in the CONSORT diagram (Fig. 1). The median age (Interquartile range [IQR]) of participants was 55 (40–70) years, majority (57.2%) were females and mostly (69.6%) inhabited rural parts of the country. There was no significant difference (p = 0.33) in the distribution of clinical symptoms between the overall population and those positive for NTM (see Table 1).
Pulmonary NTM prevalence and risk factors
We estimated the prevalence of NTM in AFB positive/MPT64 negative pulmonary samples using the two statistical approaches described as shown in Table 2. The lowest prevalence estimate (25.4% [95% CI 8.8–18.4]) was seen with the most conservative model (model 2). This was the lowest limit of NTM prevalence estimate, if all unknown samples from 328 participants were negative. The prevalence estimate from model 1 (39.8% [95% CI 35.8–44.0]) in this case was the better option because it used most of the available data.
Stratification of NTM results by the different participant categories showed that the group without respiratory symptoms and normal CXR had the highest proportion of NTM positive participants compared to other groups (Table 3). It was also the smallest group, n = 16. However, these observed differences were not significant (p = 0.23).
Univariate and multivariable regression analyses are shown in Table 4. In the adjusted regression model, the odds of a positive NTM result were approximately 4 times higher in the 25–44-year-olds and significantly higher in the > 65 age group (p = 0.003) compared to the youngest age group and females were 1.5 times more likely to be NTM positive than males (Table 4). Figure 2 displays the different geographical regions of the Gambia. Location was also significantly associated with an NTM positive culture with residents of Central River Region almost two times more likely to have NTM than Upper River Region residents, while those in Lower River were nearly half as likely as Upper River residents to have a positive NTM result.
Map showing The Gambia’s six regions. Map was created using The Gambia’s administrative national and subnational boundaries shapefile downloaded from https://data.humdata.org/dataset/cod-ab-gmb?—an opensource humanitarian data exchange using QGIS version 3.24.
Culture results and Mycobacterium species identification and distribution
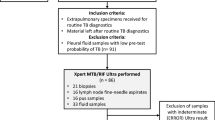
Figure 3 is the flowchart showing all individual samples processed for Mycobacterial speciation. There were 1145 samples from 903 participants with suspected PNTM. Sequences from 91.7% (278/303) of samples amplified as Mycobacteria were included in the final analysis. Almost all, 98.2% (273/278) of them were identified as NTM while 1.8% (5/278) cultures yielded additional MTBC. Nearly a tenth (20/273) of NTM in the study could not be characterised to species level (see Fig. 3).
Sequence data analyses
The phylogenetic tree (Fig. 4) constructed using the NTM sequences obtained shows 71.1% (194/273) of all NTM sequences in this study clustered with M. avium and M. intracellulare (M. avium complex) reference strains while 10.0% (26/273) clustered with M. fortuitum and M. boenickei (M. fortuitum complex). Other NTM identified—M. nonchromogenicum, M. thermoresistible, M. terrae and M. flavescens, were 100% concordant in clustering with respective reference strains. However, 20 NTM sequences could not be identified especially as they did not cluster with any of the NTM and MTBC reference sequences. The five MTBC strains identified in the study also clustered with MTBC reference strains. Table 5 displays the Mycobacteria species identified, stratified by the different participant groupings in the present study.
Phylogenetic tree of NTM in The Gambia based on 16S rRNA gene sequence analysis of regions A and B of the mycobacteria gene. Red coloured fonts represent unknown sequences from the present study while fonts in black colour represent NTM and MTBC reference sequences of ATCC strains downloaded from GenBank.
Prevalent pulmonary non-tuberculous mycobacteria disease
On applying the ATS/IDSA criteria to determine the clinical relevance of isolated PNTM to the 44 of 229 (19.2%) participants who grew NTM in both sputa, only 9 of 44 (20.5%) had PNTM disease, and all were caused by MAC. These nine patients met the full criteria for confirmed PNTM disease as summarised in Table 6. One participant who grew M. fortuitum in both sputa but did not meet clinical criteria for PNTM because they had a normal CXR. All other participants not meeting the ATS/IDSA criteria were classified as being colonised by NTM.
Discussion
In this study we have described the epidemiology and population biology of NTM in colonisation and disease from a nationally representative population sample in a West African country.
We estimated the prevalence of NTM in pulmonary samples amongst AFB culture positives/MPT64 negative strains using two statistical approaches but settled for the complete case analysis derived estimate (39.8% [95% CI 35.8–44.0]) because it used most of the available data in the present study.
The availability of detailed clinical, radiological, and microbiologic data enabled the discrimination of pulmonary NTM carriage from disease through the application of the ATS/IDSA diagnostic criteria. When we separated the proportion of participants with NTM in both samples from those with NTM of same species in both samples, we found only 20.5% (9/44) had confirmed PNTM disease which were caused by the same NTM species—MAC, that was also the most prevalent in colonisation. This study was executed using the accepted standards for identification of NTM at every step, from culture to identification by 16S rRNA gene analysis techniques optimised for this study14.
PNTM colonisation and disease rates vary globally. In North America and Europe colonisation and disease in the general population range from approximately 0.1–2/100,000 persons and 1–15/100,000 persons, respectively. However, rates are largely unknown for many countries in sub-Saharan Africa where there is a high burden of active MTBC disease15. In this study, the estimated prevalence of PNTM regardless of clinical relevance, was 39.8% which is higher than reported from Nigeria, Ghana, Zambia, Mozambique, Mali and Zimbabwe16,17,18,19,20. However, of all these other studies, only the Zambian and Zimbabwean studies with PNTM prevalence of 15.8% and 16.5%, respectively were also surveys of nationally representative population samples. Estimating the prevalence of PNTM in colonisation and disease is also hampered by the widely varied methods used in the limited number of published studies from sub-Saharan Africa.
The top three NTM isolated in this study (MAC, M. fortuitum, and M. nonchromogenicum) similar to reports from other African and high-income low MTBC countries where MAC was the predominant NTM species in pulmonary samples8,15,16. Similar to Hoefsloot et al.21, rapidly growing NTM (M. fortuitum, M. abscessus) accounted for a tenth of all NTM in our study. While in contrast to European and Canadian data22, no M. malmoense and M. xenopi were found in our study.
Distinguishing between members of the MAC family (M. intracellulare and M. avium) was incomplete in our study suggesting higher diversity in the regions A and B of the 16S rRNA gene of Gambian MAC strains compared to those isolated elsewhere. Further evidence in support of a higher NTM diversity in our setting, was the absence of 16S rRNA gene sequences in the National Centre for Biotechnology Information (NCBI) database for 7.3% of NTM in our study. This hypothesis requires further investigation using techniques of higher discriminatory power such as Whole Genome Sequencing.
Location was associated with increased odds of NTM in our study as has been reported by other investigators12. Similarly, higher risks for environmentally acquired pulmonary mycobacterial infections have also been previously reported with high soil exposure, individuals with occupational exposure to dust, and in women with high rates of biomass fuel exposure in low-income countries—all of whom are typically based in rural areas23. The areas of residence associated with NTM in our study are consistent with the aforementioned scenarios. Other commonly described risk factors for NTM in industrialised countries include structural lung damage (due to chronic obstructive pulmonary disease, cystic fibrosis, cumulative exposure to tobacco, prior PTB) and immunosenescence7,8. Increasing age and female sex were also risk factors for NTM as reported elsewhere22. The prevalence of ATS/IDSA confirmed PNTM was ~ 2.4 times higher in patients ≥ 50 years in one series in the US and others have found the same association for colonisation. Female sex was positively associated with NTM in this study and the literature suggests the epidemiology of NTM infection has changed over the last three decades to affect women more frequently than men. The reasons for this sex differential are unknown.
HIV is a major risk factor for PNTM and disseminated MAC infections were commonplace in high- and low-income settings before the widespread use of antiretroviral therapy24,25. Although we could not explore HIV as a risk factor for NTM because this data was not available, the relatively low HIV prevalence of 1.8% [1.4–2.3] in the general population at the time of the parent study suggests HIV co-infection is most likely low13. The TB prevalence survey was not primarily designed to study NTM which may be one reason for the low numbers of ATS/IDSA confirmed PNTM cases. The clinical relevance of NTM in younger children could not be ascertained as only participants aged 15 years or more were recruited in the parent study. Although PNTM is predominantly reported in older age groups, it is believed to affect children as well, but the epidemiology remains poorly described17.
WGS techniques with higher discriminatory power would have been useful for full identification of the NTM and for differentiating MAC species in this study.
In conclusion, this paper describes the contribution of NTM to colonisation and pulmonary disease in The Gambia. Only a fifth of all NTM positive participants met the rigorous ATS/IDSA diagnostic criteria confirmed disease. The high colonisation rates of NTM in pulmonary samples may contribute to the over diagnosis of PTB, especially in those with abnormal chest radiographs and where access to culture or PCR-based diagnostics do not exist as is the case in the majority of settings in The Gambia. At the time of the parent study, access to PCR diagnosis and liquid TB cultures was limited to one research laboratory in the entire country. The situation is different now with the availability of GeneXpert devices and TB culture capacity in the National Public Health Laboratory. More research and surveillance is needed to investigate the full contribution of NTM to pulmonary disease, particularly in high risk groups, and of NTM colonisation to overdiagnosis and treatment of PTB.
Materials and methods
Study participants and samples
Subjects were recruited as part of the Gambian national TB prevalence study, a multistage cluster survey to estimate the burden of smear positive and bacteriologically confirmed PTB, conducted between December 2011 and January 2013. During the survey, one or more sputum samples were obtained from participants with respiratory symptoms and/or abnormal chest X-ray suspected of PTB. Smear microscopy, decontamination, liquid cultures, and isolation of MTBC were performed using recommended standards and techniques as previously described11. The final radiological diagnosis was determined through consensus by a pulmonologist and radiologist.
From the parent survey, 903 suspected cases of NTM were identified from AFB positive cultures that tested negative for MTBC with the rapid identification assay BD MGIT™ TBc Identification (Becton, Dickinson and Company, USA). This assay tests for the MTBC-specific Mycobacterium Protein Target 64 (MPT64) antigen. All 903 were selected for inclusion in our study (Fig. 1). Following retrieval of stored sputa, only samples for 575 (63.7%) of 903 participants yielded AFB positive cultures, all of which were selected for analysis.
The study population was categorized into four categories: 1—no respiratory symptoms with abnormal chest X-ray (CXR); 2—no respiratory symptoms with normal CXR; 3—respiratory symptoms with abnormal CXR, and 4—respiratory symptoms with normal CXR. Participants with NTM positive samples were assessed for clinically relevant disease by application of the ATS/IDSA diagnostic criteria and classified as confirmed, probable, suspected and non-PNTM disease.
Laboratory procedures for NTM identification
All laboratory procedures were performed at the TB Reference Laboratory, Medical Research Council Unit The Gambia at the London School of Hygiene & Tropical Medicine (MRCG at LSHTM). Sputa corresponding to AFB positive cultures and MPT64 rapid test negative in the original prevalence survey, were retrieved. Briefly, 0.5 mL of stored, decontaminated sputum samples from each suspected NTM case were cultured in Mycobacteria Growth Indicator Tubes (MGIT) supplemented with 0.8 mL of BACTEC™ MGIT™ growth supplement and BBL™ MGIT™ PANTA™ antibiotic26. MGIT tubes were incubated at 37 °C in the automated BACTEC MGIT 960™ system (Becton Dickinson Diagnostic Instrument Systems, Franklin Lakes, United States of America) until flagged as positive. Samples that failed to show any growth after 42 days of incubation in the machine were removed and classified as negative based on the manufacturer’s protocol. All cultures confirmed as acid-fast bacilli (AFB) with Ziehl–Neelsen (ZN) staining were classified as suspected NTM while non-AFB cultures were considered contaminants and excluded from the study.
16S rRNA gene PCRs and sequencing
Mycobacterial deoxyribonucleic acid (DNA) was extracted from MGIT cultures for 16S rRNA gene Polymerase Chain Reactions (PCR) by the boiled lysate method described by Aldous et al.27. Briefly, 0.5 mL of culture was centrifuged at 10,000 rpm for 15 min. The resulting pellets were resuspended in 0.1 mL sterile Tris ethylene diamine tetra acetic acid (TE) buffer® (Sigma Aldrich, St. Louis, Missouri, United States) and heated to 99 °C in a heat block for 20 min followed by sonication for 15 min. Tubes were spun at 14,000 rpm for 5 min following which the supernatant was used for mycobacteria species-specific 16S rRNA PCRs and 2.5 μL of extracted DNA was added to 22.5 μL of master mix. We used primer pairs P1 (TGCTTAACACATGCAAGTCG) and P2 (TCTCTAGACGCGTCCTGTGC) to amplify regions A and B of the mycobacteria 16S rRNA gene. The PCR comprised a denaturation step of 5 min at 95 °C, then 45 cycles of 95 °C for 45 s, 56 °C for 45 s, 72 °C for 45 s and a final extension step at 72 °C for 10 min. Amplified DNA products were viewed on a 1% (w/v) agarose gel stained with 500 ng/μL ethidium bromide.
16S rRNA gene sequencing was performed on purified PCR products by the Sanger chain-termination DNA sequencing method28. The obtained sequences were edited and analysed in BioEdit version 7.2.5 Ibis Biosciences http://www.mbio.ncsu.edu/BioEdit/bioedit.html. Background and ambiguous non-standard nucleotides were removed and the correct DNA nucleotides inserted. Consensus sequences were computed by matching forward and reverse traces of each sample using SeqTrace 0.9.0 software29 http://seqtrace.googlecode.com. We filtered low quality base calls and performed end trimming. High quality finished unknown sequences were exported in Fasta format for downstream analyses. NTM and MTBC 16S rRNA reference sequences derived from American Type Culture Collection (ATCC) isolates were downloaded from Gen Bank to illustrate the phylogenetic relationship of unknown sequences and identify them. These sequences were aligned together with unknown sequences in BioEdit software using ClustalW2 Multiple Sequence Alignment tool. A Phylogenetic tree was constructed from the aligned sequences based on regions A and B of the Mycobacteria gene using maximum likelihood algorithm. Unknown sequences were further identified based on their relatedness to NTM and MTBC reference strains on the phylogenetic tree. All sequences were made publicly available in GenBank with accession numbers KX607141.1 to KX607408.1.
Data management and statistical analysis
Data collection and management for the parent study have been described previously11. All data analyses for this study were carried out with Stata version 12 (Stata Corp, College Station, TX, USA). The main outcome of the study was the prevalence of NTM amongst AFB positives cultures/MPT64 negative strains in pulmonary samples of a nationally representative population investigated for PTB in The Gambia. NTM prevalence and other categorical variables were summarised using frequency counts and proportions.
Following retrieval and re-culture, samples for 328 of the 903 originally AFB culture positives in the parent study failed to grow. To account for this disparity, NTM prevalence was estimated using two models: Model 1 in a complete case analysis used only results from the 575 participants with positive cultures after retrieval of stored sputum samples. Model 2 assumes the negative results for 328 of 903 participants, are truly negative.
Associations between NTM prevalence and categorical risk factors were examined using logistic regression models. Variables reaching statistical significance (p < 0.05) in univariate analyses were included in multivariable logistic regression models to obtain odds ratios and their 95% confidence intervals. All models adjusted for clustering as per the parent study design.
Ethical approval
The Joint MRCG at LSHTM/Gambia Government Ethics Committee approved this study (SCC number 1371). Subjects were recruited as part of the Gambian national TB prevalence study and gave informed consent prior to enrolment. All the laboratory methods in this study were carried out in accordance with the relevant institutional guidelines and regulations.
Data availability
All relevant data are within the manuscript and its Supplementary Information file.
References
Tortoli, E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin. Microbiol. Rev. 27, 727–752 (2014).
Falkinham, J. O. Environmental sources of nontuberculous mycobacteria. Clin. Chest Med. 36, 35–41 (2015).
Falkinham, J. O. Ecology of nontuberculous mycobacteria-where do human infections come from?. Semin. Respir. Crit. Care Med. 34, 95–102. https://pubmed.ncbi.nlm.nih.gov/23460009/ (2013).
Turenne, C. Y. Nontuberculous mycobacteria: Insights on taxonomy and evolution. Infect. Genet. Evol. 72, 159–168 (2019).
Griffith, D. E. et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175, 367–416 (2007).
van Ingen, J. et al. Nontuberculous mycobacteria: Clinically relevant. Ned. Tijdschr. Geneeskd. 154, 25 (2010).
Kendall, B. A. & Winthrop, K. L. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin. Respir. Crit. Care Med. 34, 87–94 (2013).
Horne, D. & Skerrett, S. Recent advances in nontuberculous mycobacterial lung infections. F1000Research 8, 25 (2019).
Okoi, C. et al. Non-tuberculous mycobacteria isolated from pulmonary samples in sub-Saharan Africa—a systematic review and meta analyses. Sci. Rep. 7, 25 (2017).
Fedrizzi, T. et al. Genomic characterization of nontuberculous Mycobacteria. Sci. Rep. 7, 25 (2017).
Adetifa, I. M. O. et al. A tuberculosis nationwide prevalence survey in Gambia, 2012. Bull. World Health Organ. 94, 433–441 (2016).
Jankovic, M. et al. Geographical distribution and clinical relevance of non-tuberculous mycobacteria in Croatia. Int. J. Tuberc. Lung Dis. 17, 836–841 (2013).
Winkler, W. et al. HIV country profile: 2016. Global Health Action vol. 12. http://www.who.int/hiv/data/Country_profile_Mozambique.pdf?ua=1%0A. http://www.ncbi.nlm.nih.gov/pubmed/10338786%250A. http://www.ncbi.nlm.nih.gov/pubmed/25734004%250A. https://patents.google.com/patent/US5924074A/en%250A. https://doi.org/10.1016/S2214-109X(17)3 (2014).
Tortoli, E. Standard operating procedure for optimal identification of mycobacteria using 16S rRNA gene sequences. Stand. Genom. Sci. 3, 145–152 (2010).
Pokam, B. T. & Asuquo, A. E. Acid-fast bacilli other than mycobacteria in tuberculosis patients receiving directly observed therapy short course in cross river State, Nigeria. Tuberc. Res. Treat. 2012, 1–4 (2012).
Aliyu, G. et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One 8, 25 (2013).
López-Varela, E. et al. Non-tuberculous mycobacteria in children: Muddying the waters of tuberculosis diagnosis. Lancet Respir. Med. 3, 244–256 (2015).
López-Varela, E. et al. Correction: High rates of non-tuberculous mycobacteria isolation in mozambican presumptive tuberculosis. PLoS One 12, e0169757. https://doi.org/10.1371/journal.pone.0169757 (2017).
Chanda-Kapata, P. et al. Non-tuberculous mycobacteria (NTM) in Zambia: Prevalence, clinical, radiological and microbiological characteristics. BMC Infect. Dis. 15, 25 (2015).
Chinombe, N., Muzividzi, B., Munemo, E. & Nziramasanga, P. Molecular identification of nontuberculous mycobacteria in humans in Zimbabwe using 16S ribosequencing. Open Microbiol. J. 10, 113–123 (2016).
Hoefsloot, W. et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 42, 1604–1613 (2013).
Prevots, D. R. & Marras, T. K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria a review. Clin. Chest Med. 36, 13–34 (2015).
Katale, B. Z. et al. One Health approach in the prevention and control of mycobacterial infections in Tanzania: Lessons learnt and future perspectives. One Health Outlook 1, 2 (2019).
Lapinel, N. C., Jolley, S. E., Ali, J. & Welsh, D. A. Prevalence of non-tuberculous mycobacteria in HIV-infected patients admitted to hospital with pneumonia. Int. J. Tuberc. Lung Dis. 23, 491–497 (2019).
Chan, E. D. & Iseman, M. D. Underlying host risk factors for nontuberculous mycobacterial lung disease. Semin. Respir. Crit. Care Med. 34, 110–123 (2013).
Rodrigues, C. et al. Evaluation of the bactec MGIT 960 TB system for recovery and identification of mycobacterium tuberculosis complex in a high volume tertiary care centre. Indian J. Med. Microbiol. 27, 217–221 (2009).
Aldous, W. K., Pounder, J. I., Cloud, J. L. & Woods, G. L. Comparison of six methods of extracting Mycobacterium tuberculosis DNA from processed sputum for testing by quantitative real-time PCR. J. Clin. Microbiol. 43, 2471–2473 (2005).
Sanger, F., Nicklen, S. & Coulson, A. R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74, 5463–5467 (1977).
Stucky, B. J. Seqtrace: A graphical tool for rapidly processing DNA sequencing chromatograms. J. Biomol. Tech. 23, 90–93 (2012).
Acknowledgements
We are grateful to staff and the participants of the Gambian Survey of Tuberculosis Prevalence, as well as staff of the molecular microbiology and Tuberculosis diagnostic and research laboratories at the Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, for their contributions to this study.
Disclaimers
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the institutions with which the authors are affiliated.
Funding
This study was funded by the Medical Research Council Unit The Gambia MPhil studentship in Life and Biomedical Sciences. Financial support for this project was obtained from the Institute of Tropical Medicine Antwerp Seed Grant. The Gambian national TB prevalence survey was funded by the Global Fund to fight AIDS, TB and Malaria through Grant GMB-T-MRC. The funders had no role in the study design, data analysis, decision to publish research findings, or preparation of the manuscript for publication.
Author information
Authors and Affiliations
Contributions
C.O., S.A., F.G., M.A., and I.A. led the writing of the manuscript. C.O., F.G., M.A. processed sputum samples by MGIT cultures, MPT64 rapid antigen tests and 16S RNA gene sequencing for identification of non-tuberculous mycobacteria at the Medical Research Council Unit The Gambia at London School of Hygiene and Tropical Medicine. C.O., A.W., F.G. and M.A. determined phylogenetic related of mycobacterial strains. S.M., I.A. and C.O. carried out data management and data analyses. C.O. conceived the project. M.A. and I.A. contributed equally to data acquisition, analyses, interpretation and writing of the manuscript. All authors have read and approved the article for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okoi, C., Anderson, S.T., Mulwa, S. et al. Pulmonary non-tuberculous mycobacteria in colonisation and disease in The Gambia. Sci Rep 12, 19523 (2022). https://doi.org/10.1038/s41598-022-22777-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22777-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.