Abstract
Nasal decolonization procedures against the opportunistic pathogen Staphylococcus aureus rely on topical antimicrobial drug usage, whose impact on the nasal microbiota is poorly understood. We examined this impact in healthy S. aureus carriers and noncarriers. This is a prospective interventional cohort study of 8 S. aureus carriers and 8 noncarriers treated with nasal mupirocin and chlorhexidine baths. Sequential nasal swabs were taken over 6 months. S. aureus was detected by quantitative culture and genotyped using spa typing. RNA-based 16S species-level metabarcoding was used to assess the living microbial diversity. The species Dolosigranulum pigrum, Moraxella nonliquefaciens and Corynebacterium propinquum correlated negatively with S. aureus carriage. Mupirocin treatment effectively eliminated S. aureus, D. pigrum and M. nonliquefaciens, but not corynebacteria. S. aureus recolonization in carriers occurred more rapidly than recolonization by the dominant species in noncarriers (median 3 vs. 6 months, respectively). Most recolonizing S. aureus isolates had the same spa type as the initial isolate. The impact of mupirocin-chlorhexidine treatment on the nasal microbiota was still detectable after 6 months. S. aureus recolonization predated microbiota recovery, emphasizing the strong adaptation of this pathogen to the nasal niche and the transient efficacy of the decolonization procedure.
Similar content being viewed by others
Introduction
Staphylococcus aureus is an opportunistic pathogen and a frequent cause of severe infections. Approximately 20% of the general population are persistent S. aureus carriers and another 30% are intermittent carriers1. S. aureus is commonly carried in the nose and less frequently in the throat, skin, and perineum1.
Staphylococcus aureus carriers are at higher risk of infection after invasive procedures and surgery2,3. To prevent infections, several countries recommend eliminating S. aureus from the nose prior to the at-risk intervention using a decolonization procedure4. This typically involves topical antimicrobial treatment with mupirocin nasal ointment with or without chlorhexidine cutaneous body and hair wash. Different decolonization approaches have emerged due to costs and organizational issues in health care5. While some advise to treat all patients undergoing at-risk interventions, others limit decolonization to confirmed carriers only.
While we know that the nasal microbiome composition is related to S. aureus presence6,7, the impact of decolonization procedures on the nasal microbiota is not yet fully understood. In previous nasal microbiome studies, S. aureus carriage was associated with higher relative abundances of Cutibacterium acnes, Corynebacterium accolens and non-aureus staphylococci, and with lower abundances of Corynebacterium pseudodiphtheriticum, Dolosigranulum spp. and Cutibacterium granulosum6,7. These associations suggest that the distribution of microbial species in the nose influences S. aureus persistence, possibly through competition for nutrients and epithelial binding sites8. In turn, the alteration of the microbial distribution after a decolonization procedure might impact the likelihood of persistent S. aureus recolonization and, from a clinical standpoint, of decolonization failure. However, the magnitude and duration of microbiota alterations after decolonization are not elucidated. So far, a single-patient microbiome study found shifts in the composition and biodiversity of the nasal microbiota after mupirocin treatment9, contrasting with a previous culturomics study of 5 healthy volunteers in which no significant change of microbiota richness and diversity were found up to 1 month after decolonization10.
To decipher the relationships between S. aureus nasal carriage, the nasal microbiota and decolonization procedures, we conducted a prospective interventional cohort study of S. aureus carriers and noncarriers, monitoring microbial community changes over 6 months after mupirocin-chlorhexidine treatment. Using quantitative cultures and 16S metabarcoding, we examined the impact of decolonization on bacterial communities and the delay to recolonization with S. aureus and other dominant species.
Results
Staphylococcus aureus elimination and recolonization
Of 35 volunteers, 8 carriers and 8 noncarriers were included (see flowchart of patient selection in Fig. 1). The S. aureus carrier group consisted of 3 males and 5 females of 22–71 years old (median, 26 years). Noncarriers were 2 males and 6 females aged 18–62 years (median, 56 years). No participants reported the use of antivirals, antiparasitics, immunosuppressants or probiotics in the 3 months prior or during the study. One noncarrier reported the use of amoxicillin/clavulanic acid, 5 days prior to the D0 sampling and again between the M3 and M6 sampling. This participant was retained as antimicrobial use occurred after recruitment and the microbiota composition did not differ from other noncarriers pre-decolonisation. No participants reported previous MRSA carriage. All 16 participants had at least 1 risk factor for S. aureus acquisition (Supplementary Table 1).
The dynamics of S. aureus elimination and recolonization after the decolonization treatment were examined using quantitative culture (Fig. 2A) and RNA metabarcoding (Fig. 2B). Both methods showed a steep decrease in S. aureus loads immediately after decolonization followed by a gradual increase indicating recolonization for some carriers. Failed decolonization in one carrier was confirmed in the first post-decolonization sample by both methods (Fig. 2). Recolonization was defined as a S. aureus positive culture (> 8 CFU/mL) post-decolonization. Five carriers (C1, C2, C5, C6 and C7) got recolonized during the follow-up period, including 3 carriers within 1 month post-decolonization. In the noncarrier group, 4 S. aureus positive cultures were found post-decolonization, 3 of which with only 1 CFU/mL.
Dynamics of S. aureus abundance in nasal samples of carriers and noncarriers undergoing decolonization. Shown are S. aureus abundance in quantitative culture (CFU/ml on a log scale; A) and proportion in 16S RNA metabarcoding (B) through time in 8 carriers (red) and 8 noncarriers (blue). D0 and D7 denote samples taken immediately before and after the decolonization procedure, respectively. Dashed lines denote each participant’s data. Solid lines and colored band denote the mean and 95% confidence interval. Both culture and metabarcoding analysis identified a sharp decrease of S. aureus abundance after decolonization followed by recolonization. On average, post-decolonization abundance of S. aureus was less than before decolonization.
RNA metabarcoding showed different recolonization results. While also 5 carriers (C1, C2, C3, C5 and C8) were recolonized according to RNA metabarcoding, discrepancies were found for 4 carriers (C3, C6, C7 and C8). For 2 carriers (C5 and C8), RNA metabarcoding showed recolonization without a positive culture. Another 2 carriers (C6 and C7) showed no recolonization in RNA metabarcoding despite a positive culture.
Spa types were determined in carriers exhibiting S. aureus recolonization in culture (n = 5). All but one recolonized S. aureus carriers showed the same spa type in pre- and post-decolonization samples. In 2 carriers, a different spa type was found, suggesting transient colonization by a strain different from the pre-decolonization carriage strain. Spa typing results are shown in Table 1. Details of recolonization delay and CFU/mL loads are shown in Supplementary Fig. 1. No phenotypic resistance to methicillin was found in the tested isolates.
Overall, the S. aureus decolonization remained successful over a 6-month period in only 3 participants (38%) (Fig. 2 and Supplementary Fig. 1), consistent with previous findings11. Interestingly, the metabarcoding approach detected small proportions (~ 1–5%) of S. aureus reads 2 days and 1 month after decolonization in several noncarriers (Fig. 2B). This might reflect transient invasion of the nasal niche by S. aureus isolates, possibly facilitated by the disruption of the nasal microbiome induced by decolonization, as described in gut microbiota after antibiotic-induced perturbations12. This intermittent carriage is to be expected in the normal population.
Disruption and recovery of the nasal microbiota after decolonization
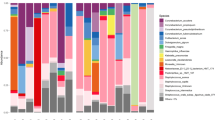
Before decolonization, nine dominant bacterial species in nasal microbiota, including S. aureus, S. epidermidis, D. pigrum, Moraxella nonliquefaciens, C. acnes and 4 Corynebactaria species were identified (Fig. 3A,C; see details for each participant in Supplementary Fig. 2). D. pigrum, a common taxon found in the anterior nares, was particularly abundant and prevalent in noncarriers. C. propinquum was present in both groups and was in average 15% more abundant in noncarriers. Mupirocin-sensitive species, including S. aureus and S. epidermidis, D. pigrum and M. nonliquefaciens, were virtually removed from the microbiota after decolonization, while mupirocin-resistant corynebacteria and C. acnes remained substantially abundant13,14. After decolonization, the average proportion of C. pseudodiphteriticum in noncarriers, but not carriers, increased tenfold after 7 days and the proportion of S. epidermidis increased tenfold after 1 month. At other time points, the average proportions of C. pseudodiphteriticum and S. epidermidis were comparable in carriers and noncarriers. In the 2 carriers and 4 noncarriers colonized with more than 10% of D. pigrum (Supplementary Fig. 2), 1 was recolonized with D. pigrum after 1 month, 2 after 3 months and all after 6 months. M. nonliquefaciens, which was observed in 2 noncarriers, recolonized only 1 participant after 6 months. The median time to recolonization with D.pigrum and M.nonliquefaciens, 2 major taxa present in noncarriers, was 6 months. In contrast, the median time to recolonization of S. aureus in carriers was 3 months. Microbiota profiles of individual participants are shown in Supplementary Fig. 2.
Evolution of the community structure of the nasal microbiota before and after mupirocin decolonization in S. aureus carriers and noncarriers. Shown are diversity bar plots of average species proportions (A,C) and the dissimilarity of these proportions (B,D) in each patient (dashed lines) and in average (solid lines; shaded area is the 95% confidence band of the mean). A high value for the Bray–Curtis dissimilarity indicates large difference in community structure relative to the initial state of the microbiota before decolonization. Nasal samples were taken immediately before decolonization (D0) and after 7 days (D7) and 1 (M1), 3 (M3), and 6 (M6) months in 8 S. aureus carriers (A,B) and noncarriers (C,D). Bacteria full names are, in order: Staphylococcus aureus, Dolosigranulum pigrum, Moraxella nonliquefaciens, Corynebacterium propinquum, Corynebacterium accolens, Corynebacterium pseudodiphtheriticum, Corynebacterium macginleyi, Staphylococcus epidermidis, Cutibacterium acnes.
To provide a more synthetic assessment of decolonization-induced changes of the microbial community structure, we computed the Bray–Curtis dissimilarity of the species assemblage at each time point, relative to the initial D0 time point in the same patient (Fig. 3B,D). The average Bray–Curtis dissimilarity was maximal immediately after decolonization in both carriers and noncarriers, denoting the most perturbed state of the microbiota. Strikingly, the dissimilarity decreased sharply in carriers but remained mostly stable in noncarriers, indicating that the microbiota of carriers (partially) reverted toward their initial state faster than in noncarriers, in line with faster recolonization by S. aureus compared to the dominant species found in noncarriers. After 6 months, the average dissimilarities from the initial state remained substantial (~ 0.5–0.7) both in carriers and noncarriers (Fig. 3B,D). Importantly, the evolution of population structure varied strongly across participants (see dashed lines in Fig. 3B,D), with microbiota recovery patterns ranging from fast recovery (dissimilarity < 0.2 after 1 month) to virtually no recovery (dissimilarity > 0.9 after 6 months) both in carriers and noncarriers.
Discussion
In this longitudinal study of S. aureus carriers and noncarriers undergoing nasal mupirocin decolonization, we find that S. aureus recolonization in carriers occurred more rapidly than recolonization by the dominant species in noncarriers. These findings highlight the transient efficacy of the S. aureus decolonization procedure and the strong adaptation of S. aureus to the nasal niche.
Next to one case of failed decolonization, we observed frequent recolonization with S. aureus during the 6-month follow-up period. The time to recolonization ranged from 1 to 3 months, in line with previous observations in which the delay to recolonization ranged from 2 weeks to 6 months after mupirocin treatment15. In another longitudinal study collecting samples of 571 participants every 2 months for > 2 years, anti-staphylococcal antibiotics increased the rate of S. aureus acquisition within 4 months after treatment16, suggesting that microbiota disruption by antibiotics facilitates the invasion by S. aureus. In our study, 4 of 5 cases of recolonization eventually occurred with the same spa-type as isolated from the carrier initially. However, transient colonization with another spa-type was also demonstrated. This is in accordance with other studies showing longitudinal carriage of the same strain, with intermittent carriage of other strains as well16,17,18. While longitudinal studies suggest that loss and acquisition of S. aureus occur as natural events16,18, another reason for recolonization could be the lack of successful decolonization. Resistance to the decolonization treatment could facilitate recolonization. However, as Dutch national surveillance for resistance in S. aureus has shown low levels of mupirocin resistance (1%)19, it seems unlikely this would drive recolonization in our study participants. Recolonization from an untreated extra-nasal body site, such as the pharynx, or through household members is a more probable explanation.
Next to the loss of S. aureus, decolonization caused the immediate removal of S. epidermidis, D. pigrum and M. nonliquefaciens from the nose. In noncarriers, a trend towards a higher abundance of C. propinquum was observed, while an increase in C. accolens and S. epidermidis was shown in effectively decolonized carriers. Indeed, mupirocin treatment has been previously tied to an increase of the relative abundance of (unclassified) corynebacteria and C. acnes, along with a decrease of S. epidermidis abundance20. Together, these results imply a rearrangement of the nasal microbiota after decolonization treatment and the removal of mupirocin-susceptible species including S. aureus, allowing new taxa to invade the nasal niche.
Our study has limitations beyond its small sample size. To enhance study participation, we adopted a self-sampling strategy which allowed participants to send in samples through regular mail service. This method has been found appropriate for detection of S. aureus previously21,22. Nevertheless, delayed transport caused 20% of samples to be processed > 48 h after sampling. As only 3 of 27 delayed samples in carriers were culture-negative, the risk of false negative S. aureus cultures due to transport can be considered low. However, the impact of delay on metabarcoding approaches is unknown. Nevertheless, delayed transport had no effect on the overall recolonization results in this study.
Discrepancies were found between quantitative culture results and RNA metabarcoding regarding the presence of S. aureus in the post-decolonization samples. We defined recolonization based on a S. aureus positive culture (> 8 CFU/mL) after decolonization, consistent with our definition of S. aureus carriage. The varying nasal bacterial load, the intrinsic microbiota composition as well as potential influence of transport to the sequencing facility added to the multi-step RNA metabarcoding analyses are amongst the many factors explaining such differences with the culture results. Both methods agreed about recolonization status in three carriers only.
Overall, our findings highlight the sensitivity of the nasal bacterial community to mupirocin treatment and stress the fact that the decolonization target, namely S. aureus, re-enters the nasal niche comparably faster than the dominant species in noncarriers. This supports the current use of mupirocin as a short-term prevention procedure preceding an identified at-risk intervention, rather than a means of eliminating circulating S. aureus isolates.
Methods
Study population and study design
This is a prospective interventional cohort study of healthy S. aureus carriers and noncarriers in the Netherlands. All experiments were performed in accordance with the Dutch Medical Research Involving Human Subjects Act (WMO). The study protocol was approved by the local Medical Ethical Committee of the Erasmus University Medical Centre Rotterdam, The Netherlands (MEC-2018-091). Written informed consent was obtained for all participants. Participants were recruited through advertisements at Dutch universities and the research teams social networks. Exclusion criteria were age < 18 years, use of antibiotics, antiparasitics, antifungals or probiotics 3 months prior to recruitment, known allergy to components of the intervention treatment, pregnant and breastfeeding women, known chronic diseases affecting the immune system, severe chronic skin diseases, immunocompromised status, or use of immunosuppressant drugs.
After filling out an eligibility questionnaire, all volunteers were screened for S. aureus carriage as described previously23. S. aureus carriage was determined by quantitative culture of 2 weekly nasal swabs. Persistent S. aureus carriers were defined as 2 positive cultures with > 8 CFU/mL for each culture. Noncarriers were defined as 2 S. aureus-negative cultures. Intermittent S. aureus carriers were excluded from further participation in the study. Eligible volunteers were enrolled on a first-come, first-served basis.
Eligible participants were asked to fill out a questionnaire regarding risk factors for S. aureus acquisition. All participants received decolonization treatment. Decolonization consisted of mupirocin nasal ointment (2%, GlaxoSmithKline BV, Zeist, the Netherlands) twice daily and chlorhexidine gluconate cutaneous solution (4%w/v, Regent Medical Overseas Limited, Oldham, UK) once daily, both for 5 days.
Nasal samples were taken 1 day before decolonization (D0) and 2 days (D7), 1 month (M1), 3 months (M3) and 6 months (M6) after decolonization. All participants received a personal demonstration for nasal sampling by the executive researcher. Thereafter, all specimens were taken by the participants by inserting a swab (ESwab, 490CE.A, Copan Italia, Brescia, Italy) into one nostril and rotating 5 times, repeating this in the second nostril using the same swab. Swabs were collected in a container filled with 1 ml modified Liquid Amies, a collection and transport solution, and sent through regular mail service (non-temperature controlled) or deposited at the laboratory personally.
Staphylococcus aureus quantitative culture
Quantitative S. aureus cultures were conducted to examine the dynamics of S. aureus carriage over the 6-month follow-up period after decolonization. Swab containers were vortexed for 20 s before plating. Serial dilutions of Amies medium were plated onto phenol mannitol salt agar (PHMA) and incubated for 2 days at 37 °C. Swabs were placed in phenol mannitol salt broth (PHMB) and incubated for 7 days at 37 °C for enrichment. S. aureus growth was confirmed by a latex agglutination test (Staph Plus Latex Kit, Diamondial, Vienna, Austria). Morphologically different S. aureus colonies were selected for spa typing and methicillin resistance screening using BBL CHROMagar MRSA II agar (BD, Breda, The Netherlands).
Spa typing
Molecular typing of S. aureus isolates was performed to infer whether recolonization with S. aureus in decolonized carriers involved the same spa-type. Typing was limited to the last S. aureus positive culture moment and the last S. aureus positive culture moment after decolonization in recolonised carriers. S. aureus DNA lysates were prepared by boiling in 10 mM Tris–HCl, 1 mM disodium EDTA, pH 8.0 or extraction with the QIAamp DNA Mini Kit (QIAGEN, Venlo, The Netherlands) according to the manufacturer's instructions. Amplification of the S. aureus protein A (spa) repeat region was performed by PCR with 2 sets of primers. One set consisted of forward primer spa-1113, 5′-TAAAGACGATCCTTCGGTGAGC-3′ and reverse primer spa-1514, 5′-CAGCAGTAGTGCCGTTTGCTT-3′24. The other set consisted of forward primers spa-F1, 5′-AACAACGTAACGGCTTCATCC-3′ and spa-F2 5′-AGACGATCCTTCAGTGAGC-3′ and reverse primer spa-R1 5′-GCTTTTGCAATGTCATTTACTG-3′. Amplicons were purified with ExoSAP-IT (Applied Biosystems) according to the manufacturer’s instructions and sent for sequence analysis (Baseclear, Leiden, The Netherlands). Resulting sequences were analysed using BioNumerics v7.6 (Applied Maths NV, Sint-Martens-Latem, Belgium) and the spa types were assigned by use of the RidomStaphType database (Ridom GmbH, Würzburg, Germany).
16S ribosomal RNA sequencing of nasal microbiota
The impact of decolonization on the nasal microbiome and the recovery of the microbiome structure after decolonization were examined by means of 16S rRNA metabarcoding. Amies medium from each nasal swab container was stored at − 80 °C on the day of receipt at the study laboratory in Rotterdam, NL, then sent at − 80 °C to the microbiome analysis laboratory in Lyon, FR. To properly capture the impact of decolonization on the living microbiota, metabarcoding used RNA-based 16S ribosomal RNA (rRNA, which is preserved in living cells but quickly cleared after cell death or lysis) rather than the DNA coding sequence, as DNA can persist for prolonged time periods after cell death25,26,27,28. RNA was extracted using the Mag Bind® Total RNA 96 Kit (Omega Bio-tek) tissue protocol from 150 µL of samples’ material. Cell lysis was performed using beads (Disruptor plate C plus—Omega Bio-tek) and proteinase K for 15 min at 2600 rpm, followed by 10 min at room temperature without agitation, and finished with a DNase I digestion of 20 min at room temperature. RNA was quantified using QuantiFluor RNA kit on Tecan Safire (TECAN). 10 ng total RNA was used for reverse transcription using FIREScript RT cDNA synthesis kit (Solis Biodyne) with random primers, then cDNA was purified with SPRIselect reagent (Beckman coulter) and quantified.
The rRNA V1–V3 region was PCR amplified using the 5× HOT BIOAmp® BlendMaster Mix 12,5 mM MgCl 2 (Biofidal), 10× GC rich Enhancer (Biofidal) and BSA 20 mg/mL. The PCR reaction consisted of 30 cycles at 56 °C using the forward primer 27F, 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG AGAGTTTGATCCTGGCTCAG-3′ and reverse primer 534R, 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGATTACCGCGGCTGCTGG-3′ in 25 µL of solution. PCR products were purified using SPRIselect beads (Beckman Coulter) in 20 µL nuclease-free water and quantified using QuantiFluor dsDNA (Promega). Samples were indexed with lllumina’s barcodes with the same PCR reagents during a 12 cycles PCR, then purified and quantified as previously mentioned. Samples were normalized and pooled, then sequenced using Illumina MiSeq V3 Flow Cell following the constructor’s recommendations for a 2 × 300 bp paired-end application. A mean of 130 k proofread reads per sample was obtained.
Experiment buffers were used as negative controls to detect contamination by out-of-sample bacterial RNA. RNA extraction was controlled using an in-house mix of live Staphylococcus aureus ATCC29213 and Escherichia coli ATCC25922 in equal proportions, allowing for assessing extraction bias in Gram-positive and -negative bacteria. PCR amplification bias was controlled using a commercial DNA mix of 8 bacterial species (ZymoBIOMICS™ Microbial Community DNA Standard).
Bioinformatics and statistical analyses
Sequencing reads were quality checked and trimmed. Paired-ended read pairs were merged using BBMap version 38.49 (available at https://sourceforge.net/projects/bbmap/), with default options besides a minimum single size of 150 bp with an average Phred quality score higher than 10, and a total pair size of minimum 400 bp. PCR adapters were removed with cutadapt v.2.1 (Martin 2011) then dereplicated using vsearch v.2.12.029 with the sizeout option. For species assignment, reads were aligned to sequences of NCBI blast 16S_ribosomal_RNA database (version date 03.12.2020) using Blastn v.2.11.0+30,31, keeping a maximum of 20 reference targets. Read counts per bacterial species were normalized to account for taxon-specific variations of the copy number of 16S rRNA genes using NCBI rrnDB-5.5 database based on the mean gene copy number in the taxon.
To optimize the resolution of sequencing read taxonomic assignment, we used in-house bioinformatic software publicly available at https://github.com/rasigadelab/taxonresolve. Briefly, when a read matches sequences from several species with identical alignment scores, taxonomic assignment pipelines typically output the higher taxonomic level such as the genus (e.g., Staphylococcus spp. when a read matches S. aureus and S. epidermidis). This loss of information can be problematic when species-level discrimination is important. To prevent losing species-level information, the taxonresolve software assigns reads with uncertain species to groups of species rather than to genera.
Bacterial species deemed present from contaminating sources such as kits reagents and found in negative controls, mostly from the Bacillus genera, were removed. A total of 1376 species or group of species were retained. The rarefaction curves corresponding to the sequencing effort to assess the species richness within samples are shown in Supplementary Fig. 3. Most samples reached a plateau after 40,000 sequences.
Given the small sample size compared to the number of variables and species considered in this study, no hypothesis testing was performed, and we provide a descriptive assessment of the results. In figures, 95% confidence intervals of the means were computed based on normal approximation, after log transformation for CFU/mL and log odds transformation for quantities restricted to the [0, 1] interval, such as proportions.
In microbial diversity analyses, we retained the 9 most prevalent bacterial species and pooled the other species into an ‘Others’ category. To assess the disruption and possible recovery of the microbiota, the divergence of sampled microbiota relative to the initial, pre-treatment microbiota (D0) was assessed using the Bray–Curtis dissimilarity at each sampling time point relative to the first sample of the same patient.
Software code of the analyses are available at https://github.com/rasigadelab/macotra-metabarcoding. Data are available at https://zenodo.org/record/6382657. Analyses and figures used R software v3.6.032 with packages dplyr33, ggplot234, vegan35, and MicrobiomAnalyst available at https://www.microbiomeanalyst.ca36,37.
Data availability
Datasets generated for this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.6382657.
References
Wertheim, H. F. et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5, 751–762 (2005).
Sakr, A., Brégeon, F., Mège, J.-L., Rolain, J.-M. & Blin, O. Staphylococcus aureus nasal colonization: An update on mechanisms, epidemiology, risk factors, and subsequent infections. Front. Microbiol. 9, 2419 (2018).
Bode, L. G. M. et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N. Engl. J. Med. 362, 9–17 (2010).
World Health Organization. Evidence-based recommendations on measures for the prevention of surgical site infection. In Global Guidelines for the Prevention of Surgical Site Infection (2018).
Kalenic, S. et al. Comparison of recommendations in national/regional Guidelines for prevention and control of MRSA in thirteen European countries. Int. J. Infect. Control. https://doi.org/10.3396/ijic.V6i2.016.10 (2010).
Yan, M. et al. Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe 14, 631–640 (2013).
Liu, C. M. et al. Staphylococcus aureus and the ecology of the nasal microbiome. Sci. Adv. 1, e1400216–e1400216 (2015).
Krismer, B., Weidenmaier, C., Zipperer, A. & Peschel, A. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat. Rev. Microbiol. 15, 675–687 (2017).
Ramakrishnan, V. R. et al. Determinants of the nasal microbiome: Pilot study of effects of intranasal medication use. Allergy Rhinol. 9, 215265671878951 (2018).
Burnham, C.-A.D. et al. Topical decolonization does not eradicate the skin microbiota of community-dwelling or hospitalized adults. Antimicrob. Agents Chemother. 60, 7303–7312 (2016).
Price, A., Sarween, N., Gupta, I. & Baharani, J. Meticillin-resistant Staphylococcus aureus and meticillin-susceptible Staphylococcus aureus screening in a cohort of haemodialysis patients: Carriage, demographics and outcomes. J. Hosp. Infect. 90, 22–27 (2015).
Pérez-Cobas, A. E. et al. Gut microbiota disturbance during antibiotic therapy: A multi-omic approach. Gut 62, 1591–1601 (2013).
Sum, S., Park, H.-M. & Oh, J. Y. High-level mupirocin resistance in Gram-positive bacteria isolated from diseased companion animals. J. Vet. Sci. 21(3), e40. https://doi.org/10.3396/ijic.V6i2.016.10 (2020).
Sutcliffe, J. et al. Susceptibility of Cutibacterium acnes to topical minocycline foam. Anaerobe 62, 102169 (2020).
Mody, L., Kauffman, C. A., McNeil, S. A., Galecki, A. T. & Bradley, S. F. Mupirocin-based decolonization of Staphylococcus aureus carriers in residents of 2 long-term care facilities: A randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 37, 1467–1474 (2003).
Miller, R. R. et al. Dynamics of acquisition and loss of carriage of Staphylococcus aureus strains in the community: The effect of clonal complex. J. Infect. 68, 426–439 (2014).
Sakwinska, O. et al. Ecological temporal stability of Staphylococcus aureus nasal carriage. J. Clin. Microbiol. 48, 2724–2728 (2010).
Muthukrishnan, G. et al. Longitudinal genetic analyses of Staphylococcus aureus nasal carriage dynamics in a diverse population. BMC Infect. Dis. 13, 221 (2013).
Stichting Werkgroep Antibioticabeleid & Rijksinstituut voor Volksgezondheid en Milieu (RIVM), C. I. (CIb). NETHMAP 2012. Consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in the Netherlands. https://swab.nl/nl/nethmap (2012).
Roghmann, M.-C. et al. Effect of mupirocin for Staphylococcus aureus decolonization on the microbiome of the nose and throat in community and nursing home dwelling adults. PLoS One 16, e0252004 (2021).
van Cleef, B. A., van Rijen, M., Ferket, M. & Kluytmans, J. A. Self-sampling is appropriate for detection of Staphylococcus aureus: A validation study. Antimicrob. Resist. Infect. Control 1, 34 (2012).
Harrison, E. M. et al. Validation of self-administered nasal swabs and postage for the isolation of Staphylococcus aureus. J. Med. Microbiol. 65, 1434–1437 (2016).
Nouwen, J. L. et al. Predicting the Staphylococcus aureus nasal carrier state: Derivation and validation of a ‘culture rule’. Clin. Infect. Dis. 39, 806–811 (2004).
Aires-de-Sousa, M. et al. High interlaboratory reproducibility of DNA sequence-based typing of bacteria in a multicenter study. J. Clin. Microbiol. 44, 619–621 (2006).
Li, R. et al. Comparison of DNA-, PMA-, and RNA-based 16S rRNA Illumina sequencing for detection of live bacteria in water. Sci. Rep. 7, 5752 (2017).
Kaplan, H. B., Miranda, J. A., Gogola, G. R., Gomez, K. & Ambrose, C. G. Persistence of bacterial DNA in orthopedic infections. Diagn. Microbiol. Infect. Dis. 91, 136–140 (2018).
Levy-Booth, D. J. et al. Cycling of extracellular DNA in the soil environment. Soil Biol. Biochem. 39, 2977–2991 (2007).
Fittipaldi, M., Nocker, A. & Codony, F. Progress in understanding preferential detection of live cells using viability dyes in combination with DNA amplification. J. Microbiol. Methods 91, 276–289 (2012).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 4, e2584 (2016).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Camacho, C. et al. BLAST+: Architecture and applications. BMC Bioinform. 10, 421 (2009).
R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2018). https://www.R-project.org/. Accessed 23 September 2022.
Wickham, H., François, R., Henry, L., Müller, K. dplyr: A Grammar of Data Manipulation. (2021). https://dplyr.tidyverse.org/. Accessed 23 September 2022.
Wickham, H. ggplot2: Elegant Graphics for Data Analysis. (Springer, 2016). https://ggplot2.tidyverse.org/. Accessed 23 September 2022.
Oksanen, J. et al. vegan: Community Ecology Package (2020). https://cran.r-project.org/web/packages/vegan/index.html. Accessed 23 September 2022.
Dhariwal, A. et al. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 45, W180–W188 (2017).
Chong, J., Liu, P., Zhou, G. & Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 15, 799–821 (2020).
Acknowledgements
We thank all participants for their contribution to this study. Special acknowledgements to Willem van Wamel, Patricia Vellekoop and Willemien Zandijk for laboratory assistance, as well as to Agnès Nguyen (Biofidal, Vaulx-en-Velin, France), for insightful discussions and assistance regarding the metabarcoding strategy used in this paper. This work was part of the MACOTRA project, funded by ZonMw Grant number 547001006 and ANR Grant number 16-JPEC-0006. We acknowledge all members of the MACOTRA study group as listed in supplementary information.
Author information
Authors and Affiliations
Contributions
A.B. and V.B. wrote the original manuscript. M.V. and J.P.R reviewed and edited the manuscript. M.V., G.L, J.P.R conceptualized and supervised the project. V.B. and M.T. conducted the recruitment of the cohort. A.B., V.B. and M.T. designed in vitro methodologies and generated data and figures. A.B. developed bioinformatics and biostatistics analysis programs for the study. A.B., V.B., M.T. and J.P.R. conducted statistical analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baede, V.O., Barray, A., Tavakol, M. et al. Nasal microbiome disruption and recovery after mupirocin treatment in Staphylococcus aureus carriers and noncarriers. Sci Rep 12, 19738 (2022). https://doi.org/10.1038/s41598-022-21453-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21453-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.