Abstract
In a phase-IIa trial, we investigated the influence of 90 days continuous-delivery tenofovir (TFV) intravaginal rings (IVRs) with/without levonorgestrel (LNG) on the genital microbiota of Kenyan women. Eligible women (n = 27; 18–34 years; negative for HIV, sexually transmitted infections, and Amsel-bacterial vaginosis) were randomized 2:2:1 to use of IVRs containing TFV, TFV/LNG, or placebo. Using vaginal wall and IVR swabs at IVR insertion and removal, the genital microbial composition was determined using 16S rRNA gene sequencing. The presence of Candida spp. was determined using qPCR. The vaginal total bacterial burden appeared to decrease with TFV and TFV/LNG IVR use (log100.57 and log100.27 decrease respectively; p > 0.05). The TFV/LNG IVR was more ‘stabilizing’: 50% of the participants’ microbiota community state types remained unchanged and 50% shifted towards higher Lactobacillus abundance. Specifically, TFV/LNG IVR use was accompanied by increased abundances of Lactobacillus gasseri/hominis/johnsonii/taiwanensis (16.3-fold) and L. fermentum/reuteri/vaginalis (7.0-fold; all p < 0.01). A significant shift in the overall microbial α-diversity or β-diversity was not observed for either IVR, and IVR use did not influence Candida spp. prevalence. TFV/LNG and TFV IVRs did not adversely affect the genital microbiota and are safe to use. Our findings support further studies assessing their efficacy in preventing HIV/HSV-2 and unintended pregnancies.
Similar content being viewed by others
Introduction
With global estimates of 1.7 million incident HIV infections1 and 121 million unintended pregnancies annually2, development of a multipurpose, user-controlled product that prevents heterosexual transmission of HIV and provides contraception for women is invaluable. Daily oral emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) has revolutionized HIV prevention as the first pre-exposure prophylaxis (PrEP) option. However, a high level of adherence with the dosing regimen is essential for protection and is often difficult to achieve3,4. Long-acting contraception options not requiring daily adherence have been pivotal in the prevention of unintended pregnancies. Innovative multipurpose technologies (MPT) could be developed by leveraging current long-acting contraceptive strategies with HIV PrEP co-delivery or co-formulation to improve protective coverage.
Intravaginal rings (IVRs) are a safe, discreet and effective option for simultaneous delivery of contraception and antiretrovirals5. An IVR with dapivirine, which was found to reduce HIV incidence by 27% in two efficacy trials6,7, with up to 75–91% reduction among women with higher adherence to the IVR8, received a positive opinion from the European Medicines Agency and the World Health Organization9,10. IVRs also allow for consistent contraceptive hormone release, eliminating the high peak experienced with injectables11 or the daily fluctuations from oral contraceptives12.
Given their localized effects, the influence of IVRs on the genital microbiota is an important consideration. Microbiota shifts could influence genital health and ability of the female genital tract (FGT) to protect against infections including sexually transmitted infections (STIs). In one study of coitally-dosed 1% tenofovir (TFV) vaginal gel (a product that has not moved forward in development)13, efficacy was higher in the presence of a Lactobacillus-dominant microbiota but decreased in the presence of higher mucosal inflammation14,15.
The CONRAD co-formulated TFV/levonorgestrel (LNG) and single agent TFV IVRs have been tested for safety in a phase-I trial among women from the United States (US) and the Dominican Republic16. However, the effect of these IVRs on the genital microbiota of sub-Saharan African Black women has not been evaluated. Women in this region face a disproportionate burden of HIV and their genital microbiota has been shown to be markedly different to women with different lived experiences and ethnicities. As part of the primary goals for this phase-IIa safety trial of 90 days use TFV and TFV/LNG IVRs, we determined the effect of each IVR relative to the placebo IVR on the genital microbiota of African women. In addition, we assessed the interplay between IVR use and genital microbiota on local TFV levels and the incidence of candidiasis.
Results
Participant characteristics
Twenty-six of the twenty-seven randomized women had bacterial absolute abundance data available: TFV/LNG IVR (n = 11), TFV IVR (n = 10), and placebo IVR (n = 5). The median age was 22 years [interquartile range (IQR) 21–26], median body mass index was 22.4 [IQR 19.8–24.9], and demographic characteristics were similar across study arms (Table 1). Most women used male condoms during the study (74.1%), 7.4% previously used a copper-intrauterine device (≥ 6 months before enrolling in the study), and 18.5% used no contraceptive method. Despite being asymptomatic and BV-negative by Amsel criteria at screening, 4/27 (14.8%) women were Nugent-BV-positive at the time of IVR insertion and 11/27 (41.0%) at the IVR removal visit. With the exception of an estimated increase in the prevalence of BV-positive Nugent score, differences in clinicial characteristics at IVR removal were small.
Total bacterial load did not change with IVR use
The total FGT 16S bacterial load did not change between the baseline and IVR removal visits for women in any arm (Fig. 1A; TFV/LNG: log108.29 vs log108.14, p = 0.56; TFV: log107.84 vs log107.94, p = 0.36; placebo: log108.14 vs log108.45, p = 0.25) and the difference in the degree of change across arms relative to the placebo arm was small (Fig. 1B). At IVR removal, no participant-level differences were observed in any arms between the lateral vaginal wall bacterial load and that of the IVR surface (Fig. S1; TFV/LNG: log108.14 vs log108.56, p = 0.11; TFV: log107.94 vs log108.22, p = 0.43; placebo: log108.45 vs log108.18, p = 0.25). Compared to the placebo arm, a higher estimated bacterial load was found on the TFV/LNG IVR surface (median difference of log100.33, p = 0.05) than on the TFV IVR (median difference of log100.04, p = 0.20 vs placebo median difference of log10− 0.34, Fig. 1C) relative to the vaginal wall bacterial load. Overall, genital bacterial load was positively correlated with the total abundance of the Lactobacillus genus, with a log100.95 increase in total bacterial load with every log10 increase in Lactobacillus abundance (Linear regression p = 0.03 after adjusting for multiple comparisons).
Change in bacterial load between intravaginal ring (IVR) insertion and removal among women randomized to use tenofovir/levonorgestrel (TFV/LNG), tenofovir (TFV), and placebo IVRs, Kisumu, Kenya, 2019. Comparing (A) vaginal log10 total 16S copies per swab between the two visits, (B) the degree of change in vaginal wall log10 total bacterial 16S copies from baseline to the IVR removal visit among women and (C) difference in total log10 16S copies between the vaginal wall and IVR surface (log10 bacterial load of vaginal wall − log10 bacterial load of IVR) at the time of IVR removal. Values above and below the dotted red line indicate an increase and decrease in bacterial loads respectively. p values were determined using the Wilcoxon Signed Rank test (A) and Mann–Whitney U test (B,C).
Change in bacterial load was not dependent on length of IVR use
There was no association between the vaginal bacterial load and the number of days a woman used the TFV/LNG IVR (Slope = − 0.009; 95% CI [− 0.032, 0.012]; p = 0.34; adjusted coefficient of determination (R2) = − 0.00019) or the TFV IVR (Slope = 0.003; 95% CI [− 0.031, 0.012]; p = 0.80; adjusted R2 = − 0.13). Women using the placebo IVR had a greater estimated increase in bacterial load with longer IVR use (Fig. 2; R2 = 0.88) although this estimate is highly imprecise since only 4 women were in this arm. Similarly, the correlation between IVR surface bacterial load and length of time of IVR use was low for either arm (Fig. S2).
Impact of length of tenofovir/levonorgestrel (TFV/LNG), tenofovir (TFV), or placebo intravaginal ring (IVR) use on total bacterial load, Kisumu, Kenya, 2019. Linear regressions showing the association between number of days of IVR use and change in bacterial load between visits. The coloured shading represents the 95% confidence interval around the slope.
The overall microbiota diversity did not change with IVR use
To measure the effect of IVR use on the genital microbial composition of women, bacterial diversity was assessed using the Shannon diversity index and Bray–Curtis distances. We did not find a large change in overall microbiota diversity between baseline and IVR removal with use of any IVR (Fig. 3A–D). Microbial diversity decreased in women using the TFV/LNG IVR and increased with the TFV IVR (Shannon index difference of − 0.36 and 0.62 respectively; Fig. 3A); however both changes were not statistically significant. We also found no difference in the microbial diversity of the IVR microbiota compared to the vaginal microbiota at the IVR removal visit (Fig. S3; PERMANOVA TFV/LNG R2 = 0.058, p = 0.32; TFV R2 = 0.011, p > 0.99; placebo R2 = 0.11, p = 0.57).
No change in overall microbiota diversity with use of tenofovir/levonorgestrel (TFV/LNG), tenofovir (TFV), or placebo intravaginal ring (IVR), Kisumu, Kenya, 2019. (A) Comparing the change in Shannon diversity (within-participant diversity) with each IVR. The dotted red line at zero indicates no change over time. (B–D) Principal component analysis plots showing the overlap between samples at baseline and the IVR removal visit for the TFV/LNG, TFV and Placebo IVRs, based on Bray–Curtis distances (between-participant diversity). p values were determined using the (A) Mann–Whitney U test or the (B–D) Adonis/PERMANOVA test based on permutations of distance matrices. NMDS non-metric multidimensional scaling.
TFV/LNG IVR use was associated with a shift towards a less diverse CST
Based on hierarchical clustering of their dominant vaginal microbiota taxa, women were categorized as having community state type (CST) I if they had a genital microbiota dominated by L. crispatus, or CST III if their microbiota was dominated by L. iners. CST IVA had a higher abundance of the BV-associated bacterium G. vaginalis and CST IVB was composed of a broader range of BV-associated bacteria (Fig. S4). We found some discordance between Nugent-BV status and CST IV categorization. At baseline, of the 11/26 (42.3%) of women with a microbiota typically associated with microbial dysbiosis (CST IV A/B), 3/11 were Nugent-BV-positive, 3/11 had an intermediate Nugent score and 5/11 were Nugent-BV-negative. Further one participant with Nugent-BV was categorized as having a CST III microbiota.
During follow up, 50.0% (5/10) of women using the TFV/LNG IVR transitioned to a CST less associated with BV (2/5 transitioned from CST IVB to CST IVA) or a higher abundance of Lactobacillus species (1/5 transitioned from CST IVB to CST III, 1/5 from CST IVA to CST I, and 1/5 from CST III to CST I) while there were no CST shifts in the other 50.0% of women (Fig. 4A, Table S1). Among women using the TFV IVR, 22.2% (2/9) of women did not experience CST shifts, while the majority transitioned to a more diverse (44.4%; 4/9) or a less diverse CST (33.0%; 3/9). With the placebo IVR, 75.0% of women (3/4) did not transition to a different CST and one participant transitioned to a more diverse CST (CST IVA to CST IVB).
Transitions in bacterial communities within each study arm, Kisumu, Kenya, 2019. (A) Shifts in community state types (CSTs) in the genital tract from baseline to the intravaginal ring (IVR) removal visit. (B) Differences in CST between the genital tract and the IVR surface at the IVR removal visit. Each line represents one participant’s transition within the tenofovir/levonorgestrel (TFV/LNG), tenofovir (TFV), or placebo study arms.
Overall, 41.6% (10/24) women had differences in assigned CST between the vaginal wall and the IVR surface at IVR removal (Fig. 4B), with 6/10 having a more diverse and 4/10 having a less diverse IVR CST, although none of these broad transitions were statistically significant (TFV/LNG p = 0.58; TFV p = 0.57; placebo p = 0.85).
Neither TFV/LNG nor TFV IVR use was associated with major increases in pathogenic bacterial taxa
Longitudinal fold-changes in relative abundances of each individual bacterial taxa measured was determined for each study arm, accounting for individual variations in time between IVR insertion and removal. Using a threshold cutoff of log2 0.05, the placebo IVR was associated with the most fluctuations, with a significant fold-change in 23 bacterial taxa (Fig. 5, Table S2): including decreases in Finegoldia spp. and Dialister spp. (~ log2 twofold), and increases in numerous taxa such as a > log2 twofold increase in Proteobacteria phylum, Atopobium vaginae, Corynebacterium coyleae/mucifaciens, Corynebacterium genitalium, Prevotella buccalis, and Porphyromonadaceae as well as a log2 9.17-fold (standard error (SE) = 2.5) increase in L. crispatus/acidophilus. Women randomized to the TFV/LNG and TFV IVRs appeared to experience a more ‘stabilizing’ effect, with less change in vaginal bacterial taxa. Women using the TFV IVR were observed to have an average 5.42-fold (SE = 1.63) increase in Dialister micraerophilus abundance, a BV-associated bacterium. Women using the TFV/LNG IVR were observed to have a 3.79-fold (SE = 1.55) decrease in the pathogenic bacterium Streptococcus anginosus/milleri/sanguinis and a 16.3-fold (SE = 2.09) and 7.0-fold (SE = 2.13) increases in L. fermentum/reuteri/vaginalis and L. gasseri respectively (all p ≤ 0.01; Fig. 5).
Fold changes in abundance of specific bacteria with the tenofovir/levonorgestrel (TFV/LNG), tenofovir (TFV), and placebo intravaginal rings (IVRs) respectively, Kisumu, Kenya, 2019. Dots on the right hand side of the gray solid line show a fold change increase in bacterial abundance and dots on the left hand side show a fold change decrease. The red and blue dotted lines represent 0.5-fold and twofold changes in bacterial abundance. Differential abundances with an adjusted p value of ≤ 0.01 are shown.
Candidiasis was similar across IVR types
The prevalence of Candida spp. were also measured at both visits. No C. parapsilosis was detected in any sample. There was an overall 30.8% prevalence of Candida at baseline and 44% at the IVR removal visit (p = 0.54; Table 2). C. krusei (23%) and C. albicans (17%) were more common (Table 2, Fig. 6). The distribution of each Candida spp. did not differ across study arms at baseline (C. albicans p > 0.99; C. glabrata p = 0.57; C. krusei p > 0.99) or at IVR removal (C. albicans p > 0.99; C. krusei p = 0.64). C. glabrata was not detected at the IVR removal visit. There were no differences in the overall microbial diversity (PERMANOVA p = 0.07) or differences in abundances of individual bacterial taxa between women who remained Candida-negative and those who acquired a Candida infection by the IVR removal visit.
Higher microbial diversity was associated with lower TFV levels
At the IVR removal visit, we found a higher estimated concentration (84.6-fold) of vaginal TFV in women with lower microbial diversity (median 53550 ng/swab with CST I vs 632.5 ng/swab with CST IVB; p = 0.16; Fig. 7), although sample sizes were small. In addition, after adjusting for the number of days of IVR use, a log10 increase in the absolute abundance of Lactobacillus genus was associated with a log10 0.41 ng/swab increase in TFV concentrations (p > 0.99).
Vaginal tenofovir (TFV) concentrations across community state types (CSTs), Kisumu, Kenya, 2019. Comparing the difference in log10 vaginal TFV concentrations at the IVR removal visit among women using the TFV and tenofovir/levonorgestrel arms categorized by their CST. The p value was determined using the Mann–Whitney U test.
Discussion
Overall, we found no evidence that either the TFV/LNG or TFV IVR significantly affected a woman’s genital bacterial or candidal composition. Our results are consistent with previous findings, including a recent safety study of the same IVR among women in the US and Dominican Republic16.
We mostly found small longitudinal changes in total bacterial load or in the overall microbial diversities in all study arms, suggesting that these IVRs do not induce major shifts in the genital microbiota. At the IVR removal visit, CSTs between the lateral vaginal wall and the IVR did not differ, similar to previous findings16. Despite no CST changes in most women using the placebo IVR, its use led to fluctuations in multiple bacterial taxa, with statistically significant fold changes in both Lactobacillus spp. and BV-associated bacteria. The TFV IVR was only associated with an increase in D. micraerophilus abundance, a BV-associated bacterium strongly associated with microbial diversity and high-inflammation states17,18,19. However, we did not see a shift to a more BV-associated microbial composition, likely because the increase in D. micraerophilus was insufficient to drive this change. Interestingly, there was a statistically significant decrease in S. anginosus/milleri/sanguinis abundance with the TFV/LNG IVR and a markedly larger fold-increase in Lactobacillus abundance, suggesting a positive effect of the TFV/LNG combination on microbial composition. Previous studies of vaginal rings found no significant destabilization of the genital microbiota, and an increase in Lactobacillus spp. in some cases16,20,21, which was attributed to ethinyl estradiol22,23,24. Here we found a similar stabilizing effect, with a decrease in microbial diversity with the TFV/LNG IVR despite containing LNG, a progestin, indicating that the decrease in microbial diversity and increase in Lactobacillus spp. could be associated with progestin use, particularly LNG, in line with a previous study reporting an increase in genital Lactobacillus spp. concentration with LNG implant use25.
Although not statistically significantly so, we found that genital TFV concentrations at the IVR removal visit were positively associated with Lactobacillus spp. abundances. This is similar to other studies showing that TFV-based HIV PrEP products were less effective in Lactobacillus-deficient states14. There was a decreasing trend in microbial diversity with increased TFV concentration, with a 84.6-fold higher TFV concentration found with an L. crispatus-dominant microbiota (CST I) compared to a more diverse BV-associated microbiota (CST IVB). Based on our differential abundance analyses, this was not driven by G. vaginalis or Prevotella spp as previously described14,26. More work is needed to determine if this decrease is clinically relevant to impact the IVR capacity to prevent HIV or HSV-2. In particular, it would be important to assess the relationship between CSTs and concentrations of the TFV active metabolite, TFV-diphosphate, in mucosal tissues.
A primary limitation was the small sample size, due to the study being a phase-IIa safety trial. Thus, we presented substantial descriptive data that examine actual numbers and trends, rather than just statistical comparisons. More in-depth comparisons, in larger cohorts, that also include women with BV at IVR insertion, might better help identify potential genital microbial changes induced by these IVRs.
In this study measuring the safety of TFV/LNG and TFV polyurethane IVRs on the genital microbiota among women living in Kenya, we found no evidence of adverse changes to genital microbial health with up to 90 days of use. We found a trend towards the establishment of high Lactobacillus states with the TFV/LNG IVR, which could positively impact genital health in a population with a high BV prevalence27,28,29. These results indicate that TFV/LNG and TFV IVRs are likely safe to use and support further studies assessing their efficacy in preventing HIV, HSV-2, and unintended pregnancy.
Methods
Participant recruitment
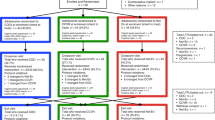
CONRAD B17-144 was a phase-IIa randomised, placebo-controlled, investigational new-drug-enabling trial assessing the safety, pharmacokinetics, pharmacodynamics, tolerability, acceptability of, and adherence to two 90 days IVRs. Women were randomised in a 2:2:1 ratio to receive a TFV-only IVR30 to prevent HIV/HSV, a combination TFV/LNG IVR that additionally may prevent pregnancy30,31,32 and a placebo IVR31. All rings were composed of polyurethane tubing31.
This study recruited women at the Jaramogi Oginga Odinga Referral Hospital, Kisumu, Kenya, who were generally healthy, non-pregnant, at lower risk for HIV (based on a validated risk score33), seronegative for HIV and hepatitis B surface antigen, and bacterial vaginosis (BV)-negative by Amsel criteria. Women diagnosed with BV by Amsel criteria during the screening procedures were treated and permitted to be re-assessed for eligibility > 2 months after treatment, and enrolled if BV was not detected at that time. Eligible women were not using hormonal contraception at enrolment and had not been diagnosed or treated for STIs in the last three months (Supplementary Methods). The study was approved by the Scientific and Ethics Review Unit at the Kenya Medical Research Institute and the Human Subjects Division at University of Washington, and was registered with ClinicalTrials.gov (Identifier: NCT03762382; registered 03/12/2018). All participants provided written informed consent and all methods were carried out in accordance with relevant guidelines and regulations.
Study procedures
Results from the parent study will be published in a separate manuscript (Mugo et al.32, in preparation). Paired data from the baseline/IVR insertion and IVR removal visits were included for this analysis, focused on microbiota changes. IVR removal was scheduled for 90 days after IVR insertion or prior to the IVR expiry date, whichever date came first (At 90 days: 12/27; before expiry date: 9/27; other reasons including pregnancy or STIs: 6/27). In cases where matched clinical data from the IVR removal visit were not available, samples collected during the next visit, which was scheduled to occur 24 h later, were used for analyses.
Sample collection at IVR insertion and removal
After randomization, the IVR was inserted deeply into the vagina by a study clinician at the baseline visit and removed at the IVR removal visit. At both baseline and the IVR removal visits, vaginal swabs were collected for (1) TFV PK measurements, (2) Nugent score and vaginal pH and (3) microbiota 16S rRNA sequencing. A swab of the IVR surface was also collected at the IVR removal visit. Nugent-BV was defined as having a Nugent score ≥ 7; women with a Nugent score 4–6 were categorized as having an intermediate vaginal microbiota (Supplementary Methods).
Genital TFV concentrations
TFV concentrations from cervicovaginal fluid from swabs were determined using liquid chromatography tandem mass spectrometry as described previously34 (Supplementary Methods).
Microbial DNA extraction
DNA extraction from lateral vaginal wall swabs was carried out (Qiagen AllPrep PowerViral® DNA/RNA kit; Supplementary Methods) and was stored at − 20 °C until used for 16S rRNA sequencing, total 16S bacterial load using real-time polymerase chain reaction (qPCR), and Candida spp. quantitation (qPCR).
16S rRNA gene sequencing and analysis
The V3–V4 hypervariable region of the bacterial 16S rRNA was amplified using modified universal primers35. Samples were sequenced using the Illumina MiSeq platform and (300 bp paired-end). DADA2 v1.12.136 was used to process, merge and filter raw reads and samples with < 2000 reads were excluded from further analyses. Taxonomic annotation was carried out using the RDP database (v11.5) and a BLAST search was carried out for further taxonomic classification of unannotated amplicon sequence variants (ASV). Taxonomic annotation was available for 993 ASVs, with 277 (27.9%) having species-level annotation. The ASVs were merged at the lowest available taxonomic level to generate relative abundances for a total of 105 bacterial taxa17 (Supplementary Methods).
The total bacterial load per swab was determined using a qPCR of the 16S rRNA gene37. The BactQuant assay targets the V3–V4 region, and gives an estimate of the total 16S rRNA copies per swab. Based on these values and the relative abundances of taxa generated by 16S rRNA sequencing, absolute bacterial abundances (copies/swab) were calculated for each taxon. One sample (out of five), in the placebo arm, without sufficient 16S DNA to pass quality control checks was excluded from analysis (final n = 4). All further analyses were carried out using absolute abundances for bacterial taxa.
Candida spp. quantification
Concentrations of C. albicans, C. glabrata, C. krusei and C. parapsilosis, were measured using species-specific qPCRs at the baseline and IVR removal visits (Supplementary Methods). The final concentrations were reported as number of log10 copies per swab.
Statistical analysis
All microbiota data analysis was carried out in R (v3.6.0). Paired comparisons between the ring insertion and ring removal visits were considered primary endpoints of this analysis. Participant microbiota were categorised into four CSTs using Ward clustering of weighted Unifrac dissimilarity matrices derived from bacterial absolute abundances. TFV and TFV/LNG study arms were compared to the placebo arm using Mann–Whitney U-tests for independent samples and permutational multivariate analysis of variance (PERMANOVA) for microbial diversity. Paired analyses comparing microbiota at IVR insertion and removal visits were carried out using Wilcoxon Signed Rank tests and generalized estimating equation models. Correlations between bacterial absolute abundance and variables such as time of IVR use and genital TFV concentrations were estimated using linear regression models. The number of days of IVR use was categorized as ≤ 47 days (< 2 menstrual cycles), 48–71 days (~ 2 menstrual cycles) and 72–95 days (~ 3 menstrual cycles), and was adjusted for when measuring fold changes in individual bacterial taxa within study arms, using generalized linear models as part of the DeSeq2 package. Log2 fold-changes were determined only for bacterial taxa found in at least 15% of samples to minimize the influence of minority taxa. Due to the small sample size, the study was not powered to estimate differences between the study arms with a high degree of precision and analyses were descriptive in nature. As a result, p-values and confidence intervals (CI) were not adjusted for multiple comparisons unless explicitly stated, in which case a false discovery rate step down procedure was used38 (Supplementary Methods).
Data availability
The dataset generated and analyzed in this study has been deposited at NCBI SRA under Accession ID PRJNA834833 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA834833). Linked data are available from the corresponding author on reasonable request.
References
UNAIDS. Global HIV & AIDS Statistics—2020 Fact Sheet. https://www.unaids.org/en/resources/fact-sheet (2020). Accessed Nov 2020.
Bearak, J. et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: Estimates from a comprehensive model for 1990–2019. Lancet Glob. Health 8, e1152–e1161 (2020).
Van Damme, L. et al. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 367, 411–422 (2012).
Marrazzo, J. M. et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 372, 509–518 (2015).
Griffin, J. B. et al. Vaginal ring acceptability and related preferences among women in low- and middle-income countries: A systematic review and narrative synthesis. PLoS ONE 14, e0224898 (2019).
van der Straten, A. et al. High acceptability of a vaginal ring intended as a microbicide delivery method for HIV prevention in African women. AIDS Behav. 16, 1775–1786 (2012).
Baeten, J. M. et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N. Engl. J. Med. 375, 2121–2132 (2016).
Brown, E. R. et al. Greater dapivirine release from the dapivirine vaginal ring is correlated with lower risk of HIV-1 acquisition: A secondary analysis from a randomized, placebo-controlled trial. J. Int. AIDS Soc. 23, e25634 (2020).
European Medicines Agency. Dapivirine Vaginal Ring 25 mg (Dapivirine) (European Medicines Agency, 2020).
WHO. WHO Recommends the Dapivirine Vaginal Ring as a New Choice for HIV Prevention for Women at Substantial Risk of HIV Infection. https://www.who.int/news/item/26-01-2021-who-recommends-the-dapivirine-vaginal-ring-as-a-new-choice-for-hiv-prevention-for-women-at-substantial-risk-of-hiv-infection (2021). Accessed Mar 2021.
Thurman, A., Kimble, T., Hall, P., Schwartz, J. L. & Archer, D. F. Medroxyprogesterone acetate and estradiol cypionate injectable suspension (Cyclofem) monthly contraceptive injection: Steady-state pharmacokinetics. Contraception 87, 738–743 (2013).
van den Heuvel, M. W., van Bragt, A. J. M., Alnabawy, A. K. M. & Kaptein, M. C. J. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: The vaginal ring, the transdermal patch and an oral contraceptive. Contraception 72, 168–174 (2005).
Abdool Karim, Q. et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329, 1168–1174 (2010).
Klatt, N. R. et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 356, 938–945 (2017).
McKinnon, L. R. et al. Genital inflammation undermines the effectiveness of tenofovir gel in preventing HIV acquisition in women. Nat. Med. https://doi.org/10.1038/nm.4506 (2018).
Thurman, A. R. et al. Vaginal microbiota and mucosal pharmacokinetics of tenofovir in healthy women using tenofovir and tenofovir/levonorgestrel vaginal rings. PLoS ONE 14, e0217229 (2019).
Lennard, K. et al. Microbial composition predicts genital tract inflammation and persistent bacterial vaginosis in South African adolescent females. Infect. Immun. https://doi.org/10.1128/IAI.00410-17 (2018).
Sabo, M. C. et al. Associations between vaginal bacteria implicated in HIV acquisition risk and proinflammatory cytokines and chemokines. Sex. Transm. Infect. https://doi.org/10.1136/sextrans-2018-053949 (2019).
De Seta, F., Campisciano, G., Zanotta, N., Ricci, G. & Comar, M. The vaginal community state types microbiome-immune network as key factor for bacterial vaginosis and aerobic vaginitis. Front. Microbiol. https://doi.org/10.3389/fmicb.2019.02451 (2019).
Hardy, L. et al. Association of vaginal dysbiosis and biofilm with contraceptive vaginal ring biomass in African women. PLoS ONE 12, e0178324 (2017).
Crucitti, T. et al. Contraceptive rings promote vaginal lactobacilli in a high bacterial vaginosis prevalence population: A randomised, open-label longitudinal study in Rwandan women. PLoS ONE 13, e0201003 (2018).
Veres, S., Miller, L. & Burington, B. A comparison between the vaginal ring and oral contraceptives. Obstet. Gynecol. 104, 555–563 (2004).
Huang, Y. et al. Effects of a one year reusable contraceptive vaginal ring on vaginal microflora and the risk of vaginal infection: An open-label prospective evaluation. PLoS ONE 10, e0134460 (2015).
De Seta, F. et al. Effects of hormonal contraception on vaginal flora. Contraception 86, 526–529 (2012).
Achilles, S. L. et al. Impact of contraceptive initiation on vaginal microbiota. Am. J. Obstet. Gynecol. 218(622), e1–e10 (2018).
Hillier, S. L. et al. Impact of vaginal microbiota on genital tissue and plasma concentrations of tenofovir (2017).
Torrone, E. A. et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med. 15, e1002511 (2018).
Jespers, V. et al. Prevalence and correlates of bacterial vaginosis in different sub-populations of women in sub-Saharan Africa: A cross-sectional study. PLoS ONE 9, e109670 (2014).
Masha, S. C. et al. High prevalence of curable sexually transmitted infections among pregnant women in a rural county hospital in Kilifi, Kenya. PLoS ONE 12, e0175166 (2017).
Johnson, T. J. et al. A 90-day tenofovir reservoir intravaginal ring for mucosal HIV prophylaxis. Antimicrob. Agents Chemother. 56, 6272–6283 (2012).
Clark, J. T. et al. Engineering a segmented dual-reservoir polyurethane intravaginal ring for simultaneous prevention of HIV transmission and unwanted pregnancy. PLoS ONE 9, e88509 (2014).
Mugo, N. R. et al. Randomized, placebo-controlled trial of safety, pharmacokinetics, and pharmacodynamics of 90-day intravaginal rings (IVRs) releasing tenofovir (TFV) with and without levonorgestrel (LNG) among women in Western Kenya. In HIV R4P (2021).
Balkus, J. E. et al. An empiric HIV risk scoring tool to predict HIV-1 acquisition in African women. J. Acquir. Immune Defic. Syndr. 72, 333–343 (2016).
Hendrix, C. W. et al. MTN-001: Randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS ONE 8, e55013 (2013).
Gohl, D. M. et al. Systematic improvement of amplicon marker gene methods for increased accuracy in microbiome studies. Nat. Biotechnol. 34, 942–949 (2016).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Liu, C. M. et al. BactQuant: An enhanced broad-coverage bacterial quantitative real-time PCR assay. BMC Microbiol. 12, 56 (2012).
Columb, M. O. & Sagadai, S. Multiple comparisons. Curr. Anaesth. Crit. Care 17, 233–236 (2006).
Acknowledgements
We are grateful to all the women who participated in this study, as well as the study teams at Kenya Medical Research Institute and the University of Washington. We would like to thank the Richard E. Haaland lab for providing the genital TFV measurements for all study participants. The authors would also like to acknowledge the efforts of the Kisumu Combined Ring Study Team: Management Team Nelly R Mugo (Center for Clinical Research (CCR), Kenya Medical Research Institute-KEMRI, Nairobi, Kenya & International Clinical Research Center (ICRC), Department of Global Health University of Washington (UW), USA), Victor Mudhune (Center for Global Health Research (CGHR)-KEMRI, Kisumu, Kenya), Renee Heffron (UW, Seattle, WA, USA), Centers for Disease Control and Prevention (CDC): Eleanor McLellan-Lemal, Siobhan O’Connor, Gustavo F Doncel (CONRAD, Eastern Virginia Medical School (EVMS)), Andrea R Thurman (CONRAD, EVMS), Betty Njoroge (CCR, KEMRI). Implementing Study Site Team (KEMRI) CGHR—KEMRI, Kisumu: Beatrice Nyagol, Eunice Ouma, Richard Ndivo, Maxcine Oguta, Haynet Opon, Dorine Awili, Anne Mithika, Epines Chavangi, Jecinter Oruko, John Okanda, Arthur Ogendo, Elizabeth Ayuo, Evans Odipo, Vitalis Sewe, Boaz Kerubo, Calvin Mbeda, Eucabeth Awuonda, Isdorah Odero, Emily Anyango, Erica Mimba, Fredrick Oloo, Richard Odipo, Valarie Opollo, Emily Kerubo, Fredrick Omondi, Angelica Gende, Kelvin Wandera, Carolyne Juma, Alice Were, Phoebe Ogutu, Susan Aremo, Philister Madiega, Daniel Ogando, Judith Arego, Margaret Otieno, Rosemary Akello, Ken Ondeng'e, Teresa Omoro, Caren Amondi. CDC-HIV Branch, Kisumu: Kimberly McCarthy, Renee Ridzon. Other Members of the KCRS Study Team ICRC, Department of Global Health, UW, USA: Susan Morrison, Meighan Krows, Connie Celum; CONRAD -EVMS: Abenan L. Ouattara, Nazita Yousefieh, Jill Schwartz, Allison Matthews. United States Agency for International Development (USAID): Shannon Allen, Elizabeth Russell, Karen Near. Centers for Disease Control and Prevention, USA: Jeffrey Wiener, Taraz Samandari, Stacie R Deaton, Lee Claypool, Josh E. Betts. Laboratory CDC, USA: Richard E. Haaland, Amy Martin (Tenofovir (TFV) pharmacokinetics (PK) assessments), Jeffrey Fountain (cytokine analysis). CONRAD EVMS: Terry A. Jacot (CBQCA and glycerin assessments on Placebo rings, Anti-HSV on genital swabs and Anti-HIV genital on swabs). Endocrine Technologies Core, Oregon National Primate Research Center: David Erikson, Steven W. Blue (Serum Levonorgestrel (LNG) and Sex Hormone-Binding Globulin (SHBG) assessments. Seattle Children's Research Institute: Heather Jaspan, Smritee Dabee, Colin Feng, Melanie Gasper (Microbiota analysis on returned Intra Vaginal rings). Particle Sciences: Barrett Remington, Bruce L. Frank (residual drug assessment on returned Intra Vaginal rings). Nina Isoherranen (UW, TFV PK Assessment), Harald Haugen (ICRC, UW, USA). Safety Monitoring Committee (SMC) Jared Baeten, Katherine Thomas, Susan Morrison (UW, Global Health, ICRC), Doncel F Gustavo (CONRAD, EVMS), Victor Mudhune (CGHR-KEMRI, Kisumu, Kenya), Eleanor McLellan-Lemal (CDC), Siobhan O'Connor (CDC), Athena Kourtis (CDC), Naomi Tepper (CDC), Shannon Allen (USAID), Nelly Mugo (CCR-KEMRI, ICRC-UW). Data management was done by DF/Net, Inc. (Seattle, USA): Lisa Ondrejcek (President), Angela Williams (Clinical Data Manager), Matt Johnson, (Biostatistician), Joe Jiang (Data Science Lead). Statistical analysis Katherine Thomas, Sue Peacock (ICRC-UW, USA), Deborah Donnell (Fred Hutchinson Cancer Research Center, Seattle, WA, USA).
Funding
This work was supported by the United States Centers for Disease Control and Prevention and the United States Agency for International Development (USAID) under the U.S. President's Emergency Plan for AIDS Relief (PEPFAR) through cooperative agreement U01PS005183, 2017–2021 (registered at ClinicalTrials.gov under the identifier NCT03762382), and was made possible by the support of the American people through these governmental agencies and programs. Funding from USAID’s Cooperative Agreements with CONRAD/Eastern Virginia Medical School (AID-OAA-A-14-00010 and AID-OAA-A-14-00011) was used to support the manufacturing and clinical qualification of the rings and CONRAD’s personnel time and effort. The contents in this article are the sole responsibility of the authors, and do not necessarily reflect the official position of the institutions, PEPFAR, USAID, Centers for Disease Control and Prevention and/or the United States Government.
Author information
Authors and Affiliations
Consortia
Contributions
Designed the study and experiments: G.F.D., R.H., N.M., H.B.J., V.M., E.M.L., S.O.C., A.R.T., R.R., J.W., S.A.A. Enrolled the cohort: B.Nj., B.Ny., E.O. Designed the specimen collection and storage procedures: H.S.H. Statistical analysis and data management: S.D., S.P. Processed samples and carried out lab experiments: S.D., C.F., M.G. Wrote the first draft of the manuscript: S.D. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dabee, S., Mugo, N., Mudhune, V. et al. Genital microbiota of women using a 90 day tenofovir or tenofovir and levonorgestrel intravaginal ring in a placebo controlled randomized safety trial in Kenya. Sci Rep 12, 12040 (2022). https://doi.org/10.1038/s41598-022-13475-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13475-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.