Abstract
Vaginal microbiota have been shown to be a modifier of protection offered by topical tenofovir in preventing HIV infection in women, an effect not observed with oral tenofovir-based pre-exposure prophylaxis (PrEP). It remains unclear whether PrEP can influence the vaginal microbiota composition. This study investigated the impact of daily oral tenofovir disoproxil fumarate in combination with emtricitabine for PrEP on the vaginal microbiota in South African women. At baseline, Lactobacillus iners or Gardnerella vaginalis dominant vaginal communities were observed in the majority of participants. In cross sectional analysis, vaginal microbiota were not affected by the initiation and use of PrEP. Longitudinal analysis revealed that Lactobacillus crispatus-dominant “cervicotypes 1 (CT1)” communities had high probability of remaining stable in PrEP group, but had a higher probability of transitioning to L. iners-dominant CT2 communities in non-PrEP group. L. iners-dominant communities were more likely to transition to communities associated with bacterial vaginosis (BV), irrespective of PrEP or antibiotic use. As expected, BV-linked CTs had a higher probability of transitioning to L. iners than L. crispatus dominant CTs and this shift was not associated with PrEP use.
Similar content being viewed by others
Introduction
Over the past decade, studies have demonstrated the efficacy of prophylactic antiretrovirals (ARVs) for the prevention of HIV, with oral tenofovir-based treatment showing markedly reduced rates of HIV infection among men who have sex with men, transgender women, injection drug users, serodiscordant couples, and heterosexual men and women1,2,3,4,5. Pre-exposure prophylaxis6 for men using either daily or intermittent dosing strategies has consistently demonstrated protective efficacy ranging from 44 to 96%1,2,3,4,5,7. Despite adherence, PrEP for women in Southern Africa has yielded mixed results, underscoring a plausible role for other biological factors that mitigate PrEP efficacy6,8,9.
Previous studies have suggested that the vaginal microbiome can significantly modulate the efficacy of topical tenofovir-based PrEP10,11, an effect not observed with oral tenofovir-based PrEP12. In addition, proteomics analyses of vaginal specimens of participants from the CAPRISA 004 1% tenofovir gel trial showed a significantly reduced risk of HIV acquisition in women with Lactobacillus dominant compared to non-Lactobacillus dominant microbiota8,10. Furthermore, the tenofovir levels were reduced in vaginal specimens from women with a Gardnerella vaginalis dominant microbial community, suggesting that the depletion of tenofovir was secondary to drug metabolism by G. vaginalis8,10. These findings are supported by high tenofovir levels in cervical tissue of women with a Nugent score of ≤ 3 (Lactobacillus dominant) and reduced tenofovir levels in women with bacterial vaginosis (BV) (G. vaginalis dominant11). However, none of these studies have characterized the potential impact of PrEP on the vaginal microbial communities of healthy women in sub-Saharan Africa. To address this gap, we investigated the impact of longitudinal oral tenofovir disoproxil fumarate + emtricitabine (TDF-FTC) PrEP on the vaginal microbiome of healthy South African women.
Materials and methods
Study design, participants, and sample collection
This is a retrospective study within the CAPRISA 08213 and CAPRISA 084 (https://www.caprisa.org/Pages/CAPRISAStudies) studies. CAPRISA 082 was a prospective observational cohort study of HIV risk factors and prevention choices in young women aged 18–30 years old from an urban and rural population in KwaZulu-Natal, South Africa. CAPRISA 084 was a PrEP demonstration project that assessed the feasibility, acceptability, uptake and patterns of daily oral TDF/FTC PrEP provided as part of sexual reproductive health services to young women and men (aged 18 years and older) at risk of acquiring HIV in eThekwini, Vulindlela and Umlazi in KwaZulu-Natal, South Africa. Women living with HIV and those who had other factors contraindicated for PrEP use were excluded from both studies. Consenting participants in both studies were followed monthly for up to 3 months, then quarterly for approximately 18 months. Some CAPRISA 082 women agreed to take PrEP while others disagreed, and all were reimbursed for attending clinic visits. All CAPRISA 084 participants were provided with PrEP at all visits and were not reimbursed for attending clinic visits. For this study, we only included cervicovaginal swab samples collected at baseline, 3, and 6 months. At each visit, cervicovaginal swabs collected from the posterior fornix and lateral vaginal walls from each participant were tested for vaginal pH using pH indicator strips, and for Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium and Trichomonas vaginalis using PCR. Gram staining was performed to confirm a diagnosis of BV based on Nugent score ≥ 7. CAPRISA 082 and 084 participants were treated with regimens recommended in the South African STI treatment guidelines for the following diagnoses: Chlamydia trachomatis (azithromycin 1 g oral), Neisseria gonorrhoeae (ceftriaxone 250 mg intramuscular and azithromycin 1 g oral), Trichomonas vaginalis (metronidazole 2 g orally) and candidiasis (clotrimazole 500 mg pessary and clotrimazole 1% cream). Women who had a Nugent score ≥ 4 were treated with metronidazole (2 g oral single dose). At follow-up visits, CAPRISA 082 participants were managed syndromically, however CAPRISA 084 participants were screened and treated for STIs and BV. All participants provided written informed consent. The study was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (BREC number: BE603/18). All the experimental procedures are in the accordance with the relevant ethical guidance and regulations.
Bacterial DNA extraction, 16S rRNA gene sequencing, taxonomic assignment and CTs assignment
Total nucleic acid was extracted using phenol–chloroform from stored vaginal swabs and the V4 region of the 16S rRNA gene was amplified and sequenced using the Illumina MiSeq as previously described14. Divisive Amplicon Denoising Algorithm (DADA) 215 was used to filter for quality, trim, and identify inference of amplicon sequence variant (ASV). ASVs were taxonomically classified to genus or higher levels using a Naïve Bayes classification approach16 and SILVA ribosomal RNA database15. The ASVs for Lactobacillus, Prevotella, Sneathia and Mobiluncus were further refined to the species level with speciateIT (version 1.0, http://ravel-lab.org/speciateIT). A phyloseq object containing a phylogenetic tree, ASV table, taxonomic table, and sample metadata was created using the phyloseq R package17. Based on composition and diversity at species-level where possible and genus-level, bacterial communities clustered into 4 basic groups, which we referred to as “cervicotypes”14.
Statistical analysis
Descriptive statistics for quantitative variables were summarized using medians and interquartile ranges (IQRs) whilst categorical data were summarized with both frequency counts and percentages. Mann–Whitney U test was used to compare continuous variables and Fisher’s Exact test for categorical comparisons. α-diversity including Richness (Chao1), evenness (Simpson’s E) and phylogenetic diversity (Faith’s PD) estimates were calculated using the R vegan library. β-diversity between samples was estimated by Bray–Curtis distances. Non-metric Multidimensional scaling (NMDS) was performed to assess differences in taxonomy profiles among samples based on the Bray–Curtis distance of ASV relative abundance using the metaMDS function in the vegan R package. The transition probabilities are calculated as proportions of the samples (stratified by antibiotic and PrEP use) shifting from one CT to the next. Permutational multivariate analysis of variance (PERMANOVA) was performed with Adonis function in the vegan R package with a similarity index using 9999 permutations to measure the differences in beta diversity between PrEP and non-PrEP groups. All statistical calculations were carried out using R and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Data availability
The datasets generated or analysed during the current study are publicly available in the NCBI BioProject respository, (http://www.ncbi.nlm.nih.gov/bioproject/858949) under accession number PRJNA858949.
Results
Study participants
A total of 100 women were included in this sub-study, of whom 60 (60%) were from CAPRISA 082 and 40 (40%) from CAPRISA 084. All CAPRISA 084 participants were provided with PrEP. For CAPRISA 082 participants, 24 (40%) were provided with PrEP and the other 36 (60%) were not (Table 1). Most women were below 30 years (median age 24 years (IQR 21.5–28) of age, but compared with non-PrEP group, PrEP group was older [median age 26.5 years (IQR 22.5–34) vs 22 years (20.5–25); p = < 0.0001]. Although majority of women reported having a stable partner (84/100, 84%), the proportion of women in the PrEP group was lower than that of non-PrEP group [49/64 (76.6%) vs 35/36 (97.2%); p = 0. 0087]. A greater proportion of participants in the PrEP group were married (12/64, 18.8%) compared to those in the non-PrEP group (0/36; p = 0.0037). A high number of women in the PrEP group (16/64, 26.2%) reported having a partner who was HIV positive compared to those in the non-PrEP group (0/36; p = 0.001). Study site, education level, vaginal sex within 30 days and contraceptive use were similar between PrEP and non-PrEP groups. Depo medroxyprogesterone acetate (49%, 49/100) was the most common contraceptive used in both groups.
Prevalence of BV and STIs in PrEP and non-PrEP group
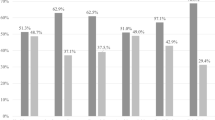
In the 100 participants, three CAPRISA 084 women at baseline, two at 3 months and four at 6 months had missing BV data. For women in the CAPRISA 082 study, BV and STIs data were performed at baseline but not at follow-up visits. At baseline, 31.6% (31/97) of women had intermediate BV (Nugent score 4–6) and 21.4% (21/97) had BV (Nugent score ≥ 7) (Table 2). Compared with PrEP group, there was a trend for non-PrEP group to be more likely to have BV (p = 0.0576). The proportion with BV in the PrEP group was 14.8% compared to 36.1% in the non-PrEP group (p = 0.0231) At 3 months follow-up, 36.8% (14/38) of CAPRISA 084 women had intermediate BV and two of them 5.3% (2/38) had BV. At 6 months, both intermediate BV rate 22.2% (8/36) and BV rate 16.7% (6/36) were almost the same. For STIs, 12% women (12/100) had C. trachomatis, 3.1% (3/98) had T. vaginalis and 2% (2/100) had N. gonorrhoea at baseline, and no difference was observed between PrEP and non-PrEP group. At the 3-month and 6-month visit, 38 and 37 of those in the PrEP group had STI data, respectively, of which 5.3% (2/38) were infected with C. trachomatis at 3 months and remained the same 5.3% (2/38) at 6 months. No women had T. vaginalis and N. gonorrhoea at 3 months and at follow-up visits (Table 2).
Composition of the vaginal microbiota at baseline
Based on the composition and relative abundance of bacterial species, we assigned bacterial communities into four CTs (CT1-4, Fig. 1)14,18. Samples with the relative majority of sequences (≥ 50%) assigned to non-iners Lactobacillus species were defined as CT1 and more than > 97% were Lactobacillus crispatus. The communities that had the highest relative abundance of Lactobacillus iners and Gardnerella vaginalis were classified as CT2, and CT3, respectively. CT4 had a mixed dominant bacterial taxon. Both PrEP and non-PrEP groups had similar vaginal microbial composition at all timepoints (Supplementary Fig. 1). At baseline, 11% (11/100) of women were assigned to CT1, 47% (47/100) to CT2, 26% (26/100) to CT3, and 16% (16/100) to CT4. Of women with intermediate BV (Nugent Score 4–6) and BV (Nugent Score > 7), 18% (2/11) were found in CT1, 36% (17/47) in CT2, 73% (19/26) in CT3 and 88% (14/16) in CT4 (Fig. 1); X2 (3, N = 100) = 21.395, p = 0.871. Of women with STI infection, 10% (1/11) were found in CT1, 13% (6/47) in CT2, 23% (6/26) in CT3 and 25% (4/16) in CT4; Fisher’s Exact test p = 0.568.
Heat map of the most abundant vaginal bacteria identified by 16S rRNA sequencing in 100 women at baseline. Relative abundance of key taxa across all of the samples was used to determine each of the four CTs: L. crispatus (CT1), L. iners (CT2), G. vaginalis (CT3), and mixed communities not dominated by other taxa (CT4). BV and STI statuses were also depicted.
Similar cervicotype composition and transition patterns among groups
We stratified the composition and structure of vaginal microbiota by PrEP use overtime (Fig. 2A). At baseline, among L. crispatus dominated CT1, 8% (3/36) and 13% (8/64) were non-PrEP and PrEP groups, respectively. Of women who were CT1 at baseline, 38% (3/8) transitioned to CT2 and 13% (1/8) to CT3 in the PrEP group while 67% (2/3) non-PrEP group transitioned to CT2 and 33% (1/3) to CT4 at the next time point. In addition, of the four women in the PrEP group who remained stable at 3 months, three women (38%, 3/8) transitioned to new CTs at 6 months. The proportions of L. iners dominated CT2 were different between PrEP (n = 32) and non-PrEP (n = 15) groups at baseline; X2 (1, N = 45) = 0.188, p = 0.665. At 3 months, 34% (11/32) of women with L. iners dominated CT2 in the PrEP group transitioned between CTs, with 13% (4/32) to CT1, 19% (6/32) to CT3 and 3% (1/32) to CT4] and 6 non-PrEP group transitioned to new CTs [7% (1/15) CT1, 27% (4/15) to CT3 and 7% (1/15) to CT4]. Furthermore, women with stable CT2 over two visits transitioned to new CTs at 6 months; PrEP group [14% (3/21) transitioned to CT1 and 24% (5/21) to CT3] and non-PrEP group [22% (2/9) to CT3 and 22% (2/9) to CT4]. More than a third of PrEP (33%, 8/24) and non-PrEP (50%, 9/18) groups with BV-associated at baseline transitioned to Lactobacillus dominated CTs at 3 months. PrEP (46%, 6/13) and non-PrEP (50%, 3/6) groups with stable BV-associated CTs over two visits transitioned to Lactobacillus dominated CTs at 6 months. No major shifts in microbial communities were detected in PrEP compared to non-PrEP group.
Composition and transitions between cervicotype in PrEP (n = 64) and non-PrEP groups (n = 36). (A) Box plots showing CTs assigned to individual participants over 3 visits at baseline, 3 months and 6 months. Blue colour depicts CT1, green CT2, pink CT3, and orange CT4. PID number that starts with 082 refers to women enrolled in CAPRISA 082 and 084 refers to CAPRISA 084 cohort. (B) Transition probabilities between CT’s from one visit to another in PrEP and non-PrEP groups who did not use or used antibiotics during the study.
We further assessed the transition probabilities between CTs in PrEP and non-PrEP groups, respectively (Fig. 2B). CT1 had an increased probability of remaining stable (0.5) in PrEP groups while were more likely to transition to CT2 (0.6) in non-PrEP group. In both groups, those with CT2 had a higher probability of remaining (0.62, respectively) compared to those with other CTs. CT3 in PrEP group without using antibiotics were less likely (0.47) to transition while those who used antibiotics were most likely to transition to CT2 (0.55). In PrEP group, CT4 had an increased probability of remaining (0.56 and 1.00 in antibiotic and non-antibiotic users, respectively) rather than transitioning to other states, while favours transitioning to CT2 (0.38) in non-PrEP group. BV-linked CTs had an increased probability of remaining rather than transitioning to Lactobacillus dominant CTs and the observed shifts were not associated with PrEP.
PrEP or antibiotics did not influence spartial segregation
To evaluate the possible impact of PrEP on vaginal microbial communities, we perfomed Non-metric Multidimensional scaling (NMDS) on Bray–Curtis distance at all timepoints. No clustering was detected based on PrEP or antibiotic use (Fig. 3). We further explored the relative contribution of PrEP to the diversity relative to other unknown factors by perfoming a permutational multivariate analysis of variance (PERMANOVA) in adonis. We found no significant differences between the two study groups (PrEP and non-PrEPgroups) at all timepoints (P = 0.467, R2 = 0.0087, P = 0.080, R2 = 0.0092 and P = 0.344, R2 = 0.0108) respectively.
Non-metric Multidimensional scaling (NMDS) of Bray–Curtis showing no clustering dissimilarity of species level relative abundances in PrEP and non-PrEP groups at baseline, 3 months and 6 months. The pink colour represents non-PrEP group and green colour represents PrEP group. Open shape represents participants on antibiotics.
Discussion
The World Health Organization recommends offering PrEP as an additional prevention choice for persons at increased risk of HIV infection as part of combination HIV prevention approaches19. There is a paucity of data on the impact of PrEP on the vaginal microbial communities of healthy women in sub-Saharan Africa. Here, we longitudinally assessed vaginal microbiota in South African women to understand whether PrEP (TDF/FTC) can influence the vaginal microbiome. We found fewer women with a high relative abundance of L. crispatus compared to that of L. iners and G. vaginalis dominant communities. Furthermore, we also observed transitions between CTs in PrEP and non-PrEP groups, with few women with stable L. crispatus over time.
Studies have revealed that European-American vaginal microbiota have high Lactobacillus abundance, while those in women of African descent is more diverse in nature14,18,20,21,22,23,24,25. In addition, Gosmann et al., (2017) demonstrated that 10% of a cohort (N = 236) of South African Black women aged 18–23 years lacked L. crispatus dominance in their cervical vaginal microbiome over time18. Another study found that only 37% of South African women had Lactobacillus-dominant communities26. However, these studies solely characterised vaginal microbial communities in women residing in South Africa and no other part of Africa. Therefore, there is a need to understand geographical and lifestyle impact on vaginal microbiome. Consistent with these studies, we demonstrate that only 8%, (3/36, non-PrEP) and 13% (8/64, PrEP group) of women had L. crispatus-dominated communities and this number was reduced to one participants (PrEP group) at the 6 month visit. In agreement with previous studies18,23,26,27, majority of South African women had vaginal community with high abundance of L. iners. L. iners-dominated communities are known to be unstable with high probability of transitioning to a high diversity community state associated with BV. Several factors have been posited to play a dominant role in shaping the vaginal microbiota, including racial lines, vaginal hygiene practices, contraceptive use, sexual behaviour, rectal colonization and host genetics. However, their individual or collective contributions to the shifts in the vaginal microbiome remains unresolved18,28.
We explored transition patterns between the observed microbial communities over time. There was no difference in changes in the microbial communities overtime between PrEP and non-PrEP groups. Although L. iners-dominant communities were less likely to transition, once they did, they favoured communities associated with BV and increased HIV risk, irrespective of PrEP or antibiotic use. This is consistent with previous studies that showed women with L. iners-dominant communities transition more frequently to ecological niche with other bacterial taxa, such as G. vaginalis, A. vaginae, and other strict and facultative anaerobes23,27,29,30,31. Furthermore, Munoz et al.27 showed that transitions away from L. crispatus and towards L. iners-dominant communities are less likely to revert to L. crispatus communities but most likely to further transition to BV-associated communities. This is particularly concerning and may explain high rates of diverse microbial communities and BV occurrence among women of African descent. These data affirm that there is a microbiome footprint that dictates the colonization of certain bacteria together more strongly, perhaps due to the synergy and mutually beneficial effects for these species to survive together. In addition, viable alternatives such as probiotics that shift transition from L. iners to L. crispatus leading to curative or preventive vaginal dysbiosis interventions are needed.
We also observed that G. vaginalis-dominant communities remained stable over time and more transitions to L. iners than L. crispatus-dominant communities, irrespective of PrEP or antibiotic use. In this study, some of the PrEP group participants diagnosed with BV were treated with metronidazole at baseline (CAPRISA 082 and CAPRISA 084), 3 months and 6 months (CAPRISA 084 only). Previous studies have demonstrated that BV treatments were associated with temporal changes in the vaginal microbiota23,32. Therefore, the observed shifts away from BV-associated communities in this study is more likely attributed to BV treatment rather than PrEP use23,29,30. This is confirmed by high numbers of observed transitions from BV associated CTs to L iners dominated CT in women actively taking antibiotics.
One of the strengths of this study is the longitudinal follow-up and sampling before and after PrEP initiation in at risk South African women. However, this study had limitations which could have influenced the composition of vaginal microbiota. Firstly, PrEP adherence data relied on pill count and self-reporting, and systemic or genital PrEP levels were measured only in a sub-set of women. Secondly, the lack of longitudinal data for STI and BV in the CAPRISA 082 trial that precluded a detailed analyses of the effect of PrEP and BV on the vaginal microbiota. Additionally, we could not measure in detail the impact of BV treatment on vaginal microbiota, instead BV scores (particularly in the CAPRISA 084 cohort- PrEP group) were used as a surrogate of treatment adherence. Furthermore, we did not investigate the impact of other potential co-factors such as STIs, human papillomavirus, herpes simplex virus, hormonal contraceptives, diets, hormonal status, and other vaginal disorders (e.g., aerobic vaginitis), although these may have different biological effects on microbial communities.
In conclusion, our results showed no major differences in vaginal microbial communities between PrEP and non-PrEP groups, suggesting that PrEP initiation does not alter the vaginal microbial communities of HIV-negative sub-Saharan African women. These findings reaffirm that PrEP rollout can be delivered in women with optimal and/or sub-optimal vaginal microbiota. Regular screening for STIs and BV during PrEP care remains an ideal option, particularly in regions with rates of HIV infection.
References
Baeten, J. M. et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 367, 399–410. https://doi.org/10.1056/NEJMoa1108524 (2012).
Grant, R. M. et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 363, 2587–2599. https://doi.org/10.1056/NEJMoa1011205 (2010).
McCormack, S., Dunn, D. & On behalf of the PROUD Study Group. in Conference on Retroviruses and Opportunistic Infections (CROI), 23–26 February (Seattle, Washington, 2015).
Molina, J.-M. et al. in Conference on Retroviruses and Opportunistic Infections.
Thigpen, M. C. et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N. Engl. J. Med. 367, 423–434 (2012).
Van Damme, L. et al. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 367, 411–422 (2012).
Choopanya, K. et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet 381, 2083–2090 (2013).
Abdool Karim, Q. et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329, 1168–1174 (2010).
Rees H. et al. in Conference on Retroviruses and Opportunistic Infections (CROI) (Seattle, Washington, 2015).
Klatt, N. R. et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 356, 938–945. https://doi.org/10.1126/science.aai9383 (2017).
Hillier, S. L. et al. in Conference on Retroviruses and Opportunistic Infections.
Heffron, R. et al. Efficacy of oral pre-exposure prophylaxis (PrEP) for HIV among women with abnormal vaginal microbiota: a post-hoc analysis of the randomised, placebo-controlled partners PrEP study. Lancet HIV https://doi.org/10.1016/S2352-3018(17)30110-8 (2017).
Mansoor, L. E. et al. Prospective study of oral pre-exposure prophylaxis initiation and adherence among young women in KwaZulu-Natal, South Africa. J. Int. AIDS Soc. 25, e25957. https://doi.org/10.1002/jia2.25957 (2022).
Anahtar, M. N. et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42, 965–976. https://doi.org/10.1016/j.immuni.2015.04.019 (2015).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. https://doi.org/10.1038/nmeth.3869 (2016).
McDonald, D. et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6, 610–618. https://doi.org/10.1038/ismej.2011.139 (2012).
McMurdie, P. J. & Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217. https://doi.org/10.1371/journal.pone.0061217 (2013).
Gosmann, C. et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 46, 29–37. https://doi.org/10.1016/j.immuni.2016.12.013 (2017).
World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV (2015).
Fettweis, J. M. et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 160, 2272–2282. https://doi.org/10.1099/mic.0.081034-0 (2014).
Ravel, J. et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U.S.A. 108(Suppl 1), 4680–4687. https://doi.org/10.1073/pnas.1002611107 (2011).
Lennard, K. et al. Microbial composition predicts genital tract inflammation and persistent bacterial vaginosis in South African adolescent females. Infect. Immun. https://doi.org/10.1128/IAI.00410-17 (2018).
Mtshali, A. et al. Temporal changes in vaginal microbiota and genital tract cytokines among South African women treated for bacterial vaginosis. Front. Immunol. https://doi.org/10.3389/fimmu.2021.730986 (2021).
Riganelli, L. et al. Structural variations of vaginal and endometrial microbiota: Hints on female infertility. Front. Cell Infect. Microbiol. 10, 350. https://doi.org/10.3389/fcimb.2020.00350 (2020).
Gajer, P. et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 4, 132ra152-132ra152 (2012).
Anahtar, M. N., Gootenberg, D. B., Mitchell, C. M. & Kwon, D. S. Cervicovaginal microbiota and reproductive health: The virtue of simplicity. Cell Host Microbe 23, 159–168. https://doi.org/10.1016/j.chom.2018.01.013 (2018).
Munoz, A. et al. Modeling the temporal dynamics of cervicovaginal microbiota identifies targets that may promote reproductive health. Microbiome 9, 163. https://doi.org/10.1186/s40168-021-01096-9 (2021).
Muzny, C. A. & Schwebke, J. R. Pathogenesis of bacterial vaginosis: Discussion of current hypotheses. J. Infect. Dis. 214(Suppl 1), S1-5. https://doi.org/10.1093/infdis/jiw121 (2016).
Joag, V. et al. Impact of standard bacterial vaginosis treatment on the genital microbiota, immune milieu, and ex vivo human immunodeficiency virus susceptibility. Clin. Infect. Dis. 68, 1675–1683. https://doi.org/10.1093/cid/ciy762 (2019).
Verwijs, M. C., Agaba, S. K., Darby, A. C. & van de Wijgert, J. Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure. Am. J. Obstet. Gynecol. https://doi.org/10.1016/j.ajog.2019.08.008 (2019).
Ravel, J. et al. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome 1, 29. https://doi.org/10.1186/2049-2618-1-29 (2013).
Ahrens, P. et al. Changes in the vaginal microbiota following antibiotic treatment for Mycoplasma genitalium, Chlamydia trachomatis and bacterial vaginosis. PLoS ONE 15, e0236036. https://doi.org/10.1371/journal.pone.0236036 (2020).
Acknowledgements
We would like to thank all CAPRISA 082 and CAPRISA 084 study participants and the study team for their contributions to this research. This research was partially supported by the Department of Science and Innovation – National Research Foundation Centre of Excellence in HIV Prevention (UID 96354) and the Bill & Melinda Gates Foundation (BMGF, INV-018075). NMM was funded by Talent Excellence Scholarship, University of KwaZulu-Natal. DA was funded through the SAMRC Self-Initiated Grant and the NRF of South Africa Thuthuka (grant#TTK160517165310), the NRF Research Career Advancement Fellowship (grant#RCA13101656388), and a European & Developing Countries Clinical Trials Partnership (EDCTP) senior fellowship (grant#TMA2017SF-1960). SN was supported by Columbia University-Southern African Fogarty AITRP Programme (grant#D43TW00231), National Research Fund Thuthuka Research Grant (grant# TTK160510164586), and Poliomyelitis Research Foundation Research Grant (grant#16/17). P.S. received support from the South African Medical Research Council (SAMRC) Special Initiative (Grant no. 96151) and S&F Scarce Skills Postdoctoral Fellowships (Grant no.132714).The CAPRISA 082 observational study was made possible by the generous support of the American people through the United States Agency for International Development (USAID) under the Cooperation Agreement No. AID-OAA-A-15-00040 and a grant funded by FHI360 under Cooperative Agreement/Grant No. AID-674-A-14-00009 funded by USAID Southern Africa. CAPRISA 084 PrEP Demonstration project was supported by FHI360 under Cooperative Agreement/Grant No. AID-674-A-14-00009 funded by USAID Southern Africa.
Author information
Authors and Affiliations
Contributions
N.M.M., P.S., D.A., D.S.K. and S.N. conceived and designed the study. N.M.M. and J.X. carried out experiments and A.M., G.M. and L.N. provided extra support during laboratory experiments. N.M.M., S.N., J.A.E. & S.E.J. performed the analysis and L.L. provided statistical support. L.E.M. and Q.A.K. were the principal investigators of the CAPRISA 082 and CAPRISA 084 studies, respectively. N.M.M., P.S., S.E.J., J.X., L.L., A.M., G.M., L.N., L.E.M., Q.A.K., S.S.A.K., D.A., D.S.K. and S.N. contributed to the interpretation and discussion of the results and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mazibuko-Motau, N., Sobia, P., Xu, J. et al. Vaginal microbial shifts are unaffected by oral pre-exposure prophylaxis in South African women. Sci Rep 12, 16187 (2022). https://doi.org/10.1038/s41598-022-20486-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20486-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.