Abstract
We aimed to validate and prove the novel risk score models of acute myeloid leukemia (AML)-specific disease risk group (AML-DRG) and AML-Hematopoietic Cell Transplant-composite risk (AML-HCT-CR) in patients with acute myeloid leukemia (AML) after allogeneic hematopoietic stem cell transplantation (AHCT). Among the 172 AML patients analysed, 48.3% (n = 83) were females. Median age was 31.5 years (range 14 to 62 years), two patients was more than 60 years old (1.2%). Median follow-up was 44 months (range 1 to 94 months). According to the AML-DRG model, 109, 49 and 14 patients were in low-, intermediate- and high-risk group, respectively. According to the AML-HCT-CR model, 108, 30, 20 and 14 patients were in low-, intermediate-, high- and very high-risk group, respectively. Our results showed that the AML-DRG and AML-HCT-CR models significantly predicted cumulative incidence of relapse (p < 0.001; p < 0.001). But AML-DRG model was not associated with NRM (p = 0.072). Univariate analysis showed that the AML-DRG model could better stratify AML patients into different risk groups compared to the AML-HCT-CR model. Multivariate analysis confirmed that prognostic impact of AML-DRG and AML-HCT-CR models on post-transplant OS was independent to age, sex, conditioning type, transplant modality, and stem cell source (p < 0.001; p < 0.001). AML-DRG and AML-HCT-CR models can be used to effectively predict post-transplant survival in patients with AML receiving AHCT. Compared to AML-HCT-CR score, the AML-DRG score allows better stratification and improved survival prediction of AML patients post-transplant.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is a clonal malignancy characterized by genetic heterogeneity due to recurrent gene mutations. Long term overall survival has stagnated in the past few decades. Even with an array of new gene mutation-targeted agents available for AML treatment1, the complete cure of leukemia still faces great challenges. Allogeneic hematopoietic stem cell transplantation (AHCT) is a curative treatment for AML patients2,3. Disease relapse and transplant-related mortality (TRM) are important for long-term survival of AML patients, especially the disease relapse4,5. How to prevent post-AHCT relapse on the basis of controlling the toxicities of conditioning regimen was important to improve the outcomes of AML patients6,7. Survival of patients after AHCT is largely dependent on disease- and patient-related factors8,9,10,11,12. Pre-transplant risk assessment is the key to optimizing transplant outcomes.
Several prognostic models have been developed in recent years. The disease risk index (DRI)13,14, representing disease-related factors, can predict overall survival (OS) but can’t integrate patient’s comorbidities and overall conditions. The hematopoietic cell transplantation-specific comorbidity index (HCT-CI) is also predictive of outcome but only includes comorbidities15,16. The disease risk comorbidity index (DRCI) and haplo-DRCI, integrating the DRI and HCT-CI, can effectively predict outcomes after AHCT17. Although previous studies have proved that minimal residual disease (MRD) is an independent predictor of survival in patients with AML18,19,20, MRD is excluded from most models. Therefore, two comprehensive new prognostic scores, AML-specific disease risk group (AML-DRG) and AML-Hematopoietic Cell Transplant-Composite Risk (AML-HCT-CR), have been shown to be predictive of OS and PFS in patients with AML received AHCT21. However, there are no published reports of the AML-DRG and AML-HCT-CR models in China. In this retrospective study, we aimed to verify the clinical effectiveness and generalizability of the AML-DRG and AML-HCT-CR models in a cohort of patients with AML receiving AHCT.
Materials and methods
Data source
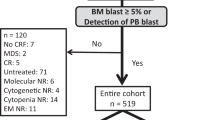
A total of 172 adult patients diagnosed with non-M3 acute myeloid leukemia who underwent the first AHCT from January 1, 2013 to December 30, 2018 in the First Affiliated Hospital of Zhengzhou University and Shandong Provincial Hospital Affiliated to Shandong First Medical University were enrolled in this study. Secondary AML was defined as AML developed after treatment with systemic chemotherapy and/or radiation therapy. All enrolled subjects in this study provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki. All patients were followed up through our outpatient clinic, medical records in hospital, or by telephone cells. The follow-up endpoint was December 30, 2020.
Rating scales
AML-DRG score assignment: 1 for secondary AML, 1 for adverse European Leukemia 2017 genetic risk (ELN2017 genetic risk), 2 for CR with MRD positive or unknown MRD status, 4 for active disease21. The AML-HCT-CR score assignment: AML-DRG score with the addition of 1 score for age ≥ 60 and 1 score for HCT-CI ≥ 321. DRI was applied as described by Armand et al.13. HCT-CI and HCT-CI/Age were applied as published by Sorror et al.15,16. ELN2017 genetic risk group was applied as described by Döhner, et al.22. MRD was assessed pre-HSCT by flow cytometry. MRD < 0.1% was judged to be negative23.
Transplant protocol
All patients received myeloablative conditioning. The major conditioning regimen for the identical sibling donor (ISD) group as follows: hydroxyurea (40 mg/kg/12 h, day − 10), cytarabine (1 ~ 1.5 g/m2/day, day − 9), busulfan (0.8 g/kg/6 h, days − 8 ~ − 6), cyclophosphamide (1.8 g/m2/day, days − 5 and − 4). For HLA-haploidentical and HLA-matched unrelated donor transplants, the major conditioning regimen as follows: cytarabine (4 g/m2/day, days − 10 and − 9), busulfan (0.8 g/kg/6 h, days − 8 to − 6), cyclophosphamide (1.8 g/ m2/day, days − 5 and − 4), anti-thymocyte globulin (ATG) (2.5 mg/kg/day, days − 5 to − 2). Four patients received total body irradiation (TBI)-based regimen: cyclophosphamide (60 mg/kg/day, day − 6 and − 5) , total body irradiation (12 to 14 Gy, day − 3 ~ − 1). The GVHD prevention scheme used cyclosporine combined with mycophenolate mofetil and methotrexate.
Outcomes
The primary endpoint was overall survival (OS) at 3 years after transplantation. The secondary endpoints were progression-free survival (PFS) at 3 years, non-relapse mortality (NRM) at 3 years, and cumulative incidence of relapse at 3 years. OS was defined as time from transplantation until death from any cause. PFS was defined as time from randomization to disease progression. Non-relapse mortality was defined as death from any cause not subsequent to relapse. Relapse was defined as either reappearance of leukemic blasts in the peripheral blood or at least 5% blasts in the bone marrow aspirate or biopsy specimen not attributable to any other cause, or reappearance or new appearance of extramedullary leukemia. Acute GVHD and chronic GVHD were diagnosed and graded according to the standard international criteria24,25.
Statistical analysis
OS, PFS were estimated using the Kaplan–Meier method and compared using the Log-rank test. Cumulative incidences of relapse, non-relapse mortality, and GVHD were calculated by accounting for competing risks. Competing risks for GVHD included death without GVHD and relapse. Relapse was a competing risk for non-relapse mortality, and non-relapse mortality was a competing risk for relapse. The comparison of the cumulative incidence in the presence of a competing risk was done using the Fine and Gray model. The impact of the AML-DRG and AML-HCT-CR models on survival outcomes were determined using univariable and multivariable Cox proportional hazards regression models. The discriminative ability of the models was assessed by Harrell’s C-statistics. p < 0.05 was considered significant. SPSS version 23.0 and R version 3.6.2 were used for data analysis.
Ethics approval and consent to participate
Informed consent was obtained for study participation from all patients, parent and/or legal guardian for minors and the protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Results
Patient characteristics
Among the 172 AML patients analysed, 48.3% (n = 83) were females. Median age was 31.5 years (range 14 to 62 years), two patients was more than 60 years old (1.2%). Median follow-up was 44 months (range 1 to 94 months). According to the AML-DRG model, 109, 49 and 14 patients were in low-, intermediate- and high-risk group, respectively. According to the AML-HCT-CR model, 108, 30, 20 and 14 patients were in low-, intermediate-, high- and very high-risk group, respectively. The basic clinical data of the patients were shown in Table 1.
GVHD
Among the 172 patients, grade II to IV acute GVHD developed in 39 patients (22.7%) and chronic GVHD developed in 38 patients (22.1%). For the entire cohort, the cumulative incidence of grade II to IV acute GVHD at day 100 was 22.7% (95% CI 20.0–25.4), the cumulative incidence of all-grade chronic GVHD at 2 years was 21.7% (95% CI 19.0–24.4). The cumulative 100-day incidence of grade II to IV acute GVHD for the low-, intermediate- and high-risk AML-DRG groups was 22.0% (95% CI 18.3–25.7), 18.4% (95% CI 11.8–25.0) and 7.1% (95% CI 0–21.1), differences are not statistically significant ( p = 0.605). The incidence for all-grade chronic GVHD at 2 years was 23.2% (95% CI 19.4–27.0), 22.4% (95% CI 15.5–29.3) and 7.1% (95% CI 0–22.1), respectively (p = 0.430) (Table 2).
For the AML-HCT-CR model, the cumulative 100-day incidence of grade II to IV acute GVHD for the low-, intermediate-, high- and very high-risk groups was 21.3% (95% CI 17.5–25.1), 13.3% (95% CI 4.3–22.3), 30.0% (95% CI 15.8–44.2) and 7.1% (95% CI 0–21.1), respectively ( p = 0.582). The incidence for all-grade chronic GVHD at 2 years was 23.4% (95% CI 19.6–27.2), 30.3% (95% CI 19.8–40.8), 10.0% (95% CI 0–21.7) and 7.1% (95% CI 0–22.7), respectively ( p = 0.287) (Table 2).
Relapse and NRM
For the entire cohort, the 3-year cumulative incidences of relapse and NRM were 21.1% (95% CI 18.5–23.7) and 24.8% (95% CI 22.1–27.5), respectively. We found that relapse and NRM occurred in 37 (21.5%) and 44 (25.6%) of 172 patients. Reasons for relapse were hematological in 30 patients (17.4%), extramedullary in 4 patients (2.3%), and hematological plus extramedullary in 5 patients (2.9%).
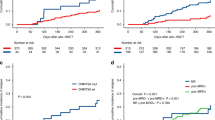
The 3-year cumulative incidence of relapse for the low-, intermediate- and high-risk AML-DRG groups was 12.2% (95% CI 9.5–16.3), 32.7% (95%CI 25.4–40.0) and 50.0% (95% CI 30.2–69.8), respectively (p < 0.001) (Fig. 1B) , with the corresponding 3-year NRM was 18.8% (95% CI 15.1–22.5), 32.9% (95% CI 25.6–40.2) and 42.9% (95% CI 22.9–62.9), differences are not statistically ( p = 0.072) (Fig. 1C). The 3-year cumulative incidence of relapse for the low-, intermediate-, high- and very high-risk AML-HCT-CR groups was 12.3% (95% CI 8.9–15.7), 26.7% (95% CI 16.4–37.0), 45.0% (95% CI 30.1–59.9) and 42.9% (95% CI 23.4–62.4), respectively (p < 0.001) (Fig. 2B), with the corresponding 3-year NRM was 19.0% (95% CI 15.3–22.7), 20.4% (95% CI 10.5–30.3), 45.0% (95% CI 29.9–60.1) and 50.0% (95% CI 30.0–70.0), respectively (p = 0.008) (Fig. 2C).
OS and PFS
The 3-year OS and PFS of the entire cohort were 56.9% (95% CI 49.9–64.9) and 53.1% (95% CI 45.9–61.3). Patients in low-, intermediate- and high-risk AML-DRG groups had median OS of 33.0 (1 ~ 94), 16.0 (1 ~ 73) and 4.5 (1 ~ 69) months, respectively (p < 0.001), with the corresponding 3-year OS of 69.6% (95% CI 61.0–79.6), 38.6% (95% CI 27.1–55.0) and 7.1% (95% CI 1.1–47.2), respectively (p < 0.001). The 3-year PFS were 67.3% (95% CI 58.8–77.2), 34.5% (95% CI 23.4–50.8) and 7.1% (95% CI 1.1–47.2), respectively (p < 0.001) (Fig. 1A). The median OS for the low-, intermediate-, high- and very high-risk AML-HCT-CR groups were 33.0 (1 ~ 94), 28.9 (2 ~ 73), 6.5 (1 ~ 43) and 4.0 (1 ~ 69) months, respectively (p < 0.001), with the corresponding 3-year OS of 69.4% (95% CI 60.7–79.4), 59.6% (95% CI 44.3–80.2), 10.0% (95% CI 2.7–37.2) and 7.1% (95% CI 1.1–47.2), respectively (p < 0.001) (Fig. 2A). The 3-year PFS were 67.1% (95% CI 58.5–77.0), 52.9% (95% CI 37.6–74.4), 10.0% (95% CI 2.7–37.2) and 7.1% (95% CI 1.1–47.2), respectively (p < 0.001) (Table 2).
In univariable analysis for OS, patients with intermediate and high-risk AML-DRG groups had a significantly increased risk of death with hazard ratio (HR) of 2.62 (95% CI 1.60–4.31; p < 0.001) and 7.08 (95% CI 3.68–13.63; p < 0.001), respectively when compared with the low-risk group. Also, the risk of death was higher in high-risk group compared with the intermediate-risk group (HR 2.63, 95% CI 1.35–5.10; p = 0.004), confirming the ability of the AML-DRG model in post-transplant survival prediction. For AML-HCT-CR groups, patients with high (HR 5.44, 95% CI 3.03–9.78; p < 0.001) and very high-risk groups (HR 8.35, 95% CI 4.32–16.14; p < 0.001) had significantly increased risk of death than low-risk group, while there was no difference between low and intermediate-risk (HR 1.39, 95% CI 0.72–2.69; p = 0.330) and between high and very high group (HR 1.53,95% CI 0.74–3.17; p = 0.251). Similar results were found in a univariable analysis for PFS as summarized in Table 3.
Multivariate analysis
Multivariable analysis confirmed that the HCT-CR and AML-HCT-CR models could be used to predict the OS of patients in different risk groups after adjusted for other variables including age, sex, conditioning type, transplant modality, and stem cell source. See Table 4 for details.
Comparison of prognostic stratification
The C-indexes of the AML-DRG, AML-HCT-CR, DRI, ELN2017 genetic risk, and HCT-CI/Age model were 0.69 (95% CI 0.61–0.78), 0.71 (95% CI 0.63–0.79), 0.61 (95% CI 0.52–0.69), 0.52 (95% CI 0.43–0.60) and 0.59 (95% CI 0.50–0.67), respectively. Compared with the DRI model and the HCT-CI/Age, the AML-DRG and AML-HCT-CR models had significantly better discrimination ability on OS prediction with C-index. The risk assessment ability of AML-DRG and AML-HCT-CR may be better than that of ELN2017 genetic risk, DRI and HCT-CI/Age models.
Discussion
Risk stratification is essential to predict the prognosis of patients with AML receiving AHCT. Recently, the AML-DRG and AML-HCT-CR models, which combines DRI, HCT-CI, ELN2017 risk classification, MRD and other important prognostic factors, was published and has demonstrated a significant impact in terms of OS and PFS21. In this study, we examined the effect of the AML-DRG and AML-HCT-CR models on clinical outcomes of AHCT. The results demonstrated that both AML-DRG and AML-HCT-CR models could significantly predict the OS, PFS and relapse. The AML-HCT-CR model could significantly predict the NRM. While the GVHD did not reach statistical significance.
In our retrospective study including 172 patients with AML, we confirmed that the AML-DRG and AML-HCT-CR models have a prognostic prediction on OS and PFS (all p < 0.001). This is consistent with the reference publication. Kongtim et al.21 reported that the OS at 5 years for low-, intermediate- and high-risk AML-DRG risk groups were 62.8%, 33.1% and 12.6%, respectively, and 5-year PFS were 60.4%, 31.1%, and 7.9%, respectively. The OS at 5 years for low-, intermediate-, high- and very high-risk AML-HCT-CR risk groups were 71.1%, 53.6%, 37.4%, and 12.7%, respectively, the corresponding PFS at 5 years were 67.4%, 51.7%, 36.2%, and 9.6%, respectively. We also performed pairwise comparisons in survival analysis among different groups. The results showed that for AML-DRG, all pairwise comparisons were statistically significant. However, unlike the reference publication, we did not find the difference of OS between AML-HCT-CR low-risk and intermediate-risk group (69.4% vs 59.6%, p = 0.330), high-risk and very high-risk group (10.0% vs 7.1%, p = 0.251). Fist, the sample of high- and very high-risk AML-HCT-CR patients was relatively small. Second, in our study, the conditioning regimens included busulfan (Bu)- and total body irradiation (TBI)-based regimens in patients, while the reference publication included busulfan (Bu)- and Melphalan-based regimens. Third, unlike the reference publication, only two patients in our sample were older than 60 years. Perhaps these reasons caused our results to be different from the reference publication. Therefore, a multi-centre clinical trial is required to confirm the findings from the reference publication. The multivariable analyses have shown that prognostic prediction of the AML-DRG and AML-HCT-CR models on post-transplant survival was independent to age, sex, conditioning type, transplant modality, and stem cell source (p < 0.001; p < 0.001). This means that the model can be applied in patients transplanted using both HLA-matched and unmatched donors.
Since the AML-DRG and AML-HCT-CR models have been developed and validated their utility in mortality prediction, we further identified the specific cause of mortality. Our study found that the AML-DRG and AML-HCT-CR models were associated with the 3-year OS, mainly due to relapse. The AML-DRG model categorized patients into 3 distinct relapse risk groups, with 3-year cumulative incidence of relapse ranging between 12.2% for the low-risk group to 50.0% for the high-risk group. Similar results were found for the AML-HCT-CR model (12.3%for the low-risk group and 42.9% for the very high-risk group). The results suggest that for the patients in the intermediate and high-risk groups of AML-DRG and AML-HCT-CR models, MRD and other related indexes should be closely monitored after transplantation, and maintenance treatment or pre-emptive treatment should be given, so as to reduce the risk of recurrence and improve the efficacy of the transplantation. Patients in the very high-risk AML-HCT-CR group had higher recurrence and NRM rates, especially high NRM rates (42.9%for relapse rate and 50.0% for NRM rate). These patients usually had advanced-stage disease and/or high comorbidities burden before AHCT, which suggested that they had a higher risk of disease relapse and may be vulnerable to drug toxicities and transplant complications. A lower intensity conditioning regimen may help to prevent the transplant-related toxicity and mortality. However, this may lead to high relapse rates after AHCT, particularly for those with relapse/refractory leukemia6,7. Therefore, how to prevent relapse after AHCT on the basis of controlling the toxicity of the conditioning regimen is important to improve the clinical outcome of patients with very high-risk AML-HCT-CR group.
Our study found that the AML-DRG and AML-HCT-CR models had a significantly better discrimination ability on OS prediction with Harrell C-index of0.69 and 0.71, respectively, when compared with the DRI model (C-index0.61), HCT-CI/Age (C-index0.59) and ELN2017 risk classification (C-index0.52). This was partially in accordance with the reference study in which the AML-DRG and AML-HCT-CR models significantly better predicted risk of death after transplant with C-indices of 0.672 and 0.715, respectively21. It demonstrated that AML-DRG and AML-HCT-CR models provide better tools for risk stratification of patients.
The AML-DRG model had no prognostic for NRM. In addition, both AML-DRG and AML-HCT-CR models were not prognostic for GVHD. Those data are also very important for HSCT choice. Further incorporation of NRM- and GVHD- related specificity indicators may help to achieve a more comprehensive scoring system. Although HCT-CI had been considered be prognostic of NRM and GVHD16,26,27, we did not find AML-HCT-CR be prognostic for GVHD. A possible explanation for our results is that combining AML-DRG and HCT-CI probably weakens the weight of HCT-CI in GVHD prognostication.
Conclusions
In conclusion, our data confirm results similar to the reference publication and provide useful information on OS, PFS, relapse, and NRM prediction. Compared to AML-HCT-CR model, the AML-DRG allows better stratification and improved survival prediction of AML patients post-transplant. Considering the absence of prognosis for NRM and GVHD, we recommend using the AML-DRG and HCT-CI separately to obtain more accurate and relevant information to guide transplant choice.
Data availability
Data and material will be available upon corresponding author approval. All data sets generated/analysed for this study are included in the manuscript and the additional files.
Abbreviations
- aGVHD:
-
Acute GVHD
- AHCT:
-
Allogeneic hematopoietic stem cell transplantation
- AML:
-
Acute myeloid leukemia
- AML-DRG:
-
AML-specific disease risk group
- AML-HCT-CR:
-
AML-Hematopoietic Cell Transplant-composite risk
- ATG:
-
Anti-thymocyte globulin
- BMSC:
-
Bone marrow stem cell
- Bu:
-
Busulfan
- cGVHD:
-
Chronic GVHD
- CI:
-
Confidence interval
- CR:
-
Complete remission
- DRCI:
-
Disease risk comorbidity index
- DRI:
-
Disease risk index
- ELN2017 genetic risk:
-
European Leukemia Net 2017 genetic risk
- HCT-CI:
-
Hematopoietic cell transplantation-specific comorbidity index
- HR:
-
Hazard ratio
- ISD:
-
Identical sibling donor
- MRD:
-
Minimal residual disease
- NRM:
-
Non-relapse mortality
- OS:
-
Overall survival
- PBSC:
-
Peripheral blood stem cell
- PFS:
-
Progression-free survival
- TBI:
-
Total body irradiation
- TRM:
-
Transplant-related mortality
References
Yu, J. et al. Advances in targeted therapy for acute myeloid leukemia. Biomark. Res. 8, 17 (2020).
Copelan, E. A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 354(17), 1813–1826 (2006).
Yang, X. & Wang, J. Precision therapy for acute myeloid leukemia. J. Hematol. Oncol. 11(1), 3 (2018).
Tsirigotis, P. et al. Relapse of AML after hematopoietic stem cell transplantation: Methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant. 11(1), 3 (2018).
Bejanyan, N. et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: A center for international blood and marrow transplant research study. Biol. Blood Marrow Transplant. 21(3), 454–459 (2015).
Abdul Wahid, S. F. et al. Comparison of reduced-intensity and myeloablative conditioning regimens for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia and acute lymphoblastic leukemia: A meta-analysis. Stem Cells Dev. 23(21), 2535–2552 (2014).
Rubio, M. T. et al. Impact of conditioning intensity in T-replete haplo-identical stem cell transplantation for acute leukemia: A report from the acute leukemia working party of the EBMT. J. Hematol. Oncol. 9, 25 (2016).
Terwijn, M. et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: Data from the HOVON/SAKK AML 42A study. J. Clin. Oncol. 31(31), 3889–3897 (2013).
Grimm, J. et al. Prognostic impact of the ELN2017 risk classification in patients with AML receiving allogeneic transplantation. Blood Adv. 4(16), 3864–3874 (2020).
Vicente, D. et al. Improved outcome in young adults with de novo acute myeloid leukemia in first remission, undergoing an allogeneic bone marrow transplant. Bone Marrow Transplant. 40(4), 349–354 (2007).
Saber, W. et al. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood 119(17), 3908–3916 (2012).
Sengsayadeth, S. et al. Transplant outcomes for secondary acute myeloid leukemia: Acute leukemia working party of the European Society for Blood and Bone Marrow Transplantation Study. Biol. Blood Marrow Transplant. 24(7), 1406–1414 (2018).
Armand, P. et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood 120(4), 905–913 (2012).
Armand, P. et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 123(23), 3664–3671 (2014).
Sorror, M. L. et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 106(8), 2912–2919 (2005).
Sorror, M. L. et al. Comorbidity-age index: A clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 32(29), 3249–3256 (2014).
Bejanyan, N. et al. Predictive value of disease risk comorbidity index for overall survival after allogeneic hematopoietic transplantation. Blood Adv. 3(3), 230–236 (2019).
Walter, R. B. et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J. Clin. Oncol. 29(9), 1190–1197 (2011).
Walter, R. B. et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood 122(10), 1813–1821 (2013).
Liu, J. et al. The significance of peri-transplantation minimal residual disease assessed by multiparameter flow cytometry on outcomes for adult AML patients receiving haploidentical allografts. Bone Marrow Transplant. 54(4), 567–577 (2019).
Kongtim, P. et al. Novel disease risk model for patients with acute myeloid leukemia receiving allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 26(1), 197–203 (2020).
Döhner, H. et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4), 424–447 (2017).
Schuurhuis, G. J. et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 131(12), 1275–1291 (2018).
Przepiorka, D. et al. 1994 Consensus Conference on Acute GVHD grading. Bone Marrow Transplant. 15(6), 825–8 (1995).
Filipovich, A. H. et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol. Blood Transplant 131(12), 1275–1291 (2005).
Sorror, M. L. et al. Pretransplant comorbidities predict severity of acute graft-versus-host disease and subsequent mortality. Blood 124(2), 287–295 (2014).
Sorror, M. L. et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: Combined FHCRC and MDACC experiences. Blood 110(13), 4606–4613 (2007).
Acknowledgements
This work was supported by the Project of Henan Provincial Education Department, China (20A320062, recipient JY), Project of Science and Technology Department of Henan Province, China (LHGJ20190039, recipient JY), Project of Science and Technology Department of Henan Province, China (SBGJ20202076, recipient JY), Talent Research Fund of the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China (recipient JY), and Key scientific research projects of colleges and universities in Henan Province, China (recipient WC).
Author information
Authors and Affiliations
Contributions
J.Y. and D.W. designed and directed the study. W. C. and X.L. wrote the manuscript. R.Z., Z.B., S.Z., L.L., H.X., C.L., X.X., Z.J. and D.W. contributed for the clinical data and patients’ treatment. All authors critically reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cao, W., Li, X., Zhang, R. et al. Prognostic prediction of novel risk scores (AML-DRG and AML-HCT-CR) in acute myeloid leukemia patients with allogeneic hematopoietic stem cell transplantation. Sci Rep 12, 19024 (2022). https://doi.org/10.1038/s41598-022-20735-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20735-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.