Abstract
In the present study, the magnetic NH2-MIL-101(Al)/chitosan nanocomposite (MIL/Cs@Fe3O4 NCs) was synthesized and used in the removal of azithromycin (AZT) from an aqueous solution for the first time. The as-synthesized MIL/Cs@Fe3O4 NCs was characterized by SEM, TEM, XRD, FTIR, BET, and VSM techniques. The effect of various key factors in the AZT adsorption process was modeled and optimized using response surface methodology based on central composite design (RSM-CCD). The low value of p-value (1.3101e−06) and RSD (1.873) parameters, along with the coefficient of determination > 0.997 implied that the developed model was well fitted with experimental data. Under the optimized conditions, including pH: 7.992, adsorbent dose: 0.279 g/L, time: 64.256 min and AZT concentration: 10.107 mg/L, removal efficiency and AZT adsorption capacity were obtained as 98.362 ± 3.24% and 238.553 mg/g, respectively. The fitting of data with the Langmuir isotherm (R2: 0.998, X2: 0.011) and Pseudo-second-order kinetics (R2: 0.999, X2: 0.013) showed that the adsorption process is monolayer and chemical in nature. ΔH° > 0, ΔS° > 0, and ∆G° < 0 indicated that AZT removal was spontaneous and endothermic in nature. The effect of Magnesium on AZT adsorption was more complicated than other background ions. Reuse of the adsorbent in 10 consecutive experiments showed that removal efficiency was reduced by about 30.24%. The performance of MIL/Cs@Fe3O4 NCs under real conditions was also tested and promising results were achieved, except in the treatment of AZT from raw wastewater.
Similar content being viewed by others
Introduction
In the last decades, the removal of antibiotics has received more attention from researchers due to their cumulative properties, high toxicity, chemical stability, biological activity, and the development of bacterial resistance even in small amounts1. So far, a variety of antibiotics including macrolides, tetracyclines, and chloramphenicols are found in environmental media2. Azithromycin (C38H72N2O12, AZT) is a broad-spectrum macrolide antibiotic with a long half-life that is primarily used to treat respiratory, enteric, and genitourinary infections3,4. The presence of azithromycin in a water body is usually related to personal hygiene products, pharmaceutical industry waste, hospital waste, therapeutic drugs, animal husbandry, agriculture, and aquaculture. The presence of trace amounts of AZT in drinking water is a public health concern because little is known about the potential chronic health effects associated with the long-term ingestion of mixtures of these compounds through drinking water5. Moreover, AZT antibiotic residues can alter the human microbiome and cause health disturbances, such as allergic reactions, chronic toxic effects after prolonged exposure, and disruption of digestive system functions6. On the other side, due to their extensive usage and negligible human metabolism, about 30–90% of AZT antibiotics are excreted unchanged in the body of humans and animals as active compounds in urine and feces7,8. Environmental exposure to active pharmaceutical ingredients (APIs) can have negative effects on the health of ecosystems and humans9. Therefore, effective removal of AZT antibiotics from wastewater before their discharge is inevitable and necessary. Conventional wastewater treatment processes are not usually able to remove pharmaceutical compounds10.
Among various wastewater treatment processes, adsorption is considered as a promising and widely-used technique for removing pharmaceutical substances from wastewater due to its flexible design, ease of operation, and cost-effectiveness11,12. Nevertheless, the development of affordable, high-performance and reusable adsorbents for the pharmaceutical wastewater treatment remains a challenge among researchers and requires more comprehensive studies. MOFs are organic–inorganic hybrid crystalline porous structures consist of metal complex or cluster joined by organic ligands. MOFs have unique properties such as large pore volumes, adjustable pore size, fine tunable pore surface property, low density, flexible framework and alterable pore functionalities, flexibility in chemistry design, pore size, shape, and structure and high affinity to polymers and environmental friendly13,14. As reported in the literature, MOFs are widely used in many fields such as electrocatalysis, electrochemical devices (fuel cells, electrolysis cells, batteries, supercapacitors, sensors, etc.), gas storage and separation, liquid separation and purification, catalysis, and sensing15. MOFs are usually synthesized through the self-linkage and coordination of metallic species with organic ligands in a cyclic frame with porosity16,17.
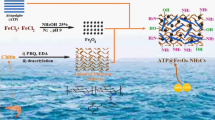
MILAl-101 is a representative of the broad family of MOFs, which is characterized by high porosity, stability, easy preparation, low cost, and surface functionality, making it an ideal choice for adsorption of contaminants18. Many researchers have proved the excellent performance of MILs toward the adsorptive removal of ibuprofen19, toxic dyes20, sulfacetamide21 and ciprofloxacin22. Although MOFs-based adsorbents provide some advantages, however, their tendency to aggregate and lose during the recovery process is considered a respective drawback for their applications23. Chitosan (Cs) is a natural, non-toxic, and inexpensive polysaccharide that with its unique physical and chemical properties including biocompatibility, biodegradability, flexibility, and various functional groups24, has been proposed as a substrate to prevent MOF agglomeration/aggregation in the present study. Furthermore, the presence of free hydroxyl and amino groups in chitosan structure and permission of their chemical modification can facilitate the adsorption of various types of pollutants in chitosan-derived nanocomposites25. Therefore, it is expected that the combination of extraordinary properties of chitosan and MILAl-101 for the synthesis of MIL/Cs NCs as a novel adsorbent will have an acceptable performance toward AZT removal. After synthesis of MIL/Cs NCs and their utilization in the removal of AZT from samples, separation and reuse of the adsorbent is necessary and inevitable to reduce the costs of the adsorption process. This process is generally performed with filtration and centrifugation which require a lot of time, cost, and manpower. Hence, induction of magnetic properties to the MIL/Cs NCs using Fe3O4 NPs and its separation using an external magnet was considered to solve this problem26,27.
Given the issues mentioned above, the purpose of present research can be summarized as follows: (a) Synthesis of novel MIL/Cs@Fe3O4 NCs and its characterization by SEM, TEM, XRD, FTIR, BET and VSM techniques, (b) Modeling and optimization of the AZT adsorption using response surface methodology based central composite design (RSM-CCD), (c) study of isotherms, kinetics and thermodynamics of process, (d) Investigation of the interfering anion presence on the performance of adsorption process, (e) Determination of MIL/Cs@Fe3O4 NCs desorption and their stability and, (f) Comparison of synthesized adsorbent ability to remove AZT in real conditions with laboratory conditions.
Materials and methods
Materials and equipment
Reagents and instruments used in this study were given in Supplementary Information, Text 1.
Preparation and characterization of adsorbents
Chitosan obtained from shrimp shell (Cs)
The chitosan (Cs) synthesis procedure in detail is provided on Supplementary Information, Text 2.
NH2-MILAl-101
NH2-MILAl-101 was synthesized by a microwave method in accordance with a previously reported procedure with some modifications28. In detail, 0.51 g of AlCl3·6H2O and 0.56 g of 2-aminoterephthalic acid were dissolved in 70 mL of DMF. After 30 min stirring, the mixture was placed in a Teflon®-lined autoclave and heated at 140 °C by microwave for 3 h. Afterwards the autoclave was allowed to naturally cool to room temperature. The resulting solid product was filtered and washed with acetone for several times, activated with methanol under reflux over-night and evacuated at 200 °C for 6 h.
NH2-MILAl-101/chitosan (MIL/Cs NCs)
The procedure for the synthesis of NH2- MILAl-101/chitosan as described by Wang et al.18, was applied with slight modifications. 0.3 g chitosan powders, 1.1 g 2-aminoterephthalic acid, and 0.89 g AlCl3·6H2O were mixed well and dispersed in 100 mL DMF. The mixture was stirred for 45 min before being introduced into a Teflon autoclave, followed the same procedure as that for synthesis of NH2-MILAl-101.
NH2-MILAl-101/chitosan@Fe3O4 (MIL/Cs@Fe3O4 NCs)
MIL/Cs@Fe3O4 NCs was prepared by co-precipitation under alkaline conditions following our previous published method29. 0. 5 g of MIL/Cs NCs was added in 400 mL of iron salt solution consisting of iron (II) chloride (0.03 M) and iron (III) chloride (0.06 M). The obtained mixture was homogenized using an US probe for 30 min (20 kHz, 585 W). Under US irradiation, 30 mL of NaOH solution added drop wise into the mixture until pH kept at 10–11. The suspension was mixed and heated at 80 °C for 1 h followed by cooling. Finally, the obtained black solid was magnetically collected and washed several times with distilled water until pH became neutral. MIL/Cs@Fe3O4 NCs was dried overnight at 105 °C in a hot air oven and stored in an airtight container for further experiments.
Characterization
Characteristic analyzes for the synthesized adsorbent are presented in Supplementary Information, Text 3.
Adsorption procedures
In this study, all the experiments were performed in a 250 mL conical flask containing 100 mL of aqueous solution. To determine the effect of key parameters on the removal efficiency, the sample solutions were agitated in an air shaker at 150 rpm at room temperature. A Central Composite Design (Four factorial CCD) approach in the series of batch tests were developed to optimize the critical parameters in adsorption process (an experimental matrix of the RSM-CCD is listed in Table 1). All experiments were performed in triplicate. The adsorption efficiency (R, %), and capacity (qe, mg/g), were calculated by the following equations:
where C0 (mg/L) and Ce (mg/L) are the initial and equilibrium AZT concentrations, respectively; V (L) is the volume of solution, and m (g) is the mass of adsorbent. After optimizing the above-mentioned parameters, kinetics (0–80 min), isotherms (10–200 mg/L) and thermodynamic (278–343 °K) of adsorption process were investigated according to a study by Azari et al.30. Desorption and economic feasibility of MIL/Cs@Fe3O4 NCs toward AZT removal were investigated with ultrasound + chemical modifications in various ethanol concentrations. Finally, effect of background electrolytes on adsorption process and adsorbent performance in real samples was investigated and reported.
Adsorption modeling by CCD-DF
To enhance the AZT removal efficiency using the smallest number of potential adsorption tests, a response surface methodology (RSM) based central composite design (CCD) technique (MODDE® Pro Software, V.11.0.2) was applied. Table 1 shows the proposed statistical method to investigate the effect of four independent parameters including pH (2–10), adsorbent dose (0.1–0.5 g/L), AZT concentration (10–50 mg/L) and time (10–90 min) on the response factor (adsorption efficiency, %). In this study, a total of 21 experiments were investigated. At a level of 0.05, ANOVA analysis was used to discover which factors had a significant impact on the response variable. The model parameters were described using analysis of variance (ANOVA), correlation coefficient (R2) value, F-test value, and contrasting of experimental Vs. model-predicted data. The model's accuracy and applicability were further evaluated using the probability value (Prob > F), relative standard deviation (RSD), pure error, and Q2 value. The operational parameters of the AZT adsorption process were optimized using desirability functions (DF). The selected models' process optimization findings were repeated (n = 5) on a laboratory scale (actual circumstances) until the best and most accurate model for AZT adsorption was found.
Results and discussion
Adsorbent characteristics
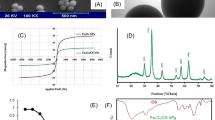
The SEM analysis of the MIL/Cs@Fe3O4 NCs is shown in Fig. 1a. As shown, NH2-MILAl-101 with hexagonal shape and average diameter of 88.5 ± 10.1 nm are distributed non-uniformly on the surface of chitosan. The surface of chitosan is observed as smooth, sheet-like and layered form. In other sides, Fe3O4 NPs with spherical structure (23.3 ± 4.0 nm) are observable on the chitosan surface. The image reveals that the surface of the adsorbent is rough and has a good porosity. This feature leads to a better contact of the adsorbent with the pollutant, which resulted in an improvement in adsorption performance. Furthermore, no agglomeration is seen in the MIL/Cs@Fe3O4 NCs structure which can be related to the presence of chitosan in the structure of the synthesized sample. A TEM analysis was applied to investigate the shape and particle sizes of MIL/Cs@Fe3O4 NCs, and its results are presented in Fig. 1b. The images indicate that the NH2-MILAl-101 and Fe3O4 nanoparticles are hexagonal and spherical respectively and distributed heterogeneously in a size range of 80–100 nm and 20–40 nm on the chitosan surface, which coincides with results obtained from SEM image. Due to the higher density of NH2-MILAl-101 and Fe3O4 than chitosan, these nanoparticles are recognized in a darker color (black), while chitosan has a light and transparent structure. DLS technique was employed to determine the particle size distribution (Figure is not shown): the obtained average sizes of Fe3O4 NPs, NH2-MILAl-101 and MIL/Cs@Fe3O4 NCs were 21.75 nm, 85.34 nm and 108.96 nm, respectively. The FTIR spectrum of the adsorbents at a wavelength within 400–4000 cm−1 is shown in Fig. 1c. The peak at 609 cm−1 is attributed to the Fe–O vibration of Fe3O4 NPs31. The medium peak at 466 cm−1 is due to Al–O vibrations, which confirms the formation of the organometallic structure. The broad peak from 3382 cm−1 is attributed to the –NH2 and –OH groups and the peaks at 1689 and 1598 cm−1 are related to the CONH2 and NH2 groups in the chitosan structure, respectively32. The peaks at 1438 and 1496 cm−1 were assigned to symmetric and asymmetric stretching of carboxyl groups, respectively. The peak at 1393 and 771 cm−1 correspond to the –COO– bond and C–H vibration of the aromatic cycles in the phenyl ring. These bonds at 1438, 1496, 1393 and 771 cm−1 suggest the presence of the organic ligands of 2-aminoterephthalic acid. Two peaks appearing at 1259 and 1116 cm−1 can be attributed to the stretching vibrations of aromatic functional epoxy C–O and alkoxy C–O groups, respectively. XRD pattern of synthesised MIL/Cs@Fe3O4 NCs in the range of 2θ = 5–70° is represented in Fig. 1d. The characteristic peaks of NH2-MILAl-101 are appeared at 2θ = 6.28°, 7.12°, 8.89°, 10.27° and 17°, which were relatively similar to the patterns of previous references33,34. The diffraction peaks at 10.96°, 19.86° and 21.64° are assigned to crystal forms of chitosan. On the other hand, the obtained peaks at 26.3°, 30.22°, 35.57°, 43.21°, 53.57°, 62.74°, and 65.88° related to the diffraction planes of 172°, 220°, 342°, 400°, 511°, 122°, and 106°, respectively confirm the presence of Fe3O4 crystals in the MIL/Cs@Fe3O4 NCs structure (0866-088-01 JCPDS NO.). The above results indicate that NH2-MILAl-101 and Fe3O4 NPs are loaded onto the CS and MIL/Cs@Fe3O4 NCs was synthesized successfully. Magnetic measurements and separation of MIL/Cs@Fe3O4 NCs were taken with a VSM analysis at room temperature, as presented in Fig. 1e. This analysis was performed in a magnetic field of ± 10 KOe and saturation magnetization around ± 80 emu/g. The results showed that the saturation magnetization (Ms) of MIL/Cs@Fe3O4 NCs was observed around 60.77 emu/g. The result clearly demonstrated the appropriate magnetic property of the synthesized adsorbent. The Nitrogen adsorption–desorption isotherms and the pore size distribution of the MIL/Cs@Fe3O4 NCs is shown in Fig. 1f and Supplementary Table S1. The isotherms are identified as type IV with H4 typical hysteresis loops, which are the characteristic isotherm of mesoporous materials according to IUPAC classification. The BET surface area and average pore diameters of the of the product were also calculated as 451.73 m2/g and 2.78 nm, respectively. The BET surface area calculated for the MIL/Cs@Fe3O4 NCs is ~ 1.5 times higher than the Fe3O4@C nanocomposite synthesized by Van Tran et al.35. Although these values are higher than all samples (Fe3O4 NPs, NH2-MILAl-101 and MIL/Cs NCs), but this difference between MIL/Cs@Fe3O4 NCs and MIL/Cs NCs is not too large. This phenomenon may be due to the occupation of the porosity of MIL/Cs NCs by magnetic nanoparticles (Fe3O4) and/or clotting (agglomeration) of the nanocomposite. Thermal stabilities of MIL/Cs@Fe3O4 NCs were investigated by using TGA analysis (Fig. 1g). MIL/Cs@Fe3O4 NCs showed gradual weight loss within three steps. The first step occurred in the temperature range of 50–250 °C with a weight loss of 7%, attributed to the evaporation of solvent or water molecules inside the framework. A weight loss of 20% in the temperature range of 150–440 °C could be ascribed to the decomposition of guest molecules or unreacted products inside the ligand structure. In the final step, beyond 440 °C, MIL/Cs@Fe3O4 NCs structure degraded. The thermal stability results revealed the good stability of the synthesized adsorbent.
AZT removal under different systems
Figure 2 shows the ability of MIL/Cs@Fe3O4 NCs compared to Cs, Fe3O4, NH2-MILAl-101 and MIL/Cs NCs for AZT removal under similar experimental conditions (experiment conditions: Contact time: 60 min, pH: 7 ± 0.4, Sample dose: 0.5 g/L, AZT Conc.: 50 mg/L). AZT removal efficiencies by Cs and Fe3O4 were negligible (33.43 and 28%, respectively), showing that AZT cannot be effectively removed by Cs and Fe3O4. However, higher removal efficiencies were observed when Cs and Fe3O4 were coupled with NH2-MILAl-101 i.e., MIL/Cs NCs and MIL/Cs@Fe3O4 NCs. The removal efficiencies of AZT by NH2-MILAl-101, MIL/Cs NCs and MIL/Cs@Fe3O4 NCs were 44.23, 57.54 and 73.21% within 60 min, respectively. Higher surface area, better average pore volume/ size and more functional groups seem to be the reasons for the superior performance of MIL/Cs@Fe3O4 NCs over other adsorbents. The priority and order of samples in AZT removal were obtained as MIL/Cs@Fe3O4 NCs > MIL/Cs NCs > NH2-MILAl-101 > Cs > Fe3O4, respectively. Accordingly, MIL/Cs@Fe3O4 NCs was selected as the preferred adsorbent in AZT removal and was used in following experiments.
Effect of key parameters on adsorption of AZT
The effect of pH changes in the range of 2 to 10 on AZT adsorption by MIL/Cs@Fe3O4 NCs was investigated. As can be seen from Fig. 3, the removal efficiency is improved by increasing the pH so that the highest efficiency is observed in alkaline conditions and the lowest was obtained at pH < 5. In other side, the point of zero charge (pHpzc) values for MIL/Cs@Fe3O4 NCs was obtained to be 4.6 (Supplementary Fig. S1). The increasing trend of AZT removal with increasing the pH of the solution can be associated with the fact that pHpzc (4.6) of adsorbent is lower than the pKa (8.7) of AZT (pHpzc < pH < pKa). Therefore, at pH = ~ 8, the surface charges of the adsorbent and the adsorbate become opposites charge and an electrostatic attraction occurs between them which results in maximum adsorption. As a result, the optimal pH 8 was employed in further adsorption tests. Imani poor et al. (2021) studied the adsorption of azithromycin onto L-methionine modified montmorillonite K10 and 3-aminopropyltriethoxysilane functionalized magnesium phyllosilicate organoclays. They found that the maximum adsorption capacity obtained at a pH 8.0 ± 0.136. A similar result was observed for azithromycin removal using powdered zeolites in research by Sousa et al.37. They reported that the lowest efficiency was recorded under acidic pH (2.5–4.5), while alkaline conditions improved the performance of the azithromycin adsorption process. The effects of adsorbent dosage (from 0.1 to 0.5 g/L) on adsorption of AZT by MIL/Cs@Fe3O4 NCs was investigated at pH = 8.0, contact time = 60 min, and room temperature. Based on the results, the increase in the adsorbent dose has led to an increase in adsorption efficiency from 53 to 76%. The specific surface area and large quantity of reactive surface centers or functional groups of the adsorbent towards contaminates might be associated to adsorption percentage improvement as adsorbent increases38,39. Wahab et al. found that increasing the amount of magnetic activated carbon (MAC) from 0.01 to 0.15 g can increase the removal efficiency of AZT from ~ 10 to 100%40. In azithromycin adsorption onto modified natural clinoptilolite, Saadi et al. observed a direct relationship between process effectiveness and adsorbent dose. They reported that the initial quick adsorption was attributed to the unoccupied site and surface area of absorbent, resulting in successful adsorbate diffusion within the adsorbent41. The adsorption of AZT on MIL/Cs@Fe3O4 NCs was studied by adjusting contact time in the optimized pH and adsorbent dosage. From Fig. 3, increasing the contact time from 10 to 90 min has resulted in a 20% improvement in efficiency. Efficiency improvements with increasing time can be due to increase the contact and collisions of AZT with the MIL/Cs@Fe3O4 NCs, which subsequently improves the mass transfer of contaminates on to the adsorbent. Adsorbents generally have a limited and specific adsorption capacity and become saturated after reaching equilibrium time. The proper efficiency at the beginning of the adsorption process can be linked to the existence of large unoccupied sites and functional groups on the adsorbents that begin to fill over time42. A more detailed about the effect of contact time on azithromycin adsorption has been discussed and reported in the kinetics study section. Balarak et al. investigated the effect of contact time on AZT molecule adsorption on Azolla Filiculoides-based activated porous carbon ranging from 0 to 150 min. They found that increasing the contact time leads to an increase in percentage removal43. Other researchers have observed similar results in the adsorption of different antibiotics by modified alpha alumina nanoparticles and NaY zeolite synthesized from wheat straws ash44,45. Increasing the initial concentrations from 10 to 50 mg/L has resulted in a considerable drop in efficiency, as seen in Fig. 3. The inverse relationship between AZT concentration and efficiency is owed to the saturation of accessible active sites on MIL/Cs@Fe3O4 NCs. On the contrary, increasing the initial concentration of AZT has led to an increase in adsorbate per mass of adsorbent (qe, mg/g). The increased in qe (mg/g) at higher concentrations might be explained by the significant driving force for mass exchange at a large initial concentration46. Davoodi et al. studied the removal of AZT using raw and saponin-modified nano diatomite by varying the initial AZM concentration from 20 to 100 mg/L. They noticed that increasing the pollutant concentration causes a significant reduction in the adsorption process47. In 2020, Ardakani et al. used from PECVD film to evaluate the influence of initial concentration on AZT removal48. According to the Ardakani report’s, increasing the AZT concentration from 0 to 160 mg/L has resulted in a decrease in efficiency, and the highest efficiency has been obtained in the lowest concentration. Rapid saturation of active sites with increasing pollutant concentration was the main cause of efficiency reduction.
Statistical analysis and modeling of AZT adsorption
The validity of the model and operational parameters was investigated by RSM-CCD technique and ANOVA analysis. Table 2 summarizes the ANOVA results for the selected model. The developed model can well describe and predicted the adsorption of AZT by MIL/Cs@Fe3O4 NCs, as evidenced by the high values of R2 (> 0.997), Adj R2 (> 0.991), and Model F-value (170.46). Furthermore, the R2 value has reasonably agreed with the Adjusted R2 value; i.e., the difference is lesser than 0.2. On the other hand, RSD (1.873) confirms the model's suitability for interpreting the studied process. The signal-to-noise ratio is expressed by the adequate precision (A–P) parameter. This value was found to be 46.973 (A–P > 4) which indicates an adequate signal and model could be used to navigate the design (good match between experimental and computed results)49. The p-value of the lack of fit parameter was 6.2431e−01 (greater than 0.05), indicating that the developed model was appropriate and the predicted values are accurate50. Variance analysis was also used to investigate the effects of variables. The results show that all parameters had a considerable influence on the AZT adsorption except interaction between pH and time, pH and AZT concentration and adsorbent dose and time. The equation in terms of actual factors can be used to make predictions about the response for given levels of each factor. The equation of AZT adsorption on MIL/Cs@Fe3O4 NCs can be defined as follows:
It can be seen that the \(\mathrm{pH}\), \(\mathrm{Ads}.\mathrm{ dose}\), \(\mathrm{Time}\), \(\mathrm{pH}\times \mathrm{ AZT Conc}.\) and \(\mathrm{Ads}.\mathrm{ dose}\times \mathrm{ Time}\) parameters have positive signs, and the others are negative. These findings show that increasing the mentioned factors has a synergistic effect on the adsorption process; nevertheless, increasing other parameters reduces the efficiency. According to Eq. (3) and Table 2, pH with the highest coefficient has the greatest impact on the AZT adsorption process by MIL/Cs@Fe3O4 NCs. Plotting actual data Vs. predicted data with R2 > 0.948 illustrated that the developed model has a strong ability to predict removal efficiency (Supplementary Fig. S2a). The normal probability plot of the studentized residuals (Supplementary Fig. S2b), shows that the plotted points are close to a straight line (balanced and uniform distribution of residuals around the normalcy line.) that indicates the model has been well fitted with the experimental results. Supplementary Fig. S2c shows the residuals are randomly scattered near the horizontal zero reference (± 3) indicating a good fit of the model for AZT adsorption by MIL/Cs@Fe3O4 NCs.
Process optimization
After determination of the effect of each parameter (positive and negative roles) on the adsorption process, calculating the optimum conditions is essential and inevitable51. The purpose of optimization was improvement of AZT adsorption efficiency in the batch process, so the response parameter (AZT adsorption efficiency) was set to maximum level to determine the best performance of system. The lower and upper limit values of other variables were taken from the experimental data ranges (see Table 1). Based on the DF approach results, the maximum adsorption efficiency for AZT was 98.362 ± 3.24% at pH = 7.992, AZT conc. = 10.107 mg/L, ads. dose g/L = 0.279 and time equal to 64.256 min. To determine the accuracy of the software model results, optimized conditions (the above result) were simulated in laboratory scale and repeated 5 times. The evidence suggested that the simulated test results (96.021 ± 4.23%) are consistent with the model's results (98.362 ± 3.24%), so the result recorded by DF was used were used in the isotherms, kinetics and thermodynamic investigation. The percentage of parameters participation in maximizing the AZT adsorption process also revealed that pH and time with 64.063 and 1.206%, had the highest and lowest impact on system optimization, respectively.
Adsorption isotherms
To determine the best-fitting isotherm model with experimental data, the Langmuir, Freundlich, Temkin, and Dobbin-Radoshkvich models were studied (conditions: pH = 7.992, AZT Conc. = 10.107 mg/L, Ads. dose g/L = 0.279). From Table 3 and Supplementary data, Fig. S3, The R2 value for the Langmuir isotherm (R2 > 0.9981) is higher than other models (0.9759, 0.9396, and 0.7093 for Freundlich, Temkin, and Dobbin-Radoshkvich, respectively), indicating that experimental data is in well agreement with Langmuir model. The lower value of χ2 error (0.011) also confirms the results obtained. According to the Langmuir model, the AZT is adsorbed in a monolayer on a homogeneous surface of adsorbent with uniform energies for all the binding sites without any interaction between the molecules52,53. The adsorption capacity upon the Langmuir model was obtained as 238.5527 mg of AZT per gram of MIL/Cs@Fe3O4 NCs. The dimensionless separation factor (RL) of the Langmuir adsorption isotherm was used to determine the favourability of the adsorption process (irreversible → RL = 0, favourable → 0 < RL < 1, linear → RL = 1 and unfavourable → RL > 1). All the RL values lie in the range of 0 < RL < 1, which suggests that the AZT are favourably adsorbed on MIL/Cs@Fe3O4 NCs. Compared to Langmuir isotherm model, Freundlich isotherm model had the poorest fit with experimental data, as determined by comparison of R2 and χ2 values. However, high values of kF (60.837 L mg-1) and n parameter (greater than 1) indicated that adsorption of adsorbate on adsorbent was acceptable/feasible54,55. Table 3 reveals that the experimental data are less consistent with the Temkin isotherm model than either of the previous two models. Nevertheless, the positivity of Temkin isotherm constants (B = 32.042) proves that the adsorption process in the present study is endothermic in nature. The results indicated that experimental data fitted with the D–R isotherm with R2 > 0.709 under the optimized condition. Magnitude of adsorption energy, E, for AZT adsorption on the MIL/Cs@Fe3O4 NCs adsorbent was calculated to be 2704.0065 kJ/mol (> 40 kJ/mol), representing that the adsorption is chemisorption process56,57. Non-linear results of the studied isotherms (Fig. S3e) also clearly showed compliance of the experimental data with the Langmuir isotherm.
Adsorption kinetics
As can be seen from the Supplementary data, Fig. S4e, the adsorption efficiency increased sharply at the initial 60 min of the experiment, whereas negligible changes were observed thereafter, which indicates that the adsorption process has reached equilibrium state. The high adsorption rate at the beginning of process may be explained by the high affinity of MIL/Cs@Fe3O4 NCs for trapping pollutants due to the huge number of vacant sites (active sites) on adsorbent. Over time, the active sites in the adsorbent structure are occupied (filled) by pollutant molecules, which results in reduced efficiency and rate of AZT adsorption58,59. In the following, four conventional kinetic models including pseudo-first-order, pseudo-second-order, Elovich, and intraparticle diffusion, were employed to investigate the kinetics of AZT adsorption on MIL/Cs@Fe3O4 NCs under optimized conditions (see Supplementary data, Fig. S4a–d). Furthermore, the parameters related to the kinetic models i.e., error functions, and regression correlation coefficients (R2), are listed in Table 3. A pseudo-first-order model was found to be in good agreement with experimental data with a high R2 > 0.983, and χ2 values less than 0.032. Higher R2 values (0.9991) and lower χ2 rate (0.013) on the one hand and more compatibility between experimental qe (qe,exp: 35.86 mg/g) with the calculated qe (qe,cal: 37.60 mg/g) on the other hand clearly indicated that the pseudo-second-order model better describe the adsorption behaviour of AZT onto MIL/Cs@Fe3O4 NCs. The error values (χ2 = 0.039) and R2 parameter (0.9543) obtained from the Elovich model indicate that there is the least agreement between the experimental data and this model, and Elovich model is not suitable for describing the experimental data compared to the previous two models. The results obtained so far confirmed that the dominant mechanism of adsorption process is chemisorption60. The results also depicted that intraparticle diffusion model with R2 > 0.997 and χ2 = 0.024 has a higher ability than pseudo-first-order and Elovich models in interpreting data, while it has a weaker performance than pseudo-second-order kinetic model. Besides, the resulting fitting line in the intraparticle diffusion plot (qt against t0.5) did not pass through the origin. This shows that intraparticle diffusion was a rate-controlling step (rate-limiting mechanism) in the AZT adsorption process, along with the chemisorption reaction61,62.
Adsorption thermodynamic
Supplementary data, Fig. S5a shows the effect of different temperatures on the AZT adsorption efficiency at the obtained optimized conditions. As it turns out, with increasing temperature from 278 to 343 °K, adsorption capacities improved from 32.68 to 36.18 mg/g. For evaluating feasibility and nature of AZT adsorption process on MIL/Cs@Fe3O4 NCs, the thermodynamic parameters i.e., Gibbs free energy (ΔG°), enthalpy change (ΔH°), and entropy change (ΔS°) were investigated ant their results are given in Table 3. The quantities of enthalpy and entropy were calculated from slope and intercept of the plot of ln ln Kd vs. 1/T (Van't Hoff plot, Supplementary data, Fig. S5b). The values of ΔH° and ΔS° were found to be 755.23 and 0.22 kJ/mol, respectively. Positive value of ΔH° and also the directed trend between ln Kd and temperature implied that AZT adsorption on the MIL/Cs@Fe3O4 NCs was endothermic in nature, indicating that higher temperature enhanced adsorption capacity. ΔS° value became positive, so the increased randomness can be expected at the solid/solution interface during AZT adsorption. Besides, positive ΔS° value corresponded to an increase in the freedom degree of the adsorbed species63. Negative values of ΔG° (− 8.08 to − 22.85 kJ/mol) indicated feasibility and spontaneous nature of adsorption process. Moreover, the degree of spontaneity was increased by increasing temperature.
Effect of background ions
The effect of background electrolytes at an initial concentration of 1, 10 and 20 mM on AZT adsorption by MIL/Cs@Fe3O4 NCs are shown Fig. 4. It was found that the Na+ and K+ cations had a smaller effect on antibiotic adsorption than Mg2+ and Ca2+ ions. This might be explained by the fact that the high polarization power of divalent ions caused them to exert a stronger squeezing-out effect. The following reasons are also given by the researchers: (a) A dense water hydration shell might surround the adsorbed Mg2+ and Ca2+ ions could hinder the available adsorption sites by blocking the hydrophobic adsorption region, and (b) It is possible that Mg2+ and Ca2 compete directly for adsorption sites on MIL/Cs@Fe3O4 NCs due to inner-sphere complexation, which inhibits the formation of charge-assisted H-bonds with antibiotics. AZT adsorption by MIL/Cs@Fe3O4 NCs was not significantly affected by background anions, including Cl−, NO3−, SO42− and CO32−, which may be due to the strong repellence/repulsion between anions and the negatively charged adsorbent surface64.
Desorption and stability
To investigate the desorption and economic feasibility of MIL/Cs@Fe3O4 NCs toward AZT removal, the adsorbent was recycled with ultrasound + chemical modifications in various ethanol concentrations ranging from 10 to 100 (v/v percent). Figure 5a shows the amount of desorption against time at various ethanol concentrations under optimized conditions. As shown, the desorption percentage rose dramatically at various ethanol concentrations and reached to equilibrium in 40 min. In Fig. 5a,b, it can also be seen that the efficiency (%), capacity (mg/g) and rate (k, based on pseudo-second order kinetic) of desorption process have an upward trend up to the ethanol concentrations of 75%, but after that no further improvement has been observed. In the following, the adsorbent's reusability was evaluated for 10 consecutive cycles. For this purpose, after each cycle, MIL/Cs@Fe3O4 NCs were washed with ethanol and distilled water (75% v/v). As observed from Fig. 5c, the removal efficiency has decreased by only 9.13% during the first to fifth cycles, and this reduction in the adsorption has reached 30.24% in the tenth cycle. The decrease in efficiency can be ascribed to the loss of adsorbent mass and the filling of active sites or functional groups of the adsorbent during repeated runs. The amounts of leached aluminium and iron were 0.024 and 0.147 mg/L in the first cycle, and fell to 0.009 and 0.11 mg/L in the tenth cycle. The leaching of Al and Fe amounts in repeated runs is reasonable (Less than the water standard level), which proves the stability and durability of MIL/Cs@Fe3O4 NCs for AZT treatment.
Adsorption mechanism
The mechanism of organic compounds’ adsorption is usually described by hydrogen bonding, electrostatic interaction, and π–π interaction. MIL/Cs@Fe3O4 NCs used in this study are enriched with π-electrons, amino group (–NH2), and acidic carboxyl group (–COOH) that can play an important role in the adsorption mechanism between MIL/Cs@Fe3O4 NCs and AZM through hydrogen bonding (H-bonds), electrostatic interaction, and π–π interactions. As can be seen in Fig. S6, AZM is surrounded by high amounts of –OH, −CH3, =O, –O–, –N–, –N=, and –OCH3 functional groups. The aforementioned functional groups can play a role in AZM adsorption as follows: (a) –OH involved to form hydrogen bonding, (b) =O, –O–, –N–, –N=, and –O–CH3 groups involve in anionic electrostatic interactions, and (c) –CH3 group participates in cationic electrostatic interactions. Figure 6 shows possible adsorption interactions between AZM and MIL/Cs@Fe3O4 NCs. Since there is no π-electron in AZM, it is unlikely to have π–π interactions with MIL/Cs@Fe3O4 NCs65. Based on the evidence, adsorption may occur as a result of cationic and anionic electrostatic interactions and H-bonding between AZM and MIL/Cs@Fe3O4 NCs.
Study of real samples
In order to evaluate the effectiveness and potential of the MIL/Cs@Fe3O4 NCs for AZT adsorption in real conditions, a series of experiments on tap water, surface runoff, raw wastewater, and secondary effluent were considered under optimized conditions (AZT concentration spiked: 10 mg/L). In Supplementary data, Table S2, the specifications of samples are listed. As can be seen, all samples have lower efficiency compared to DI-water. For example, the efficiency in tap water was 90.45% while the adsorbent performance in surface runoff, raw and treated wastewater were 85.34%, 48.45%, and 65%, respectively (Supplementary data, Fig. S7). The decrease in AZT adsorption efficiency can be attributed to the presence of TDS, various ions, organic compounds, and other contaminants in the actual samples. Based on the results, it can be reported that MIL/Cs@Fe3O4 NCs has an acceptable ability to remove AZT from aqueous samples under real conditions, although this performance has been significantly reduced in raw wastewater samples.
Comparison with previous research
The qm parameter of synthesized adsorbent (adsorption capability based on the Langmuir isotherm model, mg/g) was compared with similar literature in terms of adsorbent type, and the results are summarized in Table 4. It was observed that the MIL/Cs@Fe3O4 NCs adsorbent showed good ability to compete with similar systems for treatment of AZT and has the proper place among the literature. It should be noted that the differences between the adsorption capacities in the various adsorbents can be related to the type of materials used (due to variability in size, surface area, number of active sites, and functional groups), adsorbent dosage and the concentration of the AZT in experiments. Available evidence confirms that the adsorbent synthesized in the present study can have a suitable and promising performance in the removal of AZT as a representative of macrolide antibiotics.
Conclusions
Magnetic NH2-MIL-101(Al)/Chitosan nanocomposite (MIL/Cs@Fe3O4 NCs) adsorbent was synthesized and developed for AZT treatment from queues solution. RSM-CCD approach was applied to predict and optimize the effect of key factors on AZT removal. This research led to the following results: (1) he results of ANOVA test revealed that the effect of all parameters on the performance of the AZT adsorption was significant with Prob > F < = 0.05 except pH × Time, pH × AZT concentration and Adsorbent dose × Time, (2) The higher adsorption efficiency (predicted: 98.362%, Real: 96.021) is obtained at pH = 7.992, adsorbent dose = 0.279 g/L, time = 64.256 min and the initial concentration = 10.107 mg/L with DF = 0.996, (3) The isotherms and kinetics of data were best fitted with the Langmuir and pseudo-second-order models, (4) Positive value of ∆H° and negative value of ∆G° indicated that AZT adsorption process was spontaneous and endothermic in nature, (5) After 10 reuses of the MIL/Cs@Fe3O4 NCs, the AZT removal efficiency dropped from 98.36 to 68.12, and (6) The inhibition effect of background electrolytes on AZT adsorption by MIL/Cs@Fe3O4 NCs follows a trend of Mg2+ > Ca2+ > K+ = Na+ > NO3− > Cl− > SO42− > CO32−.
Data availability
The data that support the findings of this study are available from the first author, [Ali Azari], on special request. Part of the data and materials are also available within supplementary materials.
Change history
17 July 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-38418-w
References
Yuan, Q. et al. Migration, transformation and removal of macrolide antibiotics in the environment: A review. Environ. Sci. Pollut. Res. 29, 1–18 (2022).
Naraginti, S., Yu, Y.-Y., Fang, Z. & Yong, Y.-C. Visible light degradation of macrolide antibiotic azithromycin by novel ZrO2/Ag@ TiO2 nanorod composite: Transformation pathways and toxicity evaluation. Process Saf. Environ. Prot. 125, 39–49 (2019).
Tiwari, B. et al. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 224, 1–12 (2017).
Spielmeyer, A. Occurrence and fate of antibiotics in manure during manure treatments: A short review. Sustain. Chem. Pharm. 9, 76–86 (2018).
Rivera-Utrilla, J., Sánchez-Polo, M., Ferro-García, M. Á., Prados-Joya, G. & Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 93, 1268–1287 (2013).
Polianciuc, S. I., Gurzău, A. E., Kiss, B., Ştefan, M. G. & Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 93, 231 (2020).
Jelić, A., Gros, M., Petrović, M., Ginebreda, A. & Barceló, D. Occurrence and elimination of pharmaceuticals during conventional wastewater treatment. In Emerging and Priority Pollutants in Rivers, 1–23 (2012).
Almeida, A. et al. Human and veterinary antibiotics used in Portugal—A ranking for ecosurveillance. Toxics 2, 188–225 (2014).
Bhushan, P., Gautam, D., Kumar, P., Shukla, S. & Mohan, D. An overview of human health risk from opium alkaloids and related pharmaceutical products pollution in aquatic ecosystems. In Ecological Significance of River Ecosystems, 1–14 (2022).
Eniola, J. O., Kumar, R., Barakat, M. & Rashid, J. A review on conventional and advanced hybrid technologies for pharmaceutical wastewater treatment. J. Clean. Prod. 356, 131826 (2022).
Zdarta, J., Meyer, A. S., Jesionowski, T. & Pinelo, M. Multi-faceted strategy based on enzyme immobilization with reactant adsorption and membrane technology for biocatalytic removal of pollutants: A critical review. Biotechnol. Adv. 37, 107401 (2019).
Mesdaghinia, A. et al. Removal of phthalate esters (PAEs) by zeolite/Fe3O4: Investigation on the magnetic adsorption separation, catalytic degradation and toxicity bioassay. J. Mol. Liq. 233, 378–390 (2017).
Dehghankar, M., Mohammadi, T., Moghadam, M. T. & Tofighy, M. A. Metal-organic framework/zeolite nanocrystal/polyvinylidene fluoride composite ultrafiltration membranes with flux/antifouling advantages. Mater. Chem. Phys. 260, 124128 (2021).
Van Tran, T. et al. High performance of Mn2 (BDC) 2 (DMF) 2-derived MnO@ C nanocomposite as superior remediator for a series of emergent antibiotics. J. Mol. Liq. 308, 113038 (2020).
Kuppler, R. J. et al. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 253, 3042–3066 (2009).
Shah, S. S. A. et al. Recent advances on oxygen reduction electrocatalysis: Correlating the characteristic properties of metal organic frameworks and the derived nanomaterials. Appl. Catal. B 268, 118570 (2020).
Van Tran, T. et al. Hexagonal Fe-based MIL-88B nanocrystals with NH2 functional groups accelerating oxytetracycline capture via hydrogen bonding. Surf. Interfaces 20, 100605 (2020).
Wang, Y., Wang, K., Lin, J., Xiao, L. & Wang, X. The preparation of nano-MIL-101 (Fe)@ chitosan hybrid sponge and its rapid and efficient adsorption to anionic dyes. Int. J. Biol. Macromol. 165, 2684–2692 (2020).
Xiong, P., Zhang, H., Li, G., Liao, C. & Jiang, G. Adsorption removal of ibuprofen and naproxen from aqueous solution with Cu-doped Mil-101 (Fe). Sci. Total Environ. 797, 149179 (2021).
Vo, T. K., Trinh, T. P., Nguyen, V. C. & Kim, J. Facile synthesis of graphite oxide/MIL-101 (Cr) hybrid composites for enhanced adsorption performance towards industrial toxic dyes. J. Ind. Eng. Chem. 95, 224–234 (2021).
Zhou, Q. & Liu, G. Urea-functionalized MIL-101 (Cr)@ AC as a new adsorbent to remove sulfacetamide in wastewater treatment. Ind. Eng. Chem. Res. 59, 12056–12064 (2020).
Li, Z. et al. Efficiently removal of ciprofloxacin from aqueous solution by MIL-101 (Cr)-HSO 3: The enhanced electrostatic interaction. J. Porous Mater. 27, 189–204 (2020).
Li, X. et al. Water contaminant elimination based on metal–organic frameworks and perspective on their industrial applications. ACS Sustain. Chem. Eng. 7, 4548–4563 (2019).
Nasrollahzadeh, M., Shafiei, N. & Orooji, Y. Magnetic chitosan stabilized Cu (II)-tetrazole complex: An effective nanocatalyst for the synthesis of 3-imino-2-phenylisoindolin-1-one derivatives under ultrasound irradiation. Sci. Rep. 12, 1–13 (2022).
Nasrollahzadeh, M., Sajjadi, M., Iravani, S. & Varma, R. S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: A review. Carbohyd. Polym. 251, 116986 (2021).
Hemmati, S., Heravi, M. M., Karmakar, B. & Veisi, H. In situ decoration of Au NPs over polydopamine encapsulated GO/Fe3O4 nanoparticles as a recyclable nanocatalyst for the reduction of nitroarenes. Sci. Rep. 11, 1–14 (2021).
El-Sharkawy, R. M., Swelim, M. A. & Hamdy, G. B. Aspergillus tamarii mediated green synthesis of magnetic chitosan beads for sustainable remediation of wastewater contaminants. Sci. Rep. 12, 1–15 (2022).
Ma, X.-H., Yang, Z., Yao, Z.-K., Xu, Z.-L. & Tang, C. Y. A facile preparation of novel positively charged MOF/chitosan nanofiltration membranes. J. Membr. Sci. 525, 269–276 (2017).
Moazzen, M. et al. Multi-walled carbon nanotubes modified with iron oxide and silver nanoparticles (MWCNT-Fe3O4/Ag) as a novel adsorbent for determining PAEs in carbonated soft drinks using magnetic SPE-GC/MS method. Arab. J. Chem. 12, 476–488 (2019).
Azari, A., Nabizadeh, R., Mahvi, A. H. & Nasseri, S. Magnetic multi-walled carbon nanotubes-loaded alginate for treatment of industrial dye manufacturing effluent: Adsorption modelling and process optimisation by central composite face-central design. Int. J. Environ. Anal. Chem. 1–21 (2021).
Van Tran, T. et al. Effect of thermolysis condition on characteristics and nonsteroidal anti-inflammatory drugs (NSAIDs) absorbability of Fe-MIL-88B-derived mesoporous carbons. J. Environ. Chem. Eng. 7, 103356 (2019).
Tang, Z. et al. Synthesis of SiBNC-Al ceramics with different aluminum contents via polymer-derived method. J. Am. Ceram. Soc. 105, 2914–2924 (2022).
Aljaddua, H. I., Alhumaimess, M. S. & Hassan, H. M. CaO nanoparticles incorporated metal organic framework (NH2-MIL-101) for Knoevenagel condensation reaction. Arab. J. Chem. 15, 103588 (2022).
Guan, Y., Xia, M., Wang, X., Cao, W. & Marchetti, A. Water-based preparation of nano-sized NH2-MIL-53 (Al) frameworks for enhanced dye removal. Inorg. Chim. Acta 484, 180–184 (2019).
Van Tran, T. et al. Recyclable Fe3O4@ C nanocomposite as potential adsorbent for a wide range of organic dyes and simulated hospital effluents. Environ. Technol. Innov. 20, 101122 (2020).
Imanipoor, J., Mohammadi, M. & Dinari, M. Evaluating the performance of l-methionine modified montmorillonite K10 and 3-aminopropyltriethoxysilane functionalized magnesium phyllosilicate organoclays for adsorptive removal of azithromycin from water. Sep. Purif. Technol. 275, 119256 (2021).
de Sousa, D. N. R. et al. Equilibrium and kinetic studies of the adsorption of antibiotics from aqueous solutions onto powdered zeolites. Chemosphere 205, 137–146 (2018).
Li, X. et al. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 235, 116213 (2020).
Dehghani, M. H., Salari, M., Karri, R. R., Hamidi, F. & Bahadori, R. Process modeling of municipal solid waste compost ash for reactive red 198 dye adsorption from wastewater using data driven approaches. Sci. Rep. 11, 1–20 (2021).
Wahab, M. et al. Adsorption-membrane hybrid approach for the removal of azithromycin from water: An attempt to minimize drug resistance problem. Water 13, 1969 (2021).
Saadi, Z., Fazaeli, R., Vafajoo, L., Naser, I. & Mohammadi, G. Promotion of clinoptilolite adsorption for azithromycin antibiotic by Tween 80 and Triton X-100 surface modifiers under batch and fixed-bed processes. Chem. Eng. Commun. 208, 328–348 (2021).
Mahmoudian, M. H., Fazlzadeh, M., Niari, M. H., Azari, A. & Lima, E. C. A novel silica supported chitosan/glutaraldehyde as an efficient sorbent in solid phase extraction coupling with HPLC for the determination of Penicillin G from water and wastewater samples. Arab. J. Chem. 13, 7147–7159 (2020).
Balarak, D., Mahvi, A. H., Shahbaksh, S., Wahab, M. A. & Abdala, A. Adsorptive removal of azithromycin antibiotic from aqueous solution by azolla filiculoides-based activated porous carbon. Nanomaterials 11, 3281 (2021).
Nguyen, N. T., Dao, T. H., Truong, T. T., Nguyen, T. M. T. & Pham, T. D. Adsorption characteristic of ciprofloxacin antibiotic onto synthesized alpha alumina nanoparticles with surface modification by polyanion. J. Mol. Liq. 309, 113150 (2020).
Ali, M. M. M. & Ahmed, M. J. Adsorption behavior of doxycycline antibiotic on NaY zeolite from wheat (Triticum aestivum) straws ash. J. Taiwan Inst. Chem. Eng. 81, 218–224 (2017).
Dovi, E. et al. Functionalization of walnut shell by grafting amine groups to enhance the adsorption of Congo red from water in batch and fixed-bed column modes. J. Environ. Chem. Eng. 9, 106301 (2021).
Davoodi, S., Dahrazma, B., Goudarzi, N. & Gorji, H. G. Adsorptive removal of azithromycin from aqueous solutions using raw and saponin-modified nano diatomite. Water Sci. Technol. 80, 939–949 (2019).
Sobhan Ardakani, S., Cheraghi, M., Jafari, A. & Zandipak, R. PECVD synthesis of ZnO/Si thin film as a novel adsorbent for removal of azithromycin from water samples. Int. J. Environ. Anal. Chem. 1–18 (2020).
Van Tran, T. et al. Response surface methodology-optimized removal of chloramphenicol pharmaceutical from wastewater using Cu3 (BTC) 2-derived porous carbon as an efficient adsorbent. C. R. Chim. 22, 794–803 (2019).
Saber, W. I. et al. Rotatable central composite design versus artificial neural network for modeling biosorption of Cr6+ by the immobilized Pseudomonas alcaliphila NEWG-2. Sci. Rep. 11, 1–15 (2021).
Rezaei, M., Pourang, N. & Moradi, A. M. Removal of lead from aqueous solutions using three biosorbents of aquatic origin with the emphasis on the affective factors. Sci. Rep. 12, 1–20 (2022).
Azari, A., Nabizadeh, R., Nasseri, S., Mahvi, A. H. & Mesdaghinia, A. R. Comprehensive systematic review and meta-analysis of dyes adsorption by carbon-based adsorbent materials: Classification and analysis of last decade studies. Chemosphere 250, 126238 (2020).
Liu, X. et al. Banana stem and leaf biochar as an effective adsorbent for cadmium and lead in aqueous solution. Sci. Rep. 12, 1–14 (2022).
Selambakkannu, S. et al. Modification of radiation grafted banana trunk fibers for adsorption of anionic dyes. Fibers Polym. 20, 2556–2569 (2019).
Zaib, Q. & Kyung, D. Optimized removal of hexavalent chromium from water using spent tea leaves treated with ascorbic acid. Sci. Rep. 12, 1–14 (2022).
Maniyazagan, M., Chakraborty, S., Pérez-Sánchez, H. & Stalin, T. Encapsulation of triclosan within 2-hydroxypropyl-β-cyclodextrin cavity and its application in the chemisorption of rhodamine B dye. J. Mol. Liq. 282, 235–243 (2019).
Ali, I., Khan, T. A. & Hussain, I. Treatment and remediation methods for arsenic removal from the ground water. Int. J. Environ. Eng. 3, 48–71 (2011).
Mirzaei, N. et al. Modified natural zeolite using ammonium quaternary based material for Acid red 18 removal from aqueous solution. J. Environ. Chem. Eng. 5, 3151–3160 (2017).
Abdelkhalek, A., El-Latif, M. A., Ibrahim, H., Hamad, H. & Showman, M. Controlled synthesis of graphene oxide/silica hybrid nanocomposites for removal of aromatic pollutants in water. Sci. Rep. 12, 1–22 (2022).
Shao, S., Ma, B., Chen, Y., Zhang, W. & Wang, C. Behavior and mechanism of fluoride removal from aqueous solutions by using synthesized CaSO4· 2H2O nanorods. Chem. Eng. J. 426, 131364 (2021).
Alnajjar, M. et al. Silica-alumina composite as an effective adsorbent for the removal of metformin from water. J. Environ. Chem. Eng. 7, 102994 (2019).
Azari, A., Nabizadeh, R., Mahvi, A. H. & Nasseri, S. Integrated Fuzzy AHP-TOPSIS for selecting the best color removal process using carbon-based adsorbent materials: Multi-criteria decision making vs. systematic review approaches and modeling of textile wastewater treatment in real conditions. Int. J. Environ. Anal. Chem. 1–16 (2020).
Hadavifar, M., Bahramifar, N., Younesi, H. & Li, Q. Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multi-walled carbon nanotube with both amino and thiolated groups. Chem. Eng. J. 237, 217–228 (2014).
Zeng, Z.-W. et al. Comprehensive adsorption studies of doxycycline and ciprofloxacin antibiotics by biochars prepared at different temperatures. Front. Chem. 6, 80 (2018).
Upoma, B. P. et al. A fast adsorption of azithromycin on waste-product-derived graphene oxide induced by H-bonding and electrostatic interactions. ACS Omega 7, 29655–29665 (2022).
Acknowledgements
The authors would like to thank Zahedan University of Medical Sciences for funding this research. (Grant number: 10588, Ethics code: IR.ZAUMS.REC.1401.167).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. A.A. and M.M.: Visualization, writing original draft/review, editing and supervision. A.A., K.Y.: Conceptualization, methodology, software, writing-reviewing and editing. G.M. and M.R.M.: Formal analysis, methodology, investigation and writing original draft preparation. R.S. and S.H.: Investigation/writing original draft, formal analysis, data collection and methodology. H.K.: Investigation, software and validation. N.S.F.: Material preparation, data collection and analysis. N.J.: Methodology, writing, review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The Acknowledgements section in the original version of this Article was incomplete. “This work was supported by the Zahedan University of Medical Sciences, Zahedan, Iran [Grant No. 10588]. now reads: “The authors would like to thank Zahedan University of Medical Sciences for funding this research. (Grant number: 10588, Ethics code: IR.ZAUMS.REC.1401.167).”
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azari, A., Malakoutian, M., Yaghmaeain, K. et al. Magnetic NH2-MIL-101(Al)/Chitosan nanocomposite as a novel adsorbent for the removal of azithromycin: modeling and process optimization. Sci Rep 12, 18990 (2022). https://doi.org/10.1038/s41598-022-21551-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21551-3
This article is cited by
-
Equilibrium and kinetic modeling of Cr(VI) removal by novel tolerant bacteria species along with zero-valent iron nanoparticles
Scientific Reports (2024)
-
Magnetic attapulgite synthesized via Sonochemistry: an innovative strategy for efficient solid phase extraction of As3+ from simulated and unrefined crude oil samples
Journal of Porous Materials (2024)
-
Performance and mechanism of graphene oxide removal from aqueous solutions by calcite: adsorption isotherms, thermodynamics, and kinetics
Environmental Science and Pollution Research (2024)
-
Silver doped anatase nanocomposite: a novel photocatalyst with advanced activity under visible light for waste-water treatment
Discover Applied Sciences (2024)
-
The performance of temperature and acid-modified sludge in removing lead and cadmium
Environmental Science and Pollution Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.