Abstract
Existing data for the association between late-life body mass index (BMI) and the risk of Alzheimer’s disease (AD) in the underweight population are limited with conflicting results. A large population-based cohort study of 148,534 individuals aged ≥ 65 years who participated in the national health screening program from 2002 to 2005 was performed using the Korean National Health Insurance Service-Senior cohort database 2006–2015. The risk of AD according to BMI category (kg/m2) in Asians was evaluated using a multivariable Cox regression model, after adjustments for age, sex, lifestyle, low-income status, and comorbidities. To evaluate the association between BMI and AD risk, the underweight population was further subdivided according to the degree of thinness. During the 10-year follow-up period, 22,279 individuals developed AD. Relative to the normal-weight population, the estimated adjusted hazard ratio (HR) for incident AD in the underweight, overweight, and obese populations was 1.17 (95% confidence interval [CI], 1.09–1.24), 0.90 (0.87–0.93), and 0.83 (0.80–0.85), respectively. In the underweight population, AD risk increased as the degree of thinness increased (p for the trend, < .001). Late-life BMI showed a significant inverse relationship with AD risk, especially in the underweight population. Public health strategies to screen for AD more actively in the underweight population and improve their weight status may help reduce the burden of AD.
Similar content being viewed by others
Introduction
Dementia is a clinical syndrome characterized by progressive cognitive decline, behavioral disturbances, and significant interference in the ability to maintain activities of daily living1. Approximately 9.9 million individuals are estimated to develop dementia each year, and the number of people with dementia worldwide is predicted to increase to 75 million by 2030. Alzheimer’s disease (AD) is the most common form of dementia and may contribute to 60%-70% of all dementia cases2. The principal risk factor for AD is age, and the prevalence of AD has been reported to increase dramatically with age in the US: 5.3% in people aged 65–74 years and 34.6% in those aged 85 years or older3. However, AD is not a normal part of aging, and older age alone is not sufficient to cause AD4.
Addressing multiple modifiable risk factors for dementia, such as physical activity, smoking, social activity, blood pressure, or diet, can prevent or delay up to 40% of all dementia cases5. Among these modifiable risk factors, body mass index (BMI) has a complex effect on the incidence of dementia according to age. A high BMI in mid-life has been relatively well-demonstrated to increase the risk of AD, and many studies have shown consistent results in this regard6,7. However, in contrast to the findings for mid-life BMI, the late-life BMI as a continuous variable showed the inverse association with the AD risk in a recent meta-analysis study8. The exact underlying mechanisms are still unclear but one plausible explanation for this might be due to the fat-derived hormone leptin6. A previous study showed that higher plasma leptin levels were associated with a lower risk of AD9, suggesting leptin may play a role in reducing the production of amyloid beta (Aβ)10, diminishing extracellular Aβ11, and inhibiting the production of hyperphosphorylated tau which are the key pathological features of AD11,12.
However, previous longitudinal studies comparing the risk of AD between low and non-low BMI in late-life showed inconclusive results, which were primarily attributed to the differences in the criteria for low BMI between studies and the small number of participants included in the studies6,8,13. Although obese individuals (BMI ≥ 30 kg/m2) have been reported to show a significantly lower AD risk than those with lower BMI, these studies did not include an adequate number of participants to provide sufficient evidence to prove the generalization that obesity reduces the risk of AD. In addition, since these studies did not include Asians14,15,16, the applicability of these results to Asian participants with different BMI criteria is not clear.
Therefore, we conducted this nationwide cohort study with sufficient late-life participants to confirm the relationship between late-life BMI and AD risk. We also used a population-based representative sample from the Republic of Korea to determine whether the late-life weight state according to the Asia–Pacific BMI classification is associated with the incidence of AD.
Methods
Data source and study population
The Korean National Health Insurance Service (NHIS), which is a single compulsory social medical insurer operated by the government, has established a public database called the National Health Information Database (NHID). The NHID contains all records of healthcare utilization (including information on diagnosis and prescription records), the eligibility database (including sociodemographic variables), and the national health screening database17. The national health screening program consists of a questionnaire about previous history, family history, lifestyle, anthropometric measurements, and laboratory tests, and is provided biennially to all adults older than 40 years18. The NHIS-Senior database is composed of a 10% randomly sampled group of the entire elderly population aged ≥ 60 years in the NHID in 200219. All individuals in the NHIS-Senior cohort were followed up retrospectively from 2002 to 2015, except for those who were not eligible for national health insurance.
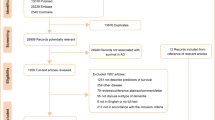
We collected data from the NHIS-Senior database for all individuals who participated in the national health screening program from 2002 to 2005. Among the 215,875 participants, we excluded 5798 individuals who died before the index date and 6729 individuals who had received a diagnosis of any type of dementia before the index date. In addition, we excluded 22,061 individuals who had been diagnosed with any type of cancer and 1966 individuals who had a history of stroke before the index date. We then excluded 20,042 participants aged < 65 years in 2006 and 10,745 individuals with missing variables. The final study population consisted of 148,534 individuals (Fig. 1). All participants were followed from the index date, January 1, 2006, to the date of AD diagnosis or December 31, 2015, whichever was earlier.
The Institutional Review Board of the Veterans Health Service Medical Center (Seoul, Republic of Korea) approved this study (IRB no. BOHUN 2021-01-059-001), and waived the requirement for obtaining written informed consent because the NHID provides anonymized and de-identified data. All research was performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Outcome and covariable definitions
AD was diagnosed on the basis of International Classification of Diseases, 10th revision codes (ICD-10) codes F00 or G30. We defined AD in cases where the diagnosis and prescription of anticholinesterases (donepezil, rivastigmine, and galantamine) or N-methyl-d-aspartate (NMDA) receptor antagonists (memantine) were claimed together on the same day20,21,22. To properly claim the prescription of anti-dementia drugs, physicians should document the evidence of cognitive decline according to the following criteria: 1) Mini Mental State Examination (MMSE) score ≤ 26 and 2) either Clinical Dementia Rating (CDR) ≥ 1 or Global Deterioration Scale (GDS) score ≥ 323.
Participants were categorized by BMI (kg/m2) as underweight (< 18.5), normal weight (18.5–22.9), overweight (23.0–24.9), or obese (≥ 25.0) using the WHO Western Pacific Region guideline strata24. The underweight population was further categorized as showing mild (17.0–18.4), moderate (16.0–16.9), or severe (< 16.0) thinness25. Participants responded to questionnaires regarding their past medical history and health behaviors, such as current smoking, current alcohol drinking, and regular exercise (at least 1 time per week) in the national health screening program. Because health insurance premiums are determined by income level or holding property in the NHIS, we defined the low-income population as individuals whose health insurance premiums were less than the lowest decile for the insured or who were medical aid beneficiaries.
Comorbidities such as hypertension, diabetes, and dyslipidemia were defined by prescription of medication for the disease using the respective ICD-10 codes (I10–13 and I15 for hypertension, E11–14 for diabetes, and E78 for dyslipidemia) at least 2 times per year before the index year or if the respective diagnostic criteria were met (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg for hypertension; fasting blood glucose ≥ 126 mg/dL for diabetes; and total cholesterol ≥ 240 mg/dL for dyslipidemia) in the results of the national health screening program. Cardiovascular disease (CVD) was identified based on the answers to the self-reported questionnaire for a physician’s diagnosis of heart disease in the national health screening program.
For each participant, the primary outcome was the occurrence of AD between January 1, 2006, and December 31, 2015, and the number of person-years of follow-up was recorded.
Statistical analysis
The baseline characteristics of the participants were compared according to BMI categories using ANOVA for continuous variables and chi-square test for categorical variables. Data are presented as the mean (standard deviation) or number (%). The AD incidence rates were calculated by dividing the number of events by 1,000 person-years (PY). Cox proportional hazards regression analyses were performed to obtain hazard ratios (HRs) and 95% confidence intervals (CIs) of AD based on baseline BMI categories. The risk of AD was analyzed after adjusting for possible confounding factors. Model 1 was adjusted for age and sex, and Model 2 was additionally adjusted for lifestyle factors (smoking status, alcohol consumption, and regular exercise) and low-income status. Model 3 was further adjusted for a history of hypertension, diabetes, dyslipidemia, and CVD. Stratified analysis was performed by dividing the participants into subgroups according to baseline age group (65–74 or ≥ 75 years), sex, low-income status, current smoking, alcohol consumption, regular exercise, underlying hypertension, diabetes, dyslipidemia, and history of CVD to test interactions between subgroups. In addition, a sensitivity analysis was performed using multiple imputation to additionally deal with missing values based on the method of fully conditional specification. Statistical analyses were performed using the SAS Enterprise Guide (version 7.1; SAS Institute, Cary, NC, USA) and STATA software (MP, version 17.0; StataCorp, College Station, TX, USA), and statistical significance was defined as two-sided p < 0.05.
Results
Baseline characteristics of the study population
Our study enrolled 148,534 individuals aged 65 years or older, and 22,279 AD events were observed during the 10-year follow-up period. As shown in Table 1, the average age of the underweight population was the highest (mean, 73.8 years), and the proportion of men was higher (51.0%) in the whole population. Average blood pressure and fasting blood sugar and total cholesterol levels increased as BMI increased (P < 0.001). The underweight population included a higher rate of current smokers (27.9%) than other populations. The obese population had the lowest percentage of current alcohol drinkers (23.7%) among all the groups. The proportion of participants who regularly exercised was lower in the underweight population (21.6%) than in the other populations. The underweight population had fewer comorbidities, such as hypertension (46.4%), diabetes mellitus (17.0%), dyslipidemia (13.8%), and cardiovascular disease (2.6%), than the other populations.
Risk of incident AD according to baseline BMI category
Table 2 shows the HRs (95% CIs) of incident AD according to baseline BMI. During the 10-year follow-up period, after adjusting the results for age, sex, smoking status, alcohol consumption, regular exercise, low-income status, hypertension, diabetes, dyslipidemia, and CVD (model 3), the risk of AD significantly decreased as BMI increased (p for trend, < 0.001). When the normal-weight population was referenced, the risk of AD increased in the underweight population (adjusted HR in the fully adjusted model = 1.17, 95% CI = 1.09–1.24). The estimated adjusted HR for incident AD in the underweight population relative to the normal-weight population increased as the degree of thinness increased (adjusted HR = 1.13, 95% CI = 1.05–1.22 for mild thinness; adjusted HR = 1.25, 95% CI = 1.09–1.44 for moderate thinness; adjusted HR = 1.24, 95% CI = 1.02–1.51 for severe thinness; p for trend, < 0.001). In contrast, the estimated HR for incident AD was inversely associated with increased BMI (adjusted HR = 0.90, 95% CI = 0.87–0.93 for the overweight population and adjusted HR = 0.83, 95% CI = 0.80–0.85 for the obese population). The cumulative hazard for 10 years according to the BMI categories is shown in Fig. 2. Sensitivity analysis performed by multiple imputation on missing values showed almost the same results (adjusted HR in the fully adjusted model = 1.18, 95% CI = 1.11–1.25 for underweight; adjusted HR = 0.91, 95% CI = 0.88–0.94 for overweight; adjusted HR = 0.83, 95% CI = 0.81–0.86 for obesity).
Risk of incident AD according to baseline BMI category in subgroups categorized by age, sex, and underlying diabetes
The interaction between BMI category and diabetes status on the risk of AD was significant, and the risk of AD according to BMI in the diabetes-free subgroup showed a greater difference (p < 0.001, Fig. 3). No significant interactions between BMI category and age, sex, low-income status, current smoking, alcohol consumption, regular exercise, underlying hypertension, dyslipidemia, and history of CVD were observed in the occurrence of AD (only some data are presented in Table 3).
Discussion
In this large nationwide population-based cohort study, BMI was significantly associated with an increased risk of incident AD in an inverse linear relationship, and this trend was maintained even when the underweight population was subdivided. In comparison with normal-weight participants, those who were underweight, overweight, and obese showed 1.17-, 0.90-, and 0.83-fold risk of AD in the fully adjusted model, respectively.
Our study showed clear evidence for the association between lower late-life BMI and the increased risk of AD and has the advantage of extending these findings to the underweight population. Previous cohort studies did not confirm definitive results as to whether the risk of AD increased in the underweight group compared to the normal-weight group because the number of participants was limited14,26,27. In addition, among the previous studies that investigated risk factors for AD in Asians, few have been conducted on the basis of late-life BMI28. Since our study was a nationwide representative cohort study and included enough underweight individuals, our findings provide more robust evidence of the association between underweight status and AD incidence in the general population. In addition, an inverse linear relationship appeared when the risk of AD was measured by dividing the late-life BMI group using the Asian-Pacific classification24 which is different from the Western classification.
Previous studies have attempted to reveal the association between late-life BMI and Aβ and tau, which are known to play key roles in the pathogenesis of AD. A recent study showed that a higher late-life BMI was associated with higher levels of cerebrospinal fluid (CSF) Aβ42 and lower levels of t-tau and t-tau/Aβ4229, which have superior accuracy for the detection of AD and could indirectly indicate the extent of AD pathology in the brain30,31. In addition, a lower load of brain cortical Aβ and larger brain volumes of AD-vulnerable regions were associated with higher late-life BMI, and the longitudinal data suggested that individuals with higher late-life BMI had less cognitive decline29. Likewise, several studies that measured the association between the accumulation of brain cortical Aβ and BMI in the elderly with normal cognitive function repeatedly proved the association between high BMI and low Aβ burden32,33. Considering the association between late-life BMI and key molecules involved in the pathogenesis of AD, it can be inferred that a certain mechanism related to late-life BMI affects the risk of AD.
The fat-derived hormone leptin has been identified as a key biomarker to explain the association between late-life BMI and the risk of developing AD. Leptin is a hormone derived from adipocytes that is secreted in proportion to the amount of adipocytes in the body, with high circulating plasma levels in obesity and low circulating plasma levels in states of starvation34. Leptin reduces the production of Aβ from amyloid precursor proteins by inhibiting the γ-secretase complex10 and diminishes extracellular Aβ by driving Aβ uptake into cells11. Leptin inhibits the production of hyperphosphorylated tau that forms neurofibrillary tangles, which are the key pathological features of AD11,12. The correlation between plasma leptin levels and body fat is known to persist with age and appear in older adults35. In addition, previous observational studies also support the notion that leptin may be protective against dementia, since low plasma leptin levels in late-life are associated with worsening cognitive decline and increased dementia risk9,36. Collectively, these findings suggest that a low leptin level state due to low late-life BMI can reduce its neuroprotective effects, which would lead to a downward cascade of worsening AD pathology and further weight loss37. Our study showed that lower BMI was associated with and increased risk of AD, which may have contributed to decreased leptin levels and the corresponding deterioration of neuroprotective effects.
In our subgroup analyses, an interaction was observed in which the underlying diabetes status affected the risk of AD according to late-life BMI, with a greater difference in the risk of AD according to the BMI category in the non-diabetic subgroup. This suggests that the neuroprotective effect of leptin, which increases with increasing body weight, decreases in individuals with diabetes. One plausible explanation for this is that diabetes attenuates the neuroprotective effects of leptin by increasing leptin resistance38. Although the exact mechanism has not been elucidated, diabetes increases resistance to leptin, which is presumed to interfere with the action of leptin39. In fact, a previous study showed that the late-life obese group with metabolic syndrome, which is known to increase leptin resistance similar to diabetes, had an increased AD risk than a normal-weight group without metabolic syndrome40. Thus, the protective action to reduce the risk of AD by hyperleptinemia caused by high late-life BMI may be modulated by other factors. Further studies are required to elucidate this hypothesis.
Thus, our study is noteworthy in that it clearly presented the relationship between late-life BMI and the risk of AD in a nationwide population-based cohort. As the BMI category changed from obese to underweight, the risk of AD increased in a weight-dependent manner, and this trend was maintained even when underweight group was subdivided. These results suggest that improving public nutritional status to prevent weight loss in older adults may be a key strategy for reducing the incidence of AD. A previous study showed that weight loss in older adults was associated with the development of AD regardless of the basal BMI status, which supports this view41. Therefore, policy support to manage the weight of older adults through active nutritional interventions may be helpful in preventing the occurrence of AD. Further studies are needed to determine whether increasing the weight of the elderly reduces the incidence of AD.
Limitation
Our study had several limitations. First, the diagnosis of AD was made using ICD-10 codes and not individual clinical diagnoses. To overcome this, the time of AD onset was defined as the simultaneous occurrence of the ICD-10 codes for AD and anti-dementia drug prescriptions that must have the patient’s result of the MMSE and the CDR or GDS to claim reimbursement23. Second, the levels of biomarkers such as leptin or genetic factors such as APOE could not be measured because they were not included in the national health screening program. Third, although our study targeted a very large population group, the participation rate for the national health screening program in the early 2000s was less than half; therefore, we could not include more participants. Selection bias may have occurred because only those participating in the national health checkup program were included. Fourth, a recent study reported that sarcopenia is associated with AD occurrence. In this study, of the two components of sarcopenia, muscle function and lean muscle mass, the former was associated with incident AD, while the latter was not42. Since our study was conducted based on BMI, it did not accurately reflect the body fat mass or lean muscle mass of the elderly participants. Further investigation regarding the incidence of AD seems necessary, considering both BMI and components of sarcopenia in the late-life population. Fifth, the difference in AD risk according to late-life BMI may have been underestimated. In another study that followed 10-year mortality among Koreans aged 65 years or older, the lower the baseline BMI, the higher the mortality rate43 which implied a higher chance of dying before AD develops. Finally, our study could not prove a causal relationship between late-life BMI and the incidence of AD because many possible confounding factors affecting the incidence of AD that cannot be identified in this database were not considered. Nevertheless, this study has value as the largest population-based study analyzing late-life BMI and AD over a 10-year follow-up. It is also meaningful because it more accurately assesses the risk of AD in Asians, including sufficient underweight populations.
Conclusion
In conclusion, the severity of underweight as defined by late-life BMI was associated with a higher risk of AD incidence in a weight-dependent manner, even in an underweight population. Strategies to gain weight in later life can help pave the way for reducing the burden of AD.
Data availability
The data that support the findings of this study are available from National Health Information Database but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of National Health Insurance Sharing Service. (https://nhiss.nhis.or.kr).
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
Amyloid beta
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- CSF:
-
Cerebrospinal fluid
- CDR:
-
Clinical dementia rating
- CIs:
-
Confidence intervals
- GDS:
-
Global Deterioration Scale
- HRs:
-
Hazard ratios
- ICD-10:
-
International Classification of Diseases, 10th revision codes
- MMSE:
-
Mini Mental State Examination
- NHIS:
-
National Health Insurance Service
- NHID:
-
National Health Information Database
- NMDA:
-
N-Methyl-d-aspartate
- PY:
-
Person-years
References
Lane, C. A., Hardy, J. & Schott, J. M. Alzheimer’s disease. Eur. J. Neurol. 25, 59–70. https://doi.org/10.1111/ene.13439 (2018).
World Health Organization. Global action plan on the public health response to dementia 2017–2025 (2017).
Alzheimer's disease facts and figures. Alzheimers Dement 17, 327–406. https://doi.org/10.1002/alz.12328 (2021).
Nelson, P. T. et al. Alzheimer’s disease is not “brain aging”: Neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 121, 571–587. https://doi.org/10.1007/s00401-011-0826-y (2011).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. https://doi.org/10.1016/s0140-6736(20)30367-6 (2020).
Emmerzaal, T. L., Kiliaan, A. J. & Gustafson, D. R. 2003–2013: A decade of body mass index, Alzheimer’s disease, and dementia. J. Alzheimers Dis. 43, 739–755. https://doi.org/10.3233/jad-141086 (2015).
Qu, Y. et al. Association of body mass index with risk of cognitive impairment and dementia: A systematic review and meta-analysis of prospective studies. Neurosci. Biobehav. Rev. 115, 189–198. https://doi.org/10.1016/j.neubiorev.2020.05.012 (2020).
Yu, J. T. et al. Evidence-based prevention of Alzheimer’s disease: Systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J. Neurol. Neurosurg. Psychiatry 91, 1201–1209. https://doi.org/10.1136/jnnp-2019-321913 (2020).
Lieb, W. et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA 302, 2565–2572. https://doi.org/10.1001/jama.2009.1836 (2009).
Niedowicz, D. M. et al. Leptin regulates amyloid β production via the γ-secretase complex. Biochim Biophys. Acta 439–444, 2013. https://doi.org/10.1016/j.bbadis.2012.12.009 (1832).
Greco, S. J. et al. Leptin inhibits glycogen synthase kinase-3beta to prevent tau phosphorylation in neuronal cells. Neurosci. Lett. 455, 191–194. https://doi.org/10.1016/j.neulet.2009.03.066 (2009).
Zhang, Z., Guo, M., Zhang, J., Du, C. & Xing, Y. Leptin regulates Tau phosphorylation through Wnt Signaling pathway in PC12 cells. Neurosignals 24, 95–101. https://doi.org/10.1159/000442616 (2016).
Rahmani, J. et al. Body mass index and risk of Parkinson, Alzheimer, Dementia, and Dementia mortality: A systematic review and dose-response meta-analysis of cohort studies among 5 million participants. Nutr. Neurosci. https://doi.org/10.1080/1028415x.2020.1758888 (2020).
Fitzpatrick, A. L. et al. Midlife and late-life obesity and the risk of dementia: Cardiovascular health study. Arch. Neurol. 66, 336–342. https://doi.org/10.1001/archneurol.2008.582 (2009).
Luchsinger, J. A., Cheng, D., Tang, M. X., Schupf, N. & Mayeux, R. Central obesity in the elderly is related to late-onset Alzheimer disease. Alzheimer Dis. Assoc. Disord. 26, 101–105. https://doi.org/10.1097/WAD.0b013e318222f0d4 (2012).
Tolppanen, A. M. et al. Midlife and late-life body mass index and late-life dementia: Results from a prospective population-based cohort. J. Alzheimers Dis. 38, 201–209. https://doi.org/10.3233/jad-130698 (2014).
Seong, S. C. et al. Data resource profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 46, 799–800. https://doi.org/10.1093/ije/dyw253 (2017).
Seong, S. C. et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 7, e016640. https://doi.org/10.1136/bmjopen-2017-016640 (2017).
Kim, Y. I. et al. Cohort profile: National health insurance service-senior (NHIS-senior) cohort in Korea. BMJ Open 9, e024344–e024344. https://doi.org/10.1136/bmjopen-2018-024344 (2019).
Jeong, S. M. et al. Anemia is associated with incidence of dementia: A national health screening study in Korea involving 37,900 persons. Alzheimers Res. Ther. 9, 94. https://doi.org/10.1186/s13195-017-0322-2 (2017).
Choi, S. et al. Association of chronic periodontitis on Alzheimer’s disease or vascular dementia. J. Am. Geriatr. Soc. 67, 1234–1239. https://doi.org/10.1111/jgs.15828 (2019).
Nam, G. E. et al. BMI, weight change, and dementia risk in patients with new-onset type 2 diabetes: A nationwide cohort study. Diabetes Care 42, 1217–1224. https://doi.org/10.2337/dc18-1667 (2019).
Lee, H. J. et al. Post-market utilization patterns of Alzheimer’s disease treatments in South Korea. Eur. J. Clin. Pharmacol. 77, 921–929. https://doi.org/10.1007/s00228-020-03065-x (2021).
World Health Organization. The Asia-Pacific perspective: Redefining obesity and its treatment (2000).
World Health Organization. Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 854, 1–452 (1995).
Hughes, T. F., Borenstein, A. R., Schofield, E., Wu, Y. & Larson, E. B. Association between late-life body mass index and dementia: The Kame Project. Neurology 72, 1741–1746. https://doi.org/10.1212/WNL.0b013e3181a60a58 (2009).
Neergaard, J. S. et al. Late-life risk factors for all-cause dementia and differential dementia diagnoses in women: A prospective cohort study. Medicine (Baltimore) 95, e3112. https://doi.org/10.1097/md.0000000000003112 (2016).
Anstey, K. J., Ee, N., Eramudugolla, R., Jagger, C. & Peters, R. A systematic review of meta-analyses that evaluate risk factors for dementia to evaluate the quantity, quality, and global representativeness of evidence. J. Alzheimers Dis. 70, S165-s186. https://doi.org/10.3233/jad-190181 (2019).
Sun, Z. et al. Late-life obesity is a protective factor for prodromal Alzheimer’s disease: A longitudinal study. Aging (Albany, NY) 12, 2005–2017. https://doi.org/10.18632/aging.102738 (2020).
Vidoni, E. D., Townley, R. A., Honea, R. A. & Burns, J. M. Alzheimer disease biomarkers are associated with body mass index. Neurology 77, 1913–1920. https://doi.org/10.1212/WNL.0b013e318238eec1 (2011).
Mathys, J., Gholamrezaee, M., Henry, H., von Gunten, A. & Popp, J. Decreasing body mass index is associated with cerebrospinal fluid markers of Alzheimer’s pathology in MCI and mild dementia. Exp. Gerontol. 100, 45–53. https://doi.org/10.1016/j.exger.2017.10.013 (2017).
Thirunavu, V. et al. Higher Body Mass Index is associated with lower cortical amyloid-β burden in cognitively normal individuals in late-life. J. Alzheimers Dis. 69, 817–827. https://doi.org/10.3233/jad-190154 (2019).
Hsu, D. C. et al. Lower late-life body-mass index is associated with higher cortical amyloid burden in clinically normal elderly. J. Alzheimers Dis. 53, 1097–1105. https://doi.org/10.3233/jad-150987 (2016).
Rohner-Jeanrenaud, F. & Jeanrenaud, B. Obesity, leptin, and the brain. N. Engl. J. Med. 334, 324–325. https://doi.org/10.1056/nejm199602013340511 (1996).
Ruhl, C. E. et al. Body mass index and serum leptin concentration independently estimate percentage body fat in older adults. Am. J. Clin. Nutr. 85, 1121–1126. https://doi.org/10.1093/ajcn/85.4.1121 (2007).
Holden, K. F. et al. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol .Aging 30, 1483–1489. https://doi.org/10.1016/j.neurobiolaging.2007.11.024 (2009).
Ishii, M. & Iadecola, C. Adipocyte-derived factors in age-related dementia and their contribution to vascular and Alzheimer pathology. Biochim Biophys Acta 966–974, 2016. https://doi.org/10.1016/j.bbadis.2015.10.029 (1862).
Katsiki, N., Mikhailidis, D. P. & Banach, M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol. Sin. 39, 1176–1188. https://doi.org/10.1038/aps.2018.40 (2018).
Davis, C., Mudd, J. & Hawkins, M. Neuroprotective effects of leptin in the context of obesity and metabolic disorders. Neurobiol. Dis. 72(Pt A), 61–71. https://doi.org/10.1016/j.nbd.2014.04.012 (2014).
Lee, J. Y. et al. Risk of incident dementia according to metabolic health and obesity status in late life: A population-based cohort study. J. Clin. Endocrinol. Metab. 104, 2942–2952. https://doi.org/10.1210/jc.2018-01491 (2019).
Kang, S. Y. et al. Body mass index trajectories and the risk for Alzheimer’s disease among older adults. Sci. Rep. 11, 3087. https://doi.org/10.1038/s41598-021-82593-7 (2021).
Beeri, M. S., Leugrans, S. E., Delbono, O., Bennett, D. A. & Buchman, A. S. Sarcopenia is associated with incident Alzheimer’s dementia, mild cognitive impairment, and cognitive decline. J. Am. Geriatr. Soc. 69, 1826–1835. https://doi.org/10.1111/jgs.17206 (2021).
Kong, J. W., Park, T., Lee, D. R. & Lee, J. Trajectories of body mass index and their associations with mortality among older adults in Korea: Analysis of the Korean longitudinal study of aging. Ann. Geriatr. Med. Res. 24, 195–203. https://doi.org/10.4235/agmr.20.0030 (2020).
Funding
This study was supported by a VHS Medical Center Research Grant from the Republic of Korea (VHSMC21038).
Author information
Authors and Affiliations
Contributions
J.M.Y. and J.W.Y. provided overall leadership and guidance for the investigation. S.H.C. performed statistical analysis and data curation. All authors performed data analysis and interpretation. S.H.C. prepared the original manuscript and all authors were involved in revising the manuscript. All authors have reviewed and approved the final manuscript. J.M.Y and J.W.Y. had the final responsibility for the decision to submit for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cho, S.H., Jang, M., Ju, H. et al. Association of late-life body mass index with the risk of Alzheimer disease: a 10-year nationwide population-based cohort study. Sci Rep 12, 15298 (2022). https://doi.org/10.1038/s41598-022-19696-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19696-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.