Abstract
Ambrosia artemisiifolia and Ambrosia trifida are annual invasive plants that cause serious harm to agriculture, animal husbandry, and human health. Based on the important characteristic of high-density, cluster distribution of their populations, it is speculated that its autotoxins have an effect on density regulation. This study explored the regulation of autotoxicity on intraspecific density. We used water extracts from two plants to compare and verify the autotoxicity of seed germination, analysed the components of autotoxins. The results showed that A. artemisiifolia and A. trifida had significant autotoxicity, and the highest inhibition rates on seed germination were 27.21% and 77.94%, respectively; ultra-performance liquid chromatography tandem mass spectrometry analysis revealed that chlorogenic acid, caffeic acid, p-coumaric acid, and vanillin were the main autotoxins of the two plants. After the seeds were washed with water, the germination recovery rate of seeds increased with the increased of inhibition degree of autotoxins treatment. Therefore, this study verified the autotoxicity of A. artemisiifolia and A. trifida, which can promote and inhibit the seed germination of A. artemisiifolia and A. trifida to regulate intraspecific competition.
Similar content being viewed by others
Introduction
Allelopathy in plants affects the growth and development of the same or other species by releasing allelochemicals into the surrounding environment1,2,3. It plays a vital role in regulating intra- and inter-specific relationships4, changing microbial communities, and affecting plant growth and litter decomposition5,6,7. This affects plant community succession and formation8,9, and has an impact on ecosystem structure and function10. Allelopathy is also an important biological mechanism for invasive plants11,12,13.
Ambrosia artemisiifolia and Ambrosia trifida are plants that are common around the world14,15, they are native to North America, belong to the ragweed genus of the Compositae family, and are annual invasive weeds. They are characterised by having robust vigour, superior adaptability, high seed yields, and rapid dispersal16. In the past 200 years, A. artemisiifolia and A. trifida have spread to 80 and 40 countries, respectively15. Invasions by these weeds damage ecological environments, reduce biodiversity, and occupy farmland, thereby significantly reducing crop yields14. Furthermore, their pollen causes allergic dermatitis and bronchial asthma, which are harmful to human health17,18. Currently, these weeds are widely distributed in Northeast China, North China, Central China, and East China19,20. In 2010, these weeds were found in the Yili River Valley of China and were widely distributed along roadsides, as well as in farmland, pastures, and scenic spots. They have negatively affected the local ecological environment, and agricultural and animal husbandry production21. Both A. artemisiifolia and A. trifida are also resistant to some herbicides22, which certainly poses a major challenge for effective prevention and control measures. It is, therefore, necessary to increase our understanding of their invasive mechanisms from an ecological perspective to lay a foundation for efficient prevention and control.
The seed densities of A. artemisiifolia and A. trifida retained in the soil seed bank are extremely high. Typically, the yield per plant of the two species can reach up to 2000–62,000 seeds23,24, while the number of plants per unit area is 300–690 plants per square meter25,26. During a ragweed seed bank study, the estimated ragweed seed number in the upper 16 cm soil layer varied between 0 and 18 820 for a square meter in Hungary27. The seed bank per unit area is large, as only about 50% of the seeds are dispersed or eaten by insects28,29. Generally, after seed germination, high-density populations are formed that exhibit a strong self-thinning phenomenon30. To avoid intra-species competition, the two species undergo density regulation to avoid mass die-offs under high-density conditions; otherwise they would be eliminated by natural selection in high-density environments. Thus, regulation of seed germination is vital for buffering competition at the seedling stage.
Many studies have demonstrated that allelochemicals have self-poisoning effects, which reduce the population density by inhibiting seed germination and seedling growth of the same plant, thereby avoiding seedling competition for nutrients that subsequently affect adult plant growth and maintain population growth31,32,33,34. In terms of autotoxicity, the allelochemicals are also called autochemicals or phytotoxins35. At present, research on autotoxicity mostly focuses on continuous cropping obstacles and plantation decline36,37,38,39,40. However, the autotoxicity of invasive species has rarely been studied. Allelopathy is an important mode of invasion; while the allelopathic effects of A. artemisiifolia and A. trifida on companion species has been examined41,42, there are few studies on their autotoxicity. Whether the allelochemicals of A. artemisiifolia and A. trifida play a role in population density regulation is currently unclear.
Seed germination is a key link in the lifecycle of plants; it is crucial for the growth, development, and continuation of the population, and the community structure of plants43,44,45. The seed germination stage is the most critical stage for the use of allelochemicals3. In areas of intense intra-species competition, allelochemicals secreted by plant roots induce seed dormancy, thereby benefiting un-germinated seeds46. Using Petri dishes to investigate the effects of allelochemicals (or plant extracts) on seed germination is a valuable method in allelopathy response research37,47,48,49. Thus, we aimed to provide useful information on the effects of autotoxicity and invasion mechanisms through the effect of autotoxicity on seed germination. The goals of this study were to determine: (a) whether A. artemisiifolia and A. trifida present autotoxicity, (b) what is the specific composition of the autotoxins, and (c) what is the role of autotoxicity in A. artemisiifolia and A. trifida populations? Thus, the role of autotoxicity in protecting population stability and improving invasiveness can be analysed.
Results
Autotoxicity of A. artemisiifolia and A. trifida extracts
Autotoxicity was observed in both A. artemisiifolia and A. trifida. Low concentrations of their extracts promoted seed germination, whereas high concentrations significantly inhibited seed germination (Fig. 1). The results revealed that the germination of the two plants was promoted at 0.1, 1, and 2 g 100 mL−1 in their respective extracts, but significant differences were not detected. Further, the promotion rate was approximately 5%. The germination inhibition rate of A. artemisiifolia seeds was 3.22% at g 100 mL−1 and 27.21% at 10 g 100 mL−1. The germination inhibition rate of A. trifida seeds was 57.97% at 5 g 100 mL−1 and 77.94% at 10 g 100 mL−1. The autotoxic effects of A. trifida were much greater than those of A. artemisiifolia at 5 and 10 g 100 mL−1, based on seed germination performance.
Autotoxicity of A. artemisiifolia and A. trifida extracts on their own seeds. Values are mean ± SE. Positive values indicate inhibited germination, and negative value indicate promoted germination. Different lowercase letters indicate that the seed germination inhibition rate of the same species at different concentrations was significantly different (p < 0.05). A. artemisiifolia seeds were treated with A. artemisiifolia plant extract, and A. trifida seeds were treated with A. trifida plant extract. EA.a: A. artemisiifolia; EA.t: A. trifida.
Similarly, the autotoxic inhibition of the two species had a significant effect on germination potential only at high concentrations (Fig. 2). At 10 g 100 mL−1, the germination inhibition rate of A. artemisiifolia seeds was the highest and the germination time was the longest, starting after 5 d. The germination potential concentration (42.67%) was significantly lower than the other concentrations. The germination potential of the other treatment concentrations was approximately 60%, with no significant differences. A. trifida was strongly affected by autotoxicity inhibition and its seeds germinated slowly. At 5 and 10 g 100 mL−1, the germination potential of A. trifida seeds was significantly reduced (14% and 7.33%, respectively), and the initial germination time was delayed by 6 d. No significant differences were detected in the germination potential at other concentrations (~ 35%).
Autotoxicity of each extract phase of A. artemisiifolia and A. trifida aqueous extracts
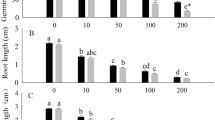
The petroleum ether, 1-butanol extract of A. artemisiifolia, and the 1-butanol extract of A. trifida promoted seed germination at low concentrations, and at high concentrations they inhibited seed germination (Fig. 3).
Effects of three organic extraction phases and residual water on seed germination rate of A. artemisiifolia (a) and A. trifida (b). In the figure, R represents the remaining water phase of the extraction, P represents the petroleum ether extraction phase, E represents the ethyl acetate extract phase and N represents the 1-butanol extract phase. Values are mean ± SE. Different lowercase letters indicate that the seed germination rate of the same species at different concentrations was significantly different (p < 0.05). A. artemisiifolia seeds were treated with A. artemisiifolia plant extract, and A. trifida seeds were treated with A. trifida plant extract. Dashed line shows the germination rate of control.
The concentration of petroleum ether extract solution which significantly promoted the germination of A. artemisiifolia seeds was 1 g 100 mL−1 (p < 0.05) and the promotion rate reached 12%. At a concentration of 10 g 100 mL−1, the germination of A. artemisiifolia seeds was significantly inhibited (p < 0.05) and the inhibition rate was 50%.
The concentration of the 1-butanol phase extract solution, which significantly inhibited the germination of A. artemisiifolia seeds, was 10 g 100 mL−1 (p < 0.05), with an inhibition rate of 47%. A concentration of 10 g 100 mL−1 significantly inhibited the seed germination of A. trifida, with an inhibition rate of 25%. The results showed that the autotoxic phases of A. artemisiifolia and A. trifida water extracts were petroleum ether phase, 1-butanol phase, and 1-butanol phase, respectively.
Using the UPLC-MS method, four autotoxins (vanillin, p-coumaric acid, caffeic acid, and chlorogenic acid) were identified in the petroleum ether phase and 1-butanol phase of A. artemisiifolia as well as in the 1-butanol phase of A. trifida. Table 1 shows the content of autotoxins and the germination promotion rate of their own seeds at different concentrations for A. artemisiifolia and A. trifida.
As shown in Table 1, the content of autotoxins per unit mass of A. artemisiifolia plants was higher than that of A. trifida. When treated separately with the four substances, the treatments of A. artemisiifolia and A. trifida seeds had certain effects on the seed germination of the two species.
Among them, vanillin promoted the germination rate of A. artemisiifolia seeds by 69% and 33% for A. trifida. The germination inhibition rate of chlorogenic acid in A. artemisiifolia seeds was 38%, and that of A. trifida was 24%. Moreover, the effect of each substance on the seed germination of A. artemisiifolia was greater than that of A. trifida.
Studies have found that the chlorogenic acid content in A. artemisiifolia and A. trifida is 263.22 μg g−1 and 75.26 μg g−1, respectively, which are much higher than those of the other three substances. Therefore, a higher concentration of chlorogenic acid is required to achieve the effect of inhibiting seed germination.
Although p-coumaric acid and caffeic acid have certain allelopathic and autotoxic effects on A. artemisiifolia and A. trifida, they are not as effective as chlorogenic acid and vanillin on the two species. Therefore, it is believed that these four substances are autotoxins in A. artemisiifolia and A. trifida, but chlorogenic acid and vanillin play a more important role.
As shown in Table 2, the concentration treatments showed promoting and inhibiting effects, which verified that chlorogenic acid and vanillin are autotoxins of A. artemisiifolia and A. trifida.
In A. artemisiifolia, chlorogenic acid and vanillin promoted seed germination at 100 μg 100 mL−1 and 4 μg 100 mL−1, and the germination promotion rates were 42% and 69%, respectively. Seed germination was inhibited at 1500 μg 100 mL−1 and 20 μg 100 mL−1, and the germination inhibition rate was 2%.
In A. trifida, chlorogenic acid and vanillin promoted seed germination at 100 μg 100 mL−1 and 20 μg 100 mL−1, and the germination promotion rates were 40% and 32%, respectively. At 500 μg 100 mL−1 and 2000 μg 100 mL−1 chlorogenic acid and vanillin inhibited seed germination, and the germination inhibition rate was 17%.
Vitality recovery of un-germinated seeds
A positive correlation was detected between recovery germination rate and the concentrations of the two sets of extracts (Fig. 4). The higher the extract concentration, the higher the seed recovery germination rate was. The recovery germination rate of A. artemisiifolia extracts treated with 10 g 100 mL−1 was different from those treated with 5 g 100 mL−1; this difference was not significant but was significantly higher than that of the other concentrations. The recovery germination rate of A. trifida extracts treated with 5 and 10 g 100 mL−1 were significantly higher than that of the control, and at 0.1 and 1 g 100 mL−1, but the difference was not significant compared to 2 g 100 mL−1.
Initially, the total germination rate of the two species increased, but then decreased as the concentration increased (Fig. 4). Seeds treated with the two extracts at 2 g 100 mL−1 had the strongest recovery after washing. The total germination rate of both species treated with 0.1, 1, and 2 g 100 mL−1 was higher than that of the control. That is, the total germination rate of both species after treatment with low-concentration extracts improved compared to the control. Additionally, the total germination rate under 5 and 10 g 100 mL−1 was lower than that of other concentrations; in other words, high concentrations negatively affected seed total germination rate.
Discussion
A. artemisiifolia and A. trifida have significant autotoxicity; low concentrations promote seed germination and high concentrations inhibit seed germination
A large number of studies have shown that allelopathy is an important invasion mechanism for many alien species50,51,52. A. artemisiifolia and A. trifida have strong allelopathic effects41, but there are few studies on the autotoxicity of these two invasive plants. This study verified the autotoxicity of A. artemisiifolia and A. trifida, and determined its effective concentrations.
In our experiment, the autotoxins of A. artemisiifolia and A. trifida significantly inhibited the germination of their own seeds. They promoted the germination of their own seeds at lower concentrations, and at the same time promoted the germination rate and germination potential. The same effect was observed when treated with separate autotoxins (chlorogenic acid and vanillin). The plant water extracts promoted seed germination at low concentrations and accelerated the germination speed 2 days and 4 days earlier, respectively, and the effect of treatment with autotoxic substances was more significant 4 days and 6 days earlier, respectively.
High concentrations of autotoxins inhibition seed germination; as the concentration of the extract increased, the germination inhibition rate was greater. At the same time, the germination rate and germination vigour of A. artemisiifolia and A. trifida seeds was reduced. The plant water extract inhibited seed germination at high concentrations and delayed the germination speed by 5 days and 6 days, respectively, and treatment with autotoxic substances was delayed by 2 days and 3 days, respectively. This low concentration promoted seed germination and the high concentrations inhibited seed germination. These findings are consistent with the "mass concentration effect" reported in most studies on allelopathy and autotoxicity53.
Kumari and Kohli54 found that the water extract of A. artemisiifolia leaves, at a concentration of 0.5 g mL−1, had a lethal rate of 50% to its own plants. Our study found that the aqueous extracts of A. artemisiifolia and A. trifida plants have a strong inhibitory effect on the germination of their own seeds, demonstrating an inhibitory rate for A. artemisiifolia of up to 27.21% at a concentration of 0.1 g mL−1. Also found that A. trifida has an inhibition rate of 77.94% on its own seed germination at a concentration of 0.1 g mL−1. Both proved that A. artemisiifolia and A. trifida have strong autotoxicity.
Chlorogenic acid, caffeic acid, p-coumaric acid, and vanillin are the main autotoxic substances in A. artemisiifolia and A. trifida, among which chlorogenic acid and vanillin have the most significant effects
There are many types of autotoxins. Common compounds can be divided into 10 categories according to their structure and properties: simple water-soluble organic acids, linearalcohols, aliphatic aldehydes, and ketones; simple unsaturated lactones; long-chain fatty acids and polyalkynes; benzoquinones, anthraquinones, and double quinones; simple phenols, benzoic acid, and their derivatives; cinnamic acids and their derivatives; coumarins; flavonoids; tannins; and terpenoids, enes, and zirconias55,56,57.
We used UPLC-MS to detect and analyse compounds from A. artemisiifolia and A. trifida and found that chlorogenic acid, caffeic acid, vanillin, and p-coumaric acid were the main autotoxins of the two species. The types of autotoxins in the two species were the same. However, the effect of the autotoxins on A. artemisiifolia was greater than that of A. trifida. Except for p-coumaric acid, the content of various substances in A. artemisiifolia was higher than that in A. trifida. For example, the content of chlorogenic acid (263.22 μg g−1) in A. artemisiifolia was close to four times that of A. trifida (75.2 μg g−1).
Further investigation found that chlorogenic acid and vanillin had the most apparent autotoxicity effects on A. artemisiifolia and A. trifida. The content of vanillin in A. artemisiifolia and A. trifida was similar, but A. artemisiifolia was more sensitive to the effects of vanillin, and a higher concentration was required in A. trifida. The autotoxicity effect of vanillin on A. artemisiifolia and A. trifida was demonstrated for the first time in this study.
Caffeic acid and p-coumaric acid have been reported in A. artemisiifolia and A. trifida. These have inhibitory effects on the seed germination and seedling growth of Digitaria ciliaris, Echinochloa crus-galli, and Cyperus microiria58. In this study, we found that there is a certain degree of autotoxicity on the seeds of A. artemisiifolia and A. trifida, but the effect on seed germination was not significant.
Autotoxicity of A. artemisiifolia and A. trifida is an important means of population density regulation
Generally speaking, under natural conditions, the allelochemicals produced by plants can enter the surrounding environment in the following ways: (1) the aerial parts of plants release volatile substances into the environment, (2) the leaching effect of rain and mist, (3) plant root exudates are secreted into the soil rhizosphere, and (4) when plant tissue litter and plant debris are decomposed, especially the decomposition of residual roots. Most plant allelochemicals enter the surrounding environment through these methods to affect the growth of the plant itself or the surrounding plants, and the longer the growth and distribution of the species, the greater the accumulation of autotoxins.
For perennial plants, it is logical to use autotoxicity to avoid sibling competition, but for annual plants, the longer a population is established, the more autotoxins accumulate. This inhibits their own seed germination and plant growth, does not prevent sibling competition, and promotes population increase. Friedman et al.59 found that the autotoxicity of annual plants can cause seeds to germinate or spread further from the parent, delay their germination, or germinate only after rainfall. From their study of the autotoxicity of Lolium rigidum, Canals et al.60 believed that the autotoxicity of annual plants improves the viability of the population by inhibiting the development of populations with fewer individuals, and chemical interference and resource competition are closely related processes. Emeterio61 believes that chemical substances have a significant density-dependent effect on plant growth. Autotoxicity may act as a self-regulator of species population density to prevent intra-specific competition44. However, there are few reports on the effects of autotoxicity on the populations of annual invasive plants.
Ambrosia artemisiifolia and A. trifida are significantly harmful, invasive plants globally, and their invasion mechanisms are a focus for current research efforts. The two species’ short distance dispersal methods and high-density cluster distributions may be an important feature that strengthens resource competition and enables invasive success28,62,63. Understanding the regulation of intra-species density at high densities is an interesting scientific challenge. Our research found that the autotoxins of the two species promote their own seed germination effects at low concentrations. Therefore, in the early stage of colonisation by both species, the population density and the concentration of autotoxins are low at this time, and this promotes seed germination and improved competitiveness, thereby gaining an inter-specific competitive advantage for the community. However, when the population has been continuously growing for many years, the population becomes densely distributed and as the intra-specific individual numbers increase, autotoxins continuously accumulate. When the self-inhibition concentration is reached, the promoting effect on seed germination is changed to an inhibitory effect, which effectively prevents population over-density. This is a common feature of plant autotoxicity64,65,66. The recovery of seed germination rates after washing with water further proved autotoxicity. In conclusion, the autotoxicity of A. artemisiifolia and A. trifida is an important means by which these populations regulate density and alleviate intra-specific competition during seed germination.
Conclusions
Based on seed bioassay method and UPLC-MS analysis technology, the autotoxicity of A. artemisiifolia and A. trifida and the main components of autotoxics were studied. The results showed that there was autotoxicity in both plants, and the main autotoxics were chlorogenic acid, caffeic acid, p-coumarin acid and vanillin. Among them, these four substances have promoting and inhibiting concentrations on the seeds of A. artemisiifolia and A. trifida under indoor test conditions, but their practical effects under natural conditions needs to be further studied.
Materials and methods
Experimental material selection and origin
Plants and seeds of A. artemisiifolia and A. trifida were collected from areas that had been invaded by these species in Xinyuan County, China. The plant material used in this study was collected under the permission from the Xinyuan County and followed the established rules and regulations. Experimental research and field studies on plants, including the collection of plant material, complied with relevant institutional, national, and international guidelines and legislation.
In September 2018, 300 plants each of A. artemisiifolia and A. trifida were randomly collected from the sampling of site, dried under natural conditions, and collected the seeds. According to the plant segments divided by Liu et al.67, the seeds on the top of the plants were selected as the research objects. The seeds were stored in a kraft paper bag at room temperature, wherein the after-ripening time of A. artemisiifolia seeds was 42 days, and the after-ripening time of ragweed A. trifida seeds was 45 days.
In October 2018, 300 complete plants each of mature A. artemisiifolia and A. trifida were randomly collected from the plot. The voucher specimens of A. artemisiifolia and A. trifida are stored in the Herbarium of Shihezi University (Xingjiang, China), and the numbers of specimen were No.11265 and No.11272 respectively.
Experimental design
Extract preparation
The concentrations used in this study were based on a preliminary experiment. In the preliminary experiment, half of the seeds treated with 2 g 100−1 mL extracts germinated successfully. To determine the concentration that completely inhibited seed germination, the highest concentration of the extract was set to 10 g 100−1 mL. This paper primarily studied the effect of autotoxin dosage, which does not need to be exactly the same as that found in natural habitats. The collected whole plants (including the root system) of both species were dried in an electric constant temperature drying box at 120 °C, then separately crushed and thoroughly mixed at room temperature to eliminate the differences between plants. All experiments in the article requiring the use of plant extracts were prepared using the whole plant (including the aerial and underground parts of the plant). And in order to ensure consistent solubility of the treatment solutions, all treatment solutions were prepared the day before the experiment.
Samples (0.1, 1, 2, 5, and 10 g) were soaked in 100 mL distilled water and filtered after 36 h. Samples were centrifuged at 3000 r min−1 for 10 min, and extracts were prepared at concentrations of 0.1, 1, 2, 5, and 10 g 100−1 mL. The 10 treatment solutions were stored in a refrigerator at 4 °C.
Preparation of organic extraction phase of aqueous extracts of A. artemisiifolia and A. trifida
Using the same method as above, 100 g of the two crushed plant samples were placed into 1 L of distilled water to prepare the water extracts.
The water extract was successively extracted with petroleum ether, ethyl acetate, and 1-butanol. Ten millilitres of each phase was reserved for qualitative and quantitative analyses. The rest was concentrated on a rotary evaporator to obtain the petroleum ether phase (A. artemisiifolia 16.71 mg g−1, A. trifida 32 mg g−1), ethyl acetate phase (A. artemisiifolia 44.78 mg g−1, A. trifida 33 mg g−1), 1-butanol phase (A. artemisiifolia 18 mg g−1, A. trifida 24.9 mg g−1), and the remaining water phase (A. artemisiifolia 34.6 mg g−1, A. trifida 41 mg g−1). The four dry substances were redissolved in distilled water to prepare the plant concentrations corresponding to the preliminary experiment. The 40 treatment solutions were stored in a refrigerator at 4 °C for the seed germination experiments.
Identification and analysis of the extraction phase of autotoxins
The 10 ml of extraction liquid reserved in the previous experiment was blow-dried withN2. The dry matter was dissolved in 1 mL methanol and centrifuged at 12,000 r min−1 for 10 min, and the supernatant was collected and filtered through a 0.22 μm membrane.
For the ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS) method, the instruments used were an ACQUITY UPLC ultra-high performance liquid chromatograph, a XEVO TQ-S triple quadrupole tandem mass spectrometer, and a MassLynx workstation (Waters company, USA; Waters ACQUITY UPLC BEH C18 column (50 mm × 2.1 mm, 1.7 μm)). The conditions were as follows: flow rate at 0.3 mL min; injection volume of 1 μL; and column oven at 30 °C. For the mass spectrometry conditions, the ion source was an electrospray ionisation source and the multi-reaction detection mode (MRM) was used for content determination. The desolventising gas temperature was 450 °C, source temperature was 150 °C, desolventising gas flow rate was 800 L h−1, cone gas was 150 L h−1, and capillary voltage was 3000 V.
The gradient elution procedures of mobile phase A (0.1% formic acid–water) and B (acetonitrile) are shown in Table 3.
Using the external standard method, solutions were prepared containing eight standard substances (all chromatographically pure) containing chlorogenic acid, borneol, acetyl borneol, caffeic acid, cinnamyl alcohol, p-coumaric acid, azelaic acid, and vanillin. The concentrations were 1, 10, 25, 50, 100, 500, and 1000 μg 10 mL−1. According to the determined chromatographic conditions, standard solutions of different concentrations were used for analysis and a standard curve was drawn. Qualitative and quantitative analyses of the samples were performed. Through this process, four chemical substances were identified and quantified: chlorogenic acid (A. artemisiifolia 263.22 μg g−1, A. trifida 75.26 μg g−1), caffeic acid (A. artemisiifolia 7.12 μg g−1, A. trifida 1.98 μg g−1), p-coumaric acid (A. artemisiifolia 0.93 μg g−1, A. trifida 1.09 μg g−1), and vanillin (A. artemisiifolia 2.19 μg g−1, A. trifida 2.13 μg g−1) (see Supplementary Fig. S2).
Seed germination experiment
The plant extracts and autotoxic substances of A. artemisiifolia and A. trifida were used to treat their own seeds. The experiment was divided into three parts.
First, the seeds were treated directly with the plant water extracts of A. artemisiifolia and A. trifida. To determine the effect of different plant extract concentrations on seed germination, we set the concentrations at 0.1, 1, 2, 5, 10 g 100 mL−1, for a total of 10 treatments.
Second, to determine the specific chemical substances, the water extracts of the plants were extracted, and the dry substances of each extract phase were formulated into the corresponding concentration according to the plant concentration. This yielded a total of 40 treatments.
Third, to determine the specific action concentrations of the autotoxins of A. artemisiifolia and A. trifida, two sets of experimental concentrations were set up. The corresponding concentrations of autotoxins (chlorogenic acid, caffeic acid, p-coumaric acid, and vanillin) were set according to the plant concentrations, at five concentrations for each substance, yielding a total of 40 treatments. To verify the germination effect of chlorogenic acid and vanillin on A. artemisiifolia and A. trifida seeds, five concentrations of chlorogenic acid and six concentrations of vanillin were set, with a total of 22 treatments (Table 4).
Experiments were carried out in Petri dishes (90 mm diameter) containing a double layer of filter paper with 25 seeds of one species evenly spread across each dish. The Petri dishes were then placed in a light growth incubator under a 12/12 h light/dark photoperiod, with a light intensity of 3000 lx at 25/15 °C to simulate day/night temperatures of the species’ original habitat from April to May. Distilled water was used as the control (control). Each factor combination was replicated thrice.
Seed germination was defined as the emergence of the radicle. The number of germinated seeds was recorded daily and a small amount of distilled water was added to keep the double-layer filter paper moist for 5 weeks. Germination and germination inhibition rates were calculated as follows:
Positive values indicated inhibited germination, whereas negative values indicated promote germination. Germination potential was calculated as follows:
The germination period of both A. artemisiifolia and A. trifida was 35 days.
Seed vitality restoration test
To study the effects of allelochemicals on A. artemisiifolia and A. trifida seed vigour, restoration experiments were conducted. Seeds that did not germinate after culturing in the two types of extracts for five weeks were washed in distilled water and cultured in new Petri dishes with clean distilled water at 15–25 °C. The recovery germination rate and total germination rate were calculated as follows:
Data analysis
Using concentration as the test factor, and germination inhibition rate as a response variable, the seed responses at different concentrations were analysed by one-way ANOVA (p < 0.05). When the significant difference was greater than 0.05, the null hypothesis was accepted and the variance was considered to be homogeneous. Duncan’s multiple range test was used to test for significant differences in the inhibition rate and germination potential between treatments with different concentrations. The germination responses of different seed sizes were analysed using an independent-sample t-test (p < 0.05). Seed size was used as a test factor and germination rate was the response variable. All analyses were performed using SPSS v19.0 (SPSS, Chicago, IL, USA) for Windows. All figures were constructed using Origin v9.0 (OriginLab, Northampton, MA, USA) for Windows.
Data availability
The data that supports the findings of this study are available in the supplementary material of this article.
References
Dorning, M. & Cipollini, D. Leaf and root extracts of the invasive shrub, Lonicera maackii, inhibit seed germination of three herbs with no autotoxic effects. Plant Ecol. 184, 287–296 (2006).
Greer, M. J., Wilson, G. W., Hickman, K. R. & Wilson, S. M. Experimental evidence that invasive grasses use allelopathic biochemicals as a potential mechanism for invasion: Chemical warfare in nature. Plant Soil 385, 165–179 (2014).
Möhler, H., Diekötter, T., Herrmann, J. D. & Donath, T. W. Allelopathic vs. autotoxic potential of a grassland weed-evidence from a seed germination experiment. Plant Ecol. Divers. 11, 539–549 (2018).
Callaway, R. M. & Aschehoug, E. T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 290, 521–523 (2000).
Niu, H. B., Liu, W. X., Wan, F. H. & Liu, B. An invasive aster (Ageratina adenophora) invades and dominates forest understories in China: Altered soil microbial communities facilitate the invader and inhibit natives. Plant Soil 294, 73–85 (2007).
Wardle, D. A., Karban, R. & Callaway, R. M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evol. 26, 655–662 (2011).
Meiners, S. J., Kong, C. H., Ladwig, L. M., Pisula, N. L. & Lang, K. A. Developing an ecological context for allelopathy. Plant Ecol. 213, 1221–1227 (2012).
Liebhold, A. M., Brockerhoff, E. G., Kalisz, S., Nunez, M. A. & Wardle, D. A. Biological invasions in forest ecosystems. Biol. Invasions 19, 3437–3458 (2017).
Liao, H. X. et al. Soil microbes regulate forest succession in a subtropical ecosystem in China: Evidence from a mesocosm experiment. Plant Soil 430, 277–289 (2018).
Wardle, D. A., Nilsson, M. C., Gallet, C. & Zackrisson, O. An ecosystem-level perspective of allelopathy. Biol. Rev. 73, 305–319 (2010).
Hierro, J. L. & Callaway, R. M. Allelopathy and exotic plant invasion. Plant Soil 256, 29–39 (2003).
Uddin, M. N., Robinson, R. W., Buultjens, A., Harun, M. A. & Shampa, S. H. Role of allelopathy of Phragmites australis in its invasion processes. J. Exp. Mar. Biol. Ecol. 486, 237–244 (2017).
Thiébaut, G., Tarayre, M. & Rodríguez-Pérez, H. Allelopathic effects of native versus invasive plants on one major invader. Front. Plant Sci. 2, 854 (2019).
Smith, M., Cecchi, L., Skjøth, C. A., Karrer, G. & Šikoparijae, B. Common ragweed: A threat to environmental health in Europe. Environ. Int. 61, 115–126 (2013).
Montagnani, C., Gentili, R., Smith, M., Guarino, M. F. & Citterio, S. The worldwide spread, success, and impact of ragweed (Ambrosia spp.). Crit. Rev. Plant Sci. 36, 1–40 (2017).
Zeng, K., Zhu, Y. Q. & Liu, J. X. Research progress on ragweed (Ambrosia). Acta Prataculturae Sin. 19, 212–219 (2010).
Jacobs, R. L. et al. Responses to ragweed pollen in a pollen challenge chamber versus seasonal exposure identify allergic rhinoconjunctivitis endotypes. J. Allergy Clin. Immun. 130, 122-127.e8 (2012).
Lake, R. I. et al. Climate change and future pollen allergy in Europe. Environ. Health Perspect. 125, 385–391 (2017).
Wang, J. J., Zhao, B. Y., Li, M. T. & Li, R. Ecological invasion plant-bitter weed (Ambrosia artemisiifolia) and integrated control strategy. Pratacultural Sci. 023, 71–75 (2006).
Deng, Z. Z., Bai, J. D., Zhao, C. Y. & Li, J. S. Advance in invasion mechanisms of Ambrosia artemisiifolia. Pratacultural Sci. 32, 54–63 (2015).
Dong, H. G. et al. Diffusion and intrusion features of Ambrosia artemisiifolia and Ambrosia trifida in Yili River Valley. J. Arid Land Resour. Environ. 31, 175–180 (2017).
Vink, J. P. et al. Glyphosate-resistant giant ragweed (Ambrosia trifida) control in dicamba-tolerant soybean. Weed Technol. 26, 422–428 (2012).
Simard, M. J. & Benoit, D. L. Effect of repetitive mowing on common ragweed (Ambrosia artemisiifolia L.) pollen and seed production. Ann. Agric. Environ. Med. 18, 55–62 (2011).
Goplen, J. J. et al. Seedbank depletion and emergence patterns of giant ragweed (Ambrosia trifida) in Minnesota cropping systems. Weed Sci. 65, 52–60 (2017).
Jurik, T. W. Population distributions of plant size and light environment of giant ragweed (Ambrosia trifida L.) at three densities. Oecologia 87, 539–550 (1991).
Patracchini, C., Vidotto, F. & Ferrero, A. Common ragweed (Ambrosia artemisiifolia) growth as affected by plant density and clipping. Weed Technol. 25, 268–276 (2011).
Kazinczi, G. Ragweed seed bank in the soils of arable fields of Transdanubia, Hungary. Hung. Weed Res. Technol. 19(1), 21–36 (2018).
Essl, F. et al. Biological flora of the British Isles: Ambrosia artemisiifolia. J. Ecol. 103, 1069–1098 (2015).
Goplen, J. J. Giant Ragweed (Ambrosia trifida) Seed Bank Dynamics and Management. (Master's dissertation, University of Minnesota.) Retrieved from https://hdl.handle.net11299174767 (2015).
Yoda, K. Self-thinning in overcrowded pure stands under cultivated and natural conditions. J. Biol. 14, 107–129 (1963).
Friedman, J. & Waller, G. R. Allelopathy and autotoxicity. Trends Biochem. Sci. 10, 47–50 (1985).
Weller, D. E. The interspecific size-density relationship among crowded plant stands and its implications for the −3/2 power rule of self-thinning. Am. Nat. 133, 20–41 (1989).
Deng, J. et al. Autotoxicity of phthalate esters in tobacco root exudates: Effects on seed germination and seedling growth. Pedosphere 27, 1073–1082 (2017).
Sudatti, D. B., Duarte, H. M., Soares, A. R., Salgado, L. T. & Pereira, R. C. New ecological role of seaweed secondary metabolites as autotoxic and allelopathic. Front. Plant Sci. 11, 347 (2020).
Singh, H. P., Batish, D. & Kohil, R. Autotoxicity: Concepts, organisms, and ecological significance. Plant Sci. 18, 757–772 (1999).
Chon, S. U. et al. Effects of alfalfa leaf extracts and phenolic allelochemicals on early seedling growth and root morphology of alfalfa and barnyard grass. Crop Prot. 21, 1077–1082 (2002).
Chen, B. M., D’Antonio, C. M., Molinari, N. & Peng, S. L. Mechanisms of influence of invasive grass litter on germination and growth of coexisting species in California. Biol. Invasions 20, 1881–1897 (2018).
Chen, L. C., Wang, S. L., Wang, P. & Kong, C. H. Autoinhibition and soil allelochemical (cyclic dipeptide) levels in replanted Chinese fir (Cunninghamia lanceolata) plantations. Plant Soil 374, 793–801 (2014).
Perry, L. G. et al. Retracted: Dual role for an allelochemical: catechin from Centaurea maculosa root exudates regulates conspecific seedling establishment. J. Ecol. 93, 1126–1135 (2005).
Yu, J. Q., Ye, S. F., Zhang, M. F. & Hu, W. H. Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber. Biochem. Syst. Ecol. 31, 129–139 (2003).
Kong, C. H., Wang, P. & Xu, X. H. Allelopathic interference of Ambrosia trifida with wheat (Triticum aestivum). Agric. Ecosyst. Environ. 119, 416–420 (2007).
Béres, I., Kazinczi, G. & Narwal, S. S. Allellopathic plants. 4. Common ragweed (Ambrosia elatior L. syn. A. artemisiifolia). Allelopathy J. 9, 27–34 (2002).
Bauer, J. T., Shannon, S. M., Stoops, R. E. & Reynolds, H. L. Context dependency of the allelopathic effects of Lonicera maackii on seed germination. Plant Ecol. 213, 1907–1916 (2012).
Renne, I. J., Sinn, B. T., Shook, G. W., Sedlacko, D. M. & Hierro, J. L. Eavesdropping in plants: Delayed germination via biochemical recognition. J. Ecol. 102, 86–94 (2014).
Loydi, A., Donath, T. W., Eckstein, R. L. & Otte, A. Non-native species litter reduces germination and growth of resident forbs and grasses: Allelopathic, osmotic or mechanical effects?. Biol. Invasions 17, 581–595 (2014).
Bais, H. P., Weir, T. L., Perry, L. G., Gilroy, S. & Vivanco, J. M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266 (2006).
Bonea, D., Bonciu, E., Niculescu, M. & Olaru, A. L. The allelopathic, cytotoxic and genotoxic effect of Ambrosia artemisiifolia on the germination and root meristems of Zea mays. Caryologia 71, 24–28 (2017).
Dadkhah, A. Allelopathic effect of sugar beet (Beta vulgaris) and eucalyptus (Eucalyptus camaldulensis) on seed germination and growth of Portulaca oleracea. Russ. Agric. Sci. 39, 117–123 (2013).
Zheng, L. & Feng, Y. L. Allelopathic effects of Eupatorium adenophorum Spreng on. seed germination and seedling growth in ten herbaceous species. Acta Ecol. Sin. 25, 2782–2787 (2005).
Brückner, D. J. The allelopathic effect of ragweed (Ambrosia artemisiifolia L.) on the germination of cultivated plants. Novenytermeles 47, 635–644 (1998).
Qin, R. M. et al. The evolution of increased competitive ability, innate competitive advantages, and novel biochemical weapons act in concert for a tropical invader. New Phytol. 197, 979–988 (2012).
Zheng, Y. L. et al. Integrating novel chemical weapons and evolutionarily increased competitive ability in success of a tropical invader. New Phytol. 205, 1350–1359 (2015).
Kaushal, R., Verma, K. S. & Singh, K. N. Effect of Grewia optiva and Populus deltoides leachatesv on field crops. Allelopathy J. 11, 229–234 (2003).
Kumari, A. & Kohli, R. Autotoxicity of ragweed parthenium (Parthenium hysterophorus). Weed Sci. 35, 629–632 (1987).
Einhellig, F. A. Allelopathy: Current status and future goals. In Allelopathy: Organisms, processes and applications (ed. Inderjit Dakshini, K. M. M.) 1–24 (Am Chem. Soc, Washington, 1995).
Hadack, F. Secondary metabolites as plant traits: Current assessment and future perspectives. Crit. Rev. Plant Sci. 21, 273–322 (2002).
Rice, E. L. Biological Control of Weeds and Plant Diseases (Oklahomka Press, 1995).
Choi, B. et al. Common ragweed-derived phenolic compounds and their effects on germination and seedling growth of weed species. Weed Turfgrass Sci. 30, 396–404 (2010).
Friedman, J. & Waller, G. R. Seeds as allelopathic agents. Chem. Ecol. 9, 1107–1117 (1983).
Canals, R. M., Emeterio, L. S. & Peralta, J. Autotoxicity in Lolium rigidum: Analyzing the role of chemically mediated interactions in annual plant populations. J. Theor. Biol. 235, 402–407 (2005).
San Emeterio, L., Damgaard, C. & Canals, R. M. Modelling the combined effect of chemical interference and resource competition on the individual growth of two herbaceous populations. Plant Soil 292, 95–103 (2007).
Dickerson, C. T. Studies on the germination, growth, development and control of Common Ragweed (Ambrosia artemisiifolia L.). PhD thesis, Cornell University, Ann Arbor (1968).
Nuutinen, V. & Butt, K. R. Homing ability widens the sphere of influence of the earthworm Lumbricus terrestris L. Soil Biol. Biochem. 37, 805–807 (2005).
Favaretto, A., Scheffer-basso, S. M. & Perez, N. B. Autotoxicity in tough lovegrass (Eragrostis plana). Planta Daninha 35(35), e017164046 (2017).
Sinkkonen, A. Modelling the effect of autotoxicity on density-dependent phytotoxicity. J. Theor. Biol. 244, 218–227 (2007).
Zhang, S. S., Shi, F. Q., Yang, W. Z., Xiang, Z. Y. & Duan, Z. L. Autotoxicity as a cause for natural regeneration failure in Nyssa yunnanensis and its implications for conservation. Isr. J. Plant Sci. 62, 187–197 (2015).
Liu, Y. et al. Relationship between seed germination and invasion of Ambrosia artemisiifolia and A. trifida at different positions. Acta Ecol. Sin. 39, 9079–9088 (2019).
Acknowledgements
In this study, we would like to thank professor T.L for her guidance, and all the authors for their joint efforts. We would like to thank Analysis and Test Center of Shihezi University (Xingjiang, China) for its assistance in the analysis of the material.
Funding
This study was supported by Key Science and Technology Project of Xinjiang Production Construction Crops (No. 2022AB010) and National Nature Science Foundation of China (No. 31770461). The funding agency has no role in the design, data collection, analysis or interpretation of the research or in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
P.S. and R.L.W. and X.L.L. conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts. T.L. conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft. W.X.Z. and M.M.S. and H.Y.W. performed the experiments, prepared figures and/or tables, and approved the final draft. Y.X.L. and Q.W. analyzed the data, and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Su, P., Liu, X., Wang, R. et al. Autotoxicity of Ambrosia artemisiifolia and Ambrosia trifida and its significance for the regulation of intraspecific populations density. Sci Rep 12, 17424 (2022). https://doi.org/10.1038/s41598-022-21344-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21344-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.