Abstract
The HDL-associated apolipoprotein M (apoM) and its ligand sphingosine-1-phosphate (S1P) may control energy metabolism. ApoM deficiency in mice is associated with increased vascular permeability, brown adipose tissue (BAT) mass and activity, and protection against obesity. In the current study, we explored the connection between plasma apoM/S1P levels and parameters of BAT as measured via 18F-FDG PET/CT after cold exposure in humans. Fixed (n = 15) vs personalized (n = 20) short-term cooling protocols decreased and increased apoM (− 8.4%, P = 0.032 vs 15.7%, P < 0.0005) and S1P (− 41.0%, P < 0.0005 vs 19.1%, P < 0.005) plasma levels, respectively. Long-term cooling (n = 44) did not affect plasma apoM or S1P levels. Plasma apoM and S1P did not correlate significantly to BAT volume and activity in the individual studies. However, short-term studies combined, showed that increased changes in plasma apoM correlated with BAT metabolic activity (β: 0.44, 95% CI [0.06–0.81], P = 0.024) after adjusting for study design but not BAT volume (β: 0.39, 95% CI [− 0.01–0.78], P = 0.054). In conclusion, plasma apoM and S1P levels are altered in response to cold exposure and may be linked to changes in BAT metabolic activity but not BAT volume in humans. This contrasts partly with observations in animals and highlights the need for further studies to understand the biological role of apoM/S1P complex in human adipose tissue and lipid metabolism.
Similar content being viewed by others
Introduction
Obesity is an increasing worldwide problem1 and is due to an imbalance in calorie in- and output causing storage of excess energy as triglycerides in white adipose tissue2. Obesity is associated with deleterious consequences such as type 2 diabetes and cardiovascular disease2. Exploring regulatory mechanisms of energy metabolism is of high importance to identify new treatment strategies to combat obesity and associated disorders.
Cold exposure leads to increased energy expenditure, peripheral vasoconstriction, and decreased peripheral blood flow to maintain core body temperature3,4. Lipids are estimated to fuel 50% of the heat production (e.g. thermogenesis) during shivering5, and exclusively during non-shivering thermogenesis induced by mild cooling6. Mild cold exposure stimulates the sympathetic nervous system and induces a release of norepinephrine, which interacts with beta-adrenergic receptors and activates brown adipose tissue (BAT)7. The presence and the metabolic activity of BAT in human adults8,9,10,11,12 may be visualized by the uptake of 18F-fluorodeoxyglucose (18F-FDG) during a positron emission tomography/computed tomography scan (PET/CT)7. Under non-shivering cold conditions in humans, BAT takes up glucose and (triglyceride-derived) non-esterified fatty acids (NEFA), which replenishes intracellular triglyceride stores13,14. Thus, activation of BAT by cold exposure, by increasing energy expenditure and the utilization of lipid stores, has potential as a treatment that targets excess adiposity and also insulin resistance, as 10 days of cold exposure improves insulin sensitivity in individuals with type 2 diabetes15.
The HDL-associated apolipoprotein M (apoM) may affect energy metabolism via regulation of BAT volume, BAT activity, and triglyceride turnover, as demonstrated in mice16. ApoM-deficient mice (apoM-KO) are protected against diet-induced obesity and have improved glucose tolerance16. ApoM transports approximately 70% of sphingosine-1-phosphate (S1P) in plasma, whereas albumin carries the remaining S1P17. Interestingly, treating wild-type mice with S1P-receptor 1-antagonist results in hyperactive BAT similar to apoM-KO mice accompanied by accelerated triglyceride turnover16. S1P exerts its effect through five G-protein coupled receptors (S1PR1-5)18 and plays also an important role in maintaining the endothelial barrier and vasodilation.
BAT is a highly vascularized tissue due to its high metabolic rate. During cold exposure, the perfusion through BAT is increased to facilitate glucose uptake and BAT perfusion correlates positively with whole-body energy expenditure in preclinical models19. The increased perfusion in BAT may lead to increased delivery of other nutrients including (triglyceride-derived) fatty acids to BAT and increased export of generated heat to peripheral tissues. For example, the apoM-KO mice, which show a decrease in S1P levels17, displays increased permeability and uptake of triglycerides in BAT16. Yet, whether apoM and S1P play a role in BAT activity in humans is unknown. Thus, was the current study aimed to investigate whether short-term cold exposure applied to a minor or larger skin surface area affects plasma S1P, its carrier apoM and whether plasma S1P and apoM are associated with 18F-FDG uptake by BAT in cold-exposed humans.
Results
Cold exposure in animals
Cold exposure of wild-type mice is associated with increased BAT activity20. We have also observed that apoM-deficiency (accompanied by a reduced level of S1P), as well as treatment with S1P-receptor 1 antagonist in wild-type mice, are associated with increased BAT activity and volume16. Whether cold per see affects the apoM/S1P metabolism is unknown. To explore whether cold exposure of mice affects plasma S1P and apoM levels, wild-type mice were housed at 23 ℃ or 4 ℃ for 16 h (Supplementary Fig. S1A and B). Plasma S1P and apoM were both increased significantly after cold exposure.
Basic characteristics of human participants exposed to cold
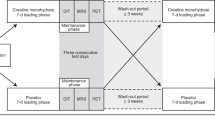
To explore whether apoM/S1P levels were affected by short-term or long-term cold exposure and associated with BAT volume and activity in humans we next included three cohorts (Fig. 1). Two cohorts represent short-term (2 h) cold applied to a minor or larger surface of the body, and one cohort represents long-term (24 h) cold exposure. Basic characteristics are presented in Supplementary Table S1 and an overview of the cohorts is presented in Fig. 2. Short-term cold-Copenhagen (STC-CPH), included 15 healthy young (24.3 ± 4.6 years) white Caucasian males with an average BMI of 23.4 kg/m2. The participants were exposed to 2 h fixed cooling protocol on the upper part of the body. Short-term cold-Leiderdorp (STC-LEI) included 20 healthy young males with Dutch white Caucasian and Dutch South Asian ethnicity with an average age of 24.4 ± 2.8 years and BMI of 21.9 kg/m2. Cold exposure was applied for 2 h by a personalized cooling protocol applied on both upper and lower parts of the body. The long-term cold (LTC) study included 44 male patients either subjected to a core body temperature of 33 or 36 ℃ for 24 h after cardiac arrest with an average age of 55.7 and 53.5 years, respectively. All patients included in LTC had an initial core body temperature of 35.2 ℃ and 35.3 ℃ for the 33 ℃ and 36 ℃ group, respectively21. Hence, the 36 ℃ group was maintained cold, whereas the 33 ℃ group was subjected to intensified cooling to reach a target temperature of 33 ℃.
Lipid parameters and response to cold
Lipid levels are presented in Supplementary Table S2. In STC-CPH, cold exposure did not affect the lipid parameters total cholesterol (TC), LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C), or triglycerides (Trig). In STC-LEI, TC increased by 7.8% (P = 0.001), LDL-C increased by 7.4% (P = 0.006), and Trig by 19.3% (P = 0.004), but no differences were observed for HDL-C after cold exposure.
The LTC study included patients exposed to either 33 ℃ or 36 ℃ for 24 h after cardiac arrest. There was no effect of target temperature, only an overall effect of cooling on all lipid parameters. Thus, TC, HDL-C, and LDL-C decreased after 24 h, whereas Trig increased. Further, analyzing the change in the difference between the two temperatures (Δ-values), showed no differences for either lipid parameter (data not shown).
Free fatty acids (FFA) increases in response to cold exposure due to liberation from white adipose tissue (WAT) during lipolysis. In both STC-CPH and STC-LEI, FFA levels were increased, but did only reach statistical significance in STC-LEI (34.1%, P < 0.005) (Supplementary Table S3).
The effect of cold on apoM plasma levels
To explore the relationship between cold and the apoM/S1P complex in humans, we measured plasma apoM and S1P in participants of the three cohorts.
Short-term cold exposure in STC-CPH decreased plasma apoM by 8.4% (P = 0.032) (Fig. 3A). In contrast, apoM levels were increased by 15.7% (P < 0.0005) in participants in STC-LEI (Fig. 3B). The increase in apoM levels was similar between ethnicity (data not shown).
ApoM plasma levels in STC-CPH (A), STC-LEI (B), and LTC (C). Thermoneutral (TN) and cold refers to pre-cooling and after cold exposure (2 h) in STC-CPH (n = 15) and STC-LEI (n = 20). In LTC samples were taken at 0 and after 24 h at either 33˚C (n = 20, intensified cold) or 36˚C (n = 24, cold maintenance). Data are presented as mean with error bars as SD. In A and B, data are analyzed with a paired t-test or Wilcoxon signed-rank test depending on normal distribution. In C, data are analyzed with a two-way mixed ANOVA with time and temperature as variables. *P < 0.05, **P < 0.01, ***P < 0.0005 for TN vs Cold. STC-CPH; short-term cold, Copenhagen. STC-LEI; short-term cold, Leiderdorp. LTC; long-term cold.
In the LTC study, there was no effect of target temperature on plasma apoM, but only an effect of cooling (P < 0.0005, Fig. 3C.). Furthermore, Δ-values (pre-cooling versus cooling) within each temperature group were not significantly changed (data not shown).
ApoM physically interacts with lipoproteins and thus correlates mainly with TC and HDL-C22,23. Therefore, differences within these lipid parameters could have caused the opposed changes in plasma apoM in the present studies. Thus, the ratio between either plasma apoM to TC or HDL-C was also reported. In STC-CPH the difference between apoM at thermoneutral (pre-cooling) and cold maintained significant after correcting for HDL-C (P = 0.02) but not TC (Supplementary Fig. S2A and B). In STC-LEI, the difference between apoM at thermoneutral and cold maintained significant after correcting for both TC and HDL-C (P = 0.031 and P = 0.0006, respectively) (Supplementary Fig. S2C and D). In the LTC study, the decrease in apoM levels after cold exposure was not maintained after correction for HDL-C or TC (Supplementary Fig. S2E and F).
The effect of cold on plasma S1P levels
Short-term cold exposure in STC-CPH decreased plasma S1P levels by 15.9% (P < 0.0005) (Fig. 4A.), whereas an increase of 19.1% (P = 0.004) was found in STC-LEI (Fig. 4B.).
S1P plasma levels in STC-CPH (A), STC-LEI (B), and LTC (C). Thermoneutral (TN) and cold refers to pre-cooling and after cold exposure (2 h) in STC-CPH (n = 15) and STC-LEI (n = 20). In LTC samples were taken at 0 and after 24 h at either 33˚C (n = 20, intensified cold) or 36˚C (n = 24, cold maintenance). Data presented as mean with error bars as SD and in A and B and analyzed with either a paired t-test or Wilcoxon signed-rank test depending on a normal distribution. In C data are analyzed with a two-way mixed ANOVA with time and temperature as variables. *P < 0.05, **P < 0.01, ***P < 0.0005 for TN vs Cold. STC-CPH; short-term cold, Copenhagen. STC-LEI; short-term cold, Leiderdorp. LTC; long-term cold.
In the LTC study, there was no significant interaction between the target temperature and cooling, thus only an effect of cooling was found (P = 0.019) (Fig. 4C.). Δ-values for time within each temperature group were not significant (data not shown).
In STC-CPH the difference between S1P at thermoneutral and cold maintained significant after correcting for both TC (P < 0.0001) and HDL-C (P < 0.0001) (Supplementary Fig. S3A and B). In STC-LEI, the difference between S1P at thermoneutral and cold maintained significant after correcting for HDL-C (P = 0.01) but not TC (Supplementary Fig. S3C and D). In the LTC study, no effect of cooling was maintained after correction for TC or HDL-C (Supplementary Fig. S3E and F).
Plasma apoM and S1P and its association with BAT parameters
To explore the association between the apoM/S1P axis and activity and volume of BAT during short-term cold exposure, participants in STC-CPH and STC-LEI were examined. BAT parameters are presented in Table 1. There was no significant association between plasma apoM or S1P with BAT volume, SUVmean or SUVmax in neither the STC-CPH nor STC-LEI (Supplementary Table S4). Combining the two studies after testing for homogeneity however, suggest that cold-induced changes in plasma apoM associate positively with BAT metabolic activity (Fig. 5). The association was maintained after adjusting for study design (β:0.44, 95% CI [0.06–0.81], P = 0.024 and adjusted R2 of 0.31, P = 0.002). No association between plasma apoM or S1P and BAT volume, SUVmean, or SUVmax was observed after adjusting for study design.
Association between brown adipose tissue (BAT) parameters and delta plasma apoM and S1P levels (ΔapoM and ΔS1P). Delta plasma apoM and S1P levels (cold-induced changes in plasma concentration) were associated with BAT volume (A and E), metabolic activity (B and F), SUVmean (C and G) and SUVmax (D and H). Data were tested for homogeneity before combining the studies in linear regression analysis adjusting for a study place. Adj R2; adjusted R-square value. β; beta-coefficient. []; 95%-confident interval. STC-CPH (n = 11); short-term cold, Copenhagen. STC-LEI (n = 19); short-term cold, Leiderdorp.
Discussion
The present study aimed to investigate the effects of cold exposure on apoM and S1P plasma levels as well as to explore whether the plasma apoM and S1P concentrations correlate with BAT volume and activity in humans. We found that both apoM and S1P plasma levels, as well as lipids, were affected by cold exposure. This effect was depending on the applied cooling protocol, fixed versus personalized. Finally, we observed that BAT metabolic activity correlated positively with cold-induced changes in apoM plasma levels only when both STC studies were combined.
Changes in plasma apoM and S1P levels during short-term cold exposure in healthy lean individuals may reflect changes in production or utilization. ApoM is mainly produced in the liver and kidney in humans24,25, while a minor part may be produced by white adipocytes26. In addition to activating BAT, cold exposure also results in sympathetic activation of other organs including WAT27. Thus, β-adrenergic activation could influence apoM secretion from WAT. Whether such stimulus also triggers the secretion of apoM/S1P from the liver or the kidney is unknown. Clearance of apoM could be another mechanism for regulating plasma apoM/S1P levels. ApoM is mainly associated with HDL particles, but can rapidly exchange onto LDL/VLDL particles28,29. Therefore, a high fractional catabolic rate of LDL in humans is related to low plasma apoM concentration28,29,30. Thus, changes in plasma lipids could affect plasma apoM. In the present study, lean healthy men exposed to short-term cold (fixed protocol, STC-CPH) via a cooling vest covering the torso showed no cold-induced differences in lipid levels, but a decrease of 8% and 42% in apoM and S1P plasma levels, respectively. In contrast, similar individuals exposed to whole-body short-term cold (personalized protocol, STC-LEI) showed an increase in lipid parameters and apoM and S1P plasma levels (16% and 18%, respectively). This effect was maintained also after correcting for changes in lipid levels. Thus, the two studies, despite comparable participants regarding BMI, age, and gender, cold exposure affected apoM and S1P levels in opposite direction. An explanation for these differences may be the cooling protocol used in STC-CPH versus STC-LEI. Careful examination of pre-analytical as well as analytical procedures was also considered but no discrepancies were observed between the two studies.
S1P is mainly carried by apoM, but 30–35% is bound to albumin31,32. S1P can be produced by various cells such as platelets, erythrocytes, and endothelial cells33. Whether any of these cells are affected by cooling and thereby contribute to cold-induced changes in plasma S1P is unknown. Moreover, apoM not only carries S1P but also stimulates the secretion of S1P from erythrocytes. A reduction or an increase in plasma apoM may therefore also directly cause a concomitant change in plasma S1P. Finally, a portion of S1P can bind to albumin, like FFA34. Although not significantly increased in STC-CPH, FFA could compete with S1P for binding to albumin resulting in the removal of S1P from the circulation. Alternatively, S1P-albumin could serve as a pool delivering S1P to apoM-containing HDL. The exchange of S1P between carriers and the function of S1P-albumin as an S1P-reservoir is currently unexplored.
The changes in apoM/S1P levels can affect vascular integrity and metabolism as shown in several animal models16,17. Hence, apoM-S1P complex bound to HDL maintains the endothelial barrier function17,35,36,37. Furthermore, HDL-S1P induces eNOS production leading to vasodilation38, but S1P also suppresses vascular leakage by promoting endothelial barrier integrity17,39. ApoM-KO mice have increased permeability in BAT, larger BAT volume, increased metabolism of triglyceride, and improved glucose tolerance compared to WT mice properly conveyed by a decrease in S1P activation of S1Pr1 on endothelial cells16. Thus, a decrease in the apoM/S1P levels in humans may lead to increased energy metabolism and the amount of BAT. We found a correlation between greater cold-induced changes in plasma apoM and BAT metabolic activity in healthy, lean human male subjects undergoing 2 h of cold exposure (combined STC-CPH and STC-LEI). Surprisingly, the correlation was positive, thus an increase in plasma apoM after short-term cold exposure was associated with a higher metabolic activity of BAT. Unexpected, but in support of our human data, we also observed an increase of plasma S1P and apoM in wild-type mice after 16 h of cold exposure. These observations do not directly align with the phenotype observed in apoM-KO mice or wild-type mice treated with S1P-receptor 1 antagonist previously reported16. In these two animal models lack of apoM, reduced plasma S1P levels, or decreased S1P-receptor 1 activity was associated with increased BAT volume and activity. This may be explained by several mechanisms. The apoM-KO mouse has a life-long deficiency of apoM and a build-up of hyperactive BAT whereas the human participants or wild-type mice in the current study have no known alterations in plasma apoM levels at baseline. In wild-type mice as well as in the human participants we observed a parallel change in apoM and S1P, suggesting that the individuals have a balanced apoM/S1P ratio. This is not the case in apoM-KO mice where S1P mainly is attached to albumin and therefore could affect the S1P-receptors differently compared to apoM-carried S1P. Thus, it is likely that the discrepancy between the present study and the previous relies on changes and shifts in the apoM/S1P versus albumin/S1P ratios. Also, we present correlations in our human studies, and more research is needed to investigate whether the correlations represent causality, thus whether the apoM/S1P complex directly affects BAT functionality. The cooling protocols used, fixed versus personalized, can potentially affect the BAT activity differently40 even though the participants in the two short-term cold studies are comparable regarding age, gender, BMI, and the associations were maintained after including the study design as a covariate in the linear regression analysis. Finally, insulin resistance is associated with a decrease in plasma apoM. In combination with our observations, it will be important to further explore whether such a reduction also can be linked to a decreased metabolic activity in BAT from humans with insulin resistance.
The effect of long-term cold exposure on plasma apoM and S1P was explored either by maintaining the core temperature at 36˚C or reducing it further to 33˚C in patients submitted after cardiac arrest. There were no effects of target temperature on apoM, S1P and lipid parameters, but only a general effect of cooling. Hence, cooling independent of the degree of cooling decreased apoM, S1P, TC, HDL-C, and LDL-C, whereas Triglycerides increased. The lack of difference between the cooling groups may be explained by the experimental setup. Hence, both groups were subjected to active cooling. The general decrease over time in apoM/S1P plasma levels after long-term cold may be supported by other studies as well. Thus, apoM and S1P plasma levels are decreased in sepsis and dengue fever both affecting vascular permeability41,42,43,44. The reduction could be explained by apoM being a negative acute-phase protein45 or simply as a consequence of a decrease in HDL-C46. In the present, LTC study patients were all hospitalized after cardiac arrest, a severe stress condition, and assisted by medical pressor treatment such as dobutamine, dopamine, and norepinephrine. Therefore, we cannot conclude whether the observed differences are an effect of the cold intervention, disease, or medical treatment. Additional studies in healthy individuals are therefore needed to elucidate whether the apoM/S1P complex is modulated due to long-term cold exposure.
The study has limitations. We see opposing effects of cold exposure on apoM and S1P plasma levels in the two short-term cold studies which may reflect the extent and method of cooling. It is known that various cooling protocols can induce different metabolic responses but no common guideline for investigations of BAT is implemented40. Both the applied fixed and personalized short-term cold protocol in the present study are standard methods. Our result underlines the need for such guidelines to translate the obtained data in such experiments into the relevant biological function of BAT in humans. Furthermore, quantification of activated BAT using a glucose tracer, i.e. 18F-FDG may be suboptimal, as cold exposure results in increased oxidation of fatty acids rather than glucose14,34. Further, cold-exposed mice increase BAT uptake of triglyceride-derived fatty acids20, mostly after selective lipolysis47. As described, apoM is linked to triglyceride metabolism and activity of BAT in mice16. Therefore, it cannot be excluded that using an FFA-tracer, preferentially incorporated within triglyceride to assess LPL-dependent lipolysis by BAT, could bring other results in the future. However, studies on lipid metabolism during non-shivering short-term cold exposure are sparse13,48. Thus, further studies in lipid metabolism during non-shivering cold using lipid tracers are needed to explore BAT metabolism. In the present cohorts, shivering was only controlled by verbal feedback and not by electromyography. We cannot exclude individuals who were not subclinical shivering, but a subanalysis did not show any correlation between plasma apoM and S1P with glucose uptake in muscles (data not shown). Changes observed in plasma apoM and S1P levels after cooling may also be affected by a 2-h extended fasting period. However, the literature does not suggest that neither total plasma apoM nor S1P concentrations are affected by fasting24,49. Our study did also not allow to investigate whether BAT-negative versus BAT-positive individuals have different plasma apoM levels. With a personalized cooling protocol, it is expected that 95% of the participants will have BAT, therefor it will be needed with significantly more participants than in the present study. Finally, the study cohorts are small in size but comparable to other published studies including quantification of BAT in humans.
In conclusion, we show that fixed versus personalized cooling procedures affect apoM and S1P plasma levels and, interestingly, are positively associated with parameters of BAT metabolic activity in humans. To our knowledge, the role of S1P signaling in human BAT is unknown. FTY720, an S1P analog, is an FDA-approved drug for multiple sclerosis50. Currently, drugs targeting the S1P receptor system are directed towards diseases involving the immune system50,51. It would be highly relevant to investigate whether drugs targeting the S1P signaling pathway in BAT could lead to increased vascular permeability and uptake of triglycerides augmenting energy expenditure and contributing to the prevention of adiposity and insulin resistance.
Materials and methods
Study setup and participants
Three different studies were included and conducted as described below (see also Fig. 1).
The STC-CPH cohort was conducted at Rigshospitalet and the University of Copenhagen. Healthy young white Caucasian men (age 18–33 years, body mass index (BMI) 20–27 kg/m2) were enrolled. Some of the participants were from a cohort published earlier52. Exclusion criteria were daily prescribed drugs and weekly alcohol and smoking consumption above 21 and 20 units, respectively. All 15 participants had given written informed consent. The study was approved by The National Committee on Health Research Ethics in Denmark (H-1–2013-064).
The STC-LEI cohort was conducted at the Alrijne Hospital in Leiderdorp, the Netherlands (Netherlands Trial Register 2473). Healthy Dutch South Asian and matched Dutch white Caucasian males with a lean phenotype (BMI < 25 kg/m2) matched for BMI and between the age of 18 and 28 years were recruited. Participants underwent medical examination including blood sampling and an oral glucose tolerance test for exclusion of type 2 diabetes. Besides type 2 diabetes, exclusion criteria were e.g. extensive exercise and smoking. Written informed consent from all participants was obtained and the study was approved by appropriate authorities6. The study has been described in detail by Bakker et al.6 and Hoeke et al.48. For the present study, we could include 10 white Caucasians and 10 South Asians.
The LTC cohort, included 44 participants from the single-center study at Rigshospitalet, Copenhagen University Hospital, Denmark (in total n = 171) as part of the Target Temperature Management (TTM) 33ºC versus 36ºC after out-of-hospital cardiac arrest (NCT01020916)53. The exclusion criteria for the TTM trial were in-hospital or presumes non-cardiac cause of arrest, severe comorbidity, admission temperature < 30 °C, and persistent cardiogenic shock 21,54. For the present study, additional exclusion criteria were women, age > 70 years, acute myocardial infarction, ischemic heart disease, arrhythmia, previous cardiac arrest, hypertension, diabetes, dialyzes, pulseless electrical activity plus asystole, and former percutaneous coronary intervention. These additional criteria were selected to align with the short-term cold studies (e.g. only men) as well as excluding diseases known to affect apoM such as diabetes and dialysis55,56. The TTM trial was designed as a multicenter, prospective investigator-initiated, randomized, parallel-group, and assessor-blinded trial including 36 intensive care units in Europe and Australia investigating the outcome of cooling patients to a core temperature of either 33 °C or 36 °C after cardiac arrest21. The LTC study was approved by the Ethics Committee of the Capital Region Copenhagen (H-1-2010-059) and the Danish Data Protection Agency, and written informed consent was in all cases obtained from the patients next of kin and general practitioner and by the patient if regaining consciousness after cardiac arrest53,57. Included patients were > 18 years of age and resuscitated after out of hospital cardiac arrest remaining unconscious (Glasgow Coma Score < 8) after sustained return to spontaneous circulation > 20 min.
All studies were conducted according to the principles of the Declaration of Helsinki.
Procedure for cold exposure
In STC-CPH, participants fasted overnight. Two hours before the PET/CT scan the participants were put in a water-perfused cooling vest covering the upper body with a continuous flow of water at a fixed temperature of 14 °C (fixed cooling protocol40). One hour before a 2-bed PET/CT scan 18F-FDG was injected intravenously (97–100 MBq, mean ± SD: 99.07 ± 0.96 MBq). The 5-min per bed PET scans were performed on a Biograph TruePoint (Siemens, Knoxville, TN) with a low dose CT and 3D-OSEM reconstruction with PSF using 3 iterations 21 subsets and a 2 mm FWHM Gaussian filter (2 × 2x3 mm voxels). Heparin blood samples were collected just before and after 2 h of cold induction. Participants were monitored for shivering (observed by the researcher or/and self-reported by the participant) due to the cold exposure. BAT parameters were analyzed using Mirada RTx 1.0.2 (Mirada Medical Ltd, Oxford, UK) analysis to calculate standardized uptake value (SUV) maximum, mean, and BAT volume on PET.
In STC-LEI, participants underwent a 10 h overnight fast. To activate BAT the participants were imbedded between two water perfused cooling blankets. Once shivering was detected visually by researchers and self-reported by the participants, water temperature increased and a personalized cooling protocol40 started for 2 h. After 1 h 2 MBq/kg 18F-FDG was injected intravenously, and after one more hour of cold exposure, the PET/CT scan was performed for BAT quantification. Blood samples were collected at thermoneutral before cold and at time 110 min after cold induction6. BAT parameters were analyzed using the Beth Israel plugin for the FIJI program as described elsewhere58,59,60.
BAT was quantified using an SUV mean threshold at > 1.5 g/ml, Hounsfield units from -250 to -10 and anatomic position defined from the vertebrae C3 to T7 in participants from both STC-CPH and STC-LEI. We observed one individual with a low amount of BAT, but all individuals had visually detected BAT, and we did not observe any BAT negative individuals.
In LTC, patients were randomized to a core body temperature of either 33 or 36 °C for 24 h after cardiac arrest. The protocol included a 4 h induction period to achieve the target temperature. Hereafter, the target temperature was maintained for 24 h with subsequent rewarming to 37 °C with a maximum of 0.5 °C/hour. Plasma samples were taken before cold induction and 28 h after (4 h of induction period + 24 h of cold). Cooling was achieved using Thermowrap21,53.
Animal experiment
Wild-type female mice (C57B6) were randomized and housed at 23 ℃ (n = 8) or 4 ℃ (n = 8) for 16 h, at the Panum Institute (University of Copenhagen) in a temperature-controlled facility with a 12-h dark/light cycle and fed a standard chow diet. Blood samples were taken after 16 h of housing at 23 ℃ or 4 ℃ and blinded for plasma analysis. All procedures were approved by the Animal Experiments Inspectorate, Ministry of Justice, Denmark, and reported according to the ARRIVE guidelines.
Ethic Declarations
All animal experiments and methods used in our animal experiments were performed in accordance with the relevant guidelines and regulations.
Plasma analysis
Plasma S1P and apoM were measured as previously described17,28 and23, respectively.
In the STC-CPH and LTC studies plasma lipids TC, HDL-C, LDL-C, and Trig were measured at the Department of Clinical Biochemistry, Rigshospitalet, Denmark. The lipids were measured using standardized enzymatic absorption photometry assays on an automated analyzer (Cobas 8000, Roche) according to the manufacturer´s instructions. In the STC-LEI study lipids were measured according to Hoeke et al.48 and FFA according to Bakker et al.6. FFA was measured by an automated spectrophotometer (ABX Pentra 400 autoanalyzer) by enzymatic kit (WAKO NEFA C Kit; TriChem Aps) in STC-CPH.
Statistics
Statistics were performed in SPSS Statistics 22 and 25. A paired t-test or a Wilcoxon t-test were performed between groups depending on whether data were normally distributed or not. Correlations were performed with Pearson or Spearman´s test depending on whether data were normally distributed. For analysis of the association between changes in plasma apoM or S1P, the STC-CPH and STC-LEI were combined after testing for homogeneity and interaction. In the linear regression analysis, the study protocol was included as a covariate. The effect of temperature and time in the LTC study was analyzed with a Two-Way Mixed ANOVA. A p-value < 0.05 was considered significant. Student´s t-test was used to compare groups in animal studies.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15, 288–298. https://doi.org/10.1038/s41574-019-0176-8 (2019).
Romieu, I. et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 28, 247–258. https://doi.org/10.1007/s10552-017-0869-z (2017).
van Marken, L. W. D. & Daanen, H. A. Cold-induced metabolism. Curr. Opin. Clin. Nutr. Metab. Care 6, 469–475. https://doi.org/10.1097/01.mco.0000078992.96795.5f (2003).
Stocks, J. M., Taylor, N. A., Tipton, M. J. & Greenleaf, J. E. Human physiological responses to cold exposure. Aviat Space Environ. Med. 75, 444–457 (2004).
Haman, F. et al. Effect of cold exposure on fuel utilization in humans: Plasma glucose, muscle glycogen, and lipids. J. Appl. Physiol. 1985(93), 77–84. https://doi.org/10.1152/japplphysiol.00773.2001 (2002).
Bakker, L. E. et al. Brown adipose tissue volume in healthy lean south Asian adults compared with white Caucasians: A prospective, case-controlled observational study. Lancet Diabetes Endocrinol. 2, 210–217. https://doi.org/10.1016/S2213-8587(13)70156-6 (2014).
Nedergaard, J., Bengtsson, T. & Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 293, E444-452. https://doi.org/10.1152/ajpendo.00691.2006 (2007).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517. https://doi.org/10.1056/NEJMoa0810780 (2009).
van Marken, L. W. D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508. https://doi.org/10.1056/NEJMoa0808718 (2009).
Virtanen, K. A. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525. https://doi.org/10.1056/NEJMoa0808949 (2009).
Saito, M. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: Effects of cold exposure and adiposity. Diabetes 58, 1526–1531. https://doi.org/10.2337/db09-0530 (2009).
Zingaretti, M. C. et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 23, 3113–3120. https://doi.org/10.1096/fj.09-133546 (2009).
Ouellet, V. et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest. 122, 545–552. https://doi.org/10.1172/JCI60433 (2012).
Cannon, B. & Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 84, 277–359. https://doi.org/10.1152/physrev.00015.2003 (2004).
Hanssen, M. J. et al. Glucose uptake in human brown adipose tissue is impaired upon fasting-induced insulin resistance. Diabetologia 58, 586–595. https://doi.org/10.1007/s00125-014-3465-8 (2015).
Christoffersen, C. et al. The apolipoprotein M/S1P axis controls triglyceride metabolism and brown fat activity. Cell Rep. 22, 175–188. https://doi.org/10.1016/j.celrep.2017.12.029 (2018).
Christoffersen, C. et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. U S A 108, 9613–9618. https://doi.org/10.1073/pnas.1103187108 (2011).
Ishii, I., Fukushima, N., Ye, X. & Chun, J. Lysophospholipid receptors: Signaling and biology. Annu. Rev. Biochem. 73, 321–354. https://doi.org/10.1146/annurev.biochem.73.011303.073731 (2004).
Orava, J. et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 14, 272–279. https://doi.org/10.1016/j.cmet.2011.06.012 (2011).
Bartelt, A. et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205. https://doi.org/10.1038/nm.2297 (2011).
Nielsen, N. et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N. Engl. J. Med. 369, 2197–2206. https://doi.org/10.1056/NEJMoa1310519 (2013).
Axler, O., Ahnstrom, J. & Dahlback, B. An ELISA for apolipoprotein M reveals a strong correlation to total cholesterol in human plasma. J. Lipid Res. 48, 1772–1780 (2007).
Bosteen, M. H., Dahlback, B., Nielsen, L. B. & Christoffersen, C. Protein unfolding allows use of commercial antibodies in an apolipoprotein M sandwich ELISA. J. Lipid. Res. 56, 754–759. https://doi.org/10.1194/jlr.D055947 (2015).
Xu, N. & Dahlback, B. A novel human apolipoprotein (apoM). J. Biol. Chem. 274, 31286–31290 (1999).
Zhang, X. Y. et al. Specific tissue expression and cellular localization of human apolipoprotein M as determined by in situ hybridization. Acta Histochem. 105, 67–72 (2003).
Sramkova, V. et al. Apolipoprotein M: A novel adipokine decreasing with obesity and upregulated by calorie restriction. Am. J. Clin. Nutr. https://doi.org/10.1093/ajcn/nqy331 (2019).
Saito, M. Brown adipose tissue as a regulator of energy expenditure and body fat in humans. Diabetes Metab. J. 37, 22–29. https://doi.org/10.4093/dmj.2013.37.1.22 (2013).
Christoffersen, C. et al. Opposing effects of apolipoprotein m on catabolism of apolipoprotein B-containing lipoproteins and atherosclerosis. Circ. Res. 106, 1624–1634 (2010).
Christoffersen, C. et al. The plasma concentration of HDL-associated apoM is influenced by LDL receptor-mediated clearance of apoB-containing particles. J. Lipid. Res. 53, 2198–2204 (2012).
Christoffersen, C. et al. Isolation and characterization of human apolipoprotein M-containing lipoproteins. J. Lipid. Res. 47, 1833–1843. https://doi.org/10.1194/jlr.M600055-JLR200 (2006).
Karuna, R. et al. Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis 219, 855–863 (2011).
Murata, N. et al. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 352, 809–815 (2000).
Ksiazek, M., Chacinska, M., Chabowski, A. & Baranowski, M. Sources, metabolism, and regulation of circulating sphingosine-1-phosphate. J. Lipid. Res. 56, 1271–1281. https://doi.org/10.1194/jlr.R059543 (2015).
Ruiz, J. R. et al. Role of human brown fat in obesity, metabolism and cardiovascular disease: Strategies to turn up the heat. Prog. Cardiovasc. Dis. 61, 232–245. https://doi.org/10.1016/j.pcad.2018.07.002 (2018).
Christensen, P. M. et al. Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J. 30, 2351–2359. https://doi.org/10.1096/fj.201500064 (2016).
Ruiz, M., Okada, H. & Dahlback, B. HDL-associated ApoM is anti-apoptotic by delivering sphingosine 1-phosphate to S1P1 & S1P3 receptors on vascular endothelium. Lipids Health Dis. 16, 36. https://doi.org/10.1186/s12944-017-0429-2 (2017).
Ruiz, M. et al. High-density lipoprotein-associated apolipoprotein M limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1. Arterioscler. Thromb. Vasc. Biol. 37, 118–129. https://doi.org/10.1161/ATVBAHA.116.308435 (2017).
Wang, X. & Wang, F. Vascular protection by high-density lipoprotein-associated sphingosine-1-phosphate. J. Geriatr. Cardiol. 14, 696–702. https://doi.org/10.11909/j.issn.1671-5411.2017.11.010 (2017).
Garcia, J. G. et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 108, 689–701 (2001).
Chen, K. Y. et al. Brown adipose reporting criteria in imaging STudies (BARCIST 1.0): Recommendations for standardized FDG-PET/CT experiments in humans. Cell Metab. 24, 210–222. https://doi.org/10.1016/j.cmet.2016.07.014 (2016).
Frej, C. et al. Sphingosine 1-phosphate and its carrier apolipoprotein M in human sepsis and in Escherichia coli sepsis in baboons. J. Cell Mol. Med. 20, 1170–1181. https://doi.org/10.1111/jcmm.12831 (2016).
Kumaraswamy, S. B., Linder, A., Akesson, P. & Dahlback, B. Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Crit. Care 16, R60. https://doi.org/10.1186/cc11305 (2012).
Gomes, L. et al. Sphingosine 1-phosphate in acute dengue infection. PLoS ONE 9, e113394. https://doi.org/10.1371/journal.pone.0113394 (2014).
Michels, M. et al. Decreased plasma levels of the endothelial protective sphingosine-1-phosphate are associated with dengue-induced plasma leakage. J. Infect. 71, 480–487. https://doi.org/10.1016/j.jinf.2015.06.014 (2015).
Feingold, K. R. et al. Infection and inflammation decrease apolipoprotein M expression. Atherosclerosis 199, 19–26. https://doi.org/10.1016/j.atherosclerosis.2007.10.007 (2008).
Lekkou, A., Mouzaki, A., Siagris, D., Ravani, I. & Gogos, C. A. Serum lipid profile, cytokine production, and clinical outcome in patients with severe sepsis. J. Crit. Care 29, 723–727. https://doi.org/10.1016/j.jcrc.2014.04.018 (2014).
Khedoe, P. P. et al. Brown adipose tissue takes up plasma triglycerides mostly after lipolysis. J. Lipid Res. 56, 51–59. https://doi.org/10.1194/jlr.M052746 (2015).
Hoeke, G. et al. Short-term cooling increases serum triglycerides and small high-density lipoprotein levels in humans. J. Clin. Lipidol. 11, 920-928 e922. https://doi.org/10.1016/j.jacl.2017.04.117 (2017).
Hammad, S. M. et al. Blood sphingolipidomics in healthy humans: Impact of sample collection methodology. J. Lipid Res. 51, 3074–3087. https://doi.org/10.1194/jlr.D008532 (2010).
Park, S. J. & Im, D. S. Sphingosine 1-phosphate receptor modulators and drug discovery. Biomol. Ther. (Seoul) 25, 80–90. https://doi.org/10.4062/biomolther.2016.160 (2017).
Chew, W. S., Wang, W. & Herr, D. R. To fingolimod and beyond: The rich pipeline of drug candidates that target S1P signaling. Pharmacol. Res. 113, 521–532. https://doi.org/10.1016/j.phrs.2016.09.025 (2016).
Ingerslev, L. R. et al. Endurance training remodels sperm-borne small RNA expression and methylation at neurological gene hotspots. Clin. Epigenetics 10, 12. https://doi.org/10.1186/s13148-018-0446-7 (2018).
Bro-Jeppesen, J. et al. The inflammatory response after out-of-hospital cardiac arrest is not modified by targeted temperature management at 33 degrees C or 36 degrees C. Resuscitation 85, 1480–1487. https://doi.org/10.1016/j.resuscitation.2014.08.007 (2014).
Nielsen, N. et al. Target temperature management after out-of-hospital cardiac arrest–a randomized, parallel-group, assessor-blinded clinical trial–rationale and design. Am. Heart J. 163, 541–548. https://doi.org/10.1016/j.ahj.2012.01.013 (2012).
Plomgaard, P. et al. Apolipoprotein M predicts pre-beta-HDL formation: Studies in type 2 diabetic and nondiabetic subjects. J. Intern. Med. 266, 258–267. https://doi.org/10.1111/j.1365-2796.2009.02095.x (2009).
Sorensen, I. M. et al. Apolipoprotein M in patients with chronic kidney disease. Atherosclerosis 275, 304–311. https://doi.org/10.1016/j.atherosclerosis.2018.06.815 (2018).
Bro-Jeppesen, J. et al. Targeted temperature management at 33 degrees C versus 36 degrees C and impact on systemic vascular resistance and myocardial function after out-of-hospital cardiac arrest: A sub-study of the Target Temperature Management Trial. Circ. Cardiovasc. Interv. 7, 663–672. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001556 (2014).
Martinez-Tellez, B. et al. The impact of using BARCIST 1.0 criteria on quantification of BAT volume and activity in three independent cohorts of adults. Sci. Rep. 8, 8567. https://doi.org/10.1038/s41598-018-26878-4 (2018).
Janssen, L. G. M. et al. Twelve weeks of exenatide treatment increases [(18)F]fluorodeoxyglucose uptake by brown adipose tissue without affecting oxidative resting energy expenditure in nondiabetic males. Metab. Clin. Exp. 106, 154167. https://doi.org/10.1016/j.metabol.2020.154167 (2020).
Nahon, K. J. et al. Effect of sitagliptin on energy metabolism and brown adipose tissue in overweight individuals with prediabetes: A randomised placebo-controlled trial. Diabetologia 61, 2386–2397. https://doi.org/10.1007/s00125-018-4716-x (2018).
Acknowledgements
The authors would like to thank Charlotte Wandel and Lis Schutt Nielsen for their excellent technical assistance. The project was funded by the Novo Nordisk Foundation Excellence Project (grant NNF13OC0003898 to C.C.), the Department of Biomedical Sciences, the University of Copenhagen (to A.B.), the Fundación Alfonso Martin Escudero (to B.M.T), the Netherlands Cardiovascular Research Initiative: an initiative with the support of the Dutch Heart Foundation (CVON2017-20 GENIUS-II to P.C.N.R) and Dutch Diabetes Research Foundation (Junior Postdoc Fellowship; 2015.81.1808 to M.R.B). Neither of the funding sources was involved in study design, data collection, analysis, interpretation of data, writing or submission of the manuscript.
Author information
Authors and Affiliations
Contributions
Study conception: A.B. and C.C.. Design of cohorts and collection of participants: I.D.; A.L., A.K., S.H.K. and R.B. designed and collected STC-CPH. M.R.B., B.M.T. and P.C.N.R. designed and collected STC-LEI. M.F., J.K., and C.H. designed and collected LTC. Data analysis: A.B., I.D., S.H.K., M.R.B., B.M.T., M.F. Data interpretation: A.B., I.D., S.H.K., B.M.T., P.C.N.R., M.F., J.K., C.H. and C.C. First draft: Written by A.B. and C.C. Critical revision and final approvement: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borup, A., Donkin, I., Boon, M.R. et al. Association of apolipoprotein M and sphingosine-1-phosphate with brown adipose tissue after cold exposure in humans. Sci Rep 12, 18753 (2022). https://doi.org/10.1038/s41598-022-21938-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21938-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.