Abstract
The seeds of Trachyspermum ammi were gathered at the ripening stage from different regions of Iran and grouped into 14 populations (P1-P14) accordingly. The essential oil (EO) extraction yielded in the 3.16–5% range. EOs were analyzed by gas chromatography-flame ionization detection (GC-FID) and gas chromatography-mass spectrometry (GC–MS) and 11 constituents were identified. Thymol (59.92–96.4%), p-cymene (0.55–21.15%), γ-terpinene (0.23–17.78%), and carvacrol (0.41–2.77%) were the major constituents. The highest contents of thymol and carvacrol were found in the Ghayen population (P2). Also, P2 and P8 (Estahban) had the highest value of total phenol (TPC) 43.2 mg gallic acid equivalent (GAE)/g DW, and total flavonoids (TFC) 8.03 mg quercetin equivalent (QE)/g DW, respectively. P1 (Kalat) had the highest total coumarin (TCC) value (0.26 mg coumarin equivalent CE/g DW). Based on EO constituents, principal component analysis (PCA) and cluster analysis classified populations into two chemotypes of thymol/p-cymene/γ-terpinene and thymol/carvacrol. The highest positive correlation coefficient was between α-terpinene and limonene (0.96), while the highest negative correlation was between thymol and p-cymene (–0.984). The antioxidant activities of extracts and EOs were evaluated by phosphomolybdenum (total antioxidant capacity; TAC), diphenylpicrylhydrazyl (DPPH IC50), and ferric ion reducing antioxidant power (FRAP) assays. Also, the antimicrobial activity of EOs was studied against Escherichia coli and Staphylococcus aureus. P8 with high thymol, EO content (%v/w), TFC, and antibacterial and antioxidant activities is recommended but further studies are needed to confirm the chemotype introduction.
Similar content being viewed by others
Introduction
Plants belonging to the Apiaceae family are mostly targeted for large-scale essential oil (EO) production with functional phytochemicals. Among them, ajwain (Trachyspermum ammi (L.) Sprague, Synonym: Carum copticum (L.) Benth. & Hook.f. ex Hiern) as an annual plant is growing in the east of India, Iran, Pakistan, and Egypt1,2. In Iran, the total vegetative herb, fruits (seeds), and roots of the plant are used as a flavoring agent and traditional medicine3. Ajwain has been confirmed that has antimicrobial, antioxidant, anticancer, anti-inflammatory, antitermitic, hypotensive, hypolipidemic, antihypertensive, antispasmodic, anti-lithiasis, diuretic, antitussive, nematicidal, antihelminthic, anti-filarial, and insecticidal activities3,4,5,6,7. Ajwain essential oil as a potent and natural antimicrobial agent through core–shell electrospun nanofibers structure has been introduced for accelerating infected wound healing8. Phytochemicals of ajwain are terpenes, phenolics, alkaloids, flavonoids, glycosides, phytosterols, ascorbic acid, chalcones, coumarins, tannins, steroids, and saponins9,10. With regards to a nutraceutical point of view, ajwain seeds are served as valuable constituents in the human diet. Trachyspermum ammi with aromatic seeds locally known as Zenian and Jajiq (Jajikh) in Iran. Several studies have reported the chemical composition of ajwain oil with different major constituents including thymol, γ-terpinene, and p-cymene11,12 carvone, limonene, and dillapiole13 and carvacrol and p-cymene14. However, no chemotype of EO has been reported in this plant, which contains a very high percentage of thymol (> 90%).
The essential oil composition and content in plants depend on several factors such as sexual, seasonal, ontogenetic, genetic variations, ecological, and environmental properties15,16. Essential oils (EOs) are used in perfumes, make-up, food preservers, and additives products17. Food and pharmaceutical products are mostly enriched with synthetic antioxidants such as butylated hydroxyanisole (BHA), Butylated hydroxytoluene (BHT), and propyl gallate (PG)18. However, applying these synthetic antioxidants might lead to serious effects on human health such as toxic and carcinogenic effects19. Natural antioxidants in comparison to synthetic ones have been preferred in terms of safety, tolerance, and non-toxicity20. Therefore in recent years EOs and extracts have been investigated to replace synthetic antioxidants21,22.
Recently phenolic monoterpenes like thymol or carvacrol with strong antioxidant and antibacterial properties are demanded23. Thymol, a phenolic monoterpenoid derivative of cymene and isomer of carvacrol is the major constituent in ajwain oil22. Thymol and carvacrol (whether chemical or natural) have been considered to be safe by the European Commission and US Food and Drug Administration (FDA) and are classified as flavoring agents; hence, they undergo regulatory requirements as additives for food preservation24. There is a little chemical difference between natural and chemical EO, such as changes in optical rotation and deuterium depleted and oxygen-18 (18O) enriched of natural thymol, which can only be determined by special methods like Isotope-ratio mass spectrometry (IRMS)25. Research has shown that the antifungal activities of synthetic thymol are lower than natural ones26. Natural thymol is mostly obtained from Thymus vulgaris L. and T. ammi27. The other Thymus L. species, Carum copticum, Oliveria decumbens Vent., Satureja thymbra L., Zataria multiflora Boiss., Majorana syriaca (L.) Raf., Origanum glandulosum Desf., and Lippia L. species were also reported as main sources of thymol with 10.4 to 81.1% in EO28.
With increasing thymol use in commercial formulations like mouthwashes, fungicides, pharmaceutical disinfectants, and insecticides, it has become a high-demanded natural antiseptic agent28. Also, antioxidant properties can cause the commercialization of natural thymol. Generally, natural disinfectants are obtained from herbs and spices, many of them are used in the human diet to enhance the flavor, color, and aroma of food29. In the last years, natural antimicrobials have been interested due to the increased consumer awareness of their quality and safety30.
The objectives of the present study are phytochemical screening, and evaluation of antioxidant and antibacterial properties of seeds of T. ammi populations in the following items; I) essential oil analysis to find chemotypes with high thymol and their classification; II) evaluation of antioxidant effects of the plant samples EOs and methanolic extracts using 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), and total antioxidant capacity (TAC) methods; III) evaluation of total phenol, flavonoids and coumarin contents of extract; and finally, IV) evaluation of the antimicrobial activity of EOs against Escherichia coli and Staphylococcus aureus.
Results and discussion
Essential oils yield and composition
Among the 14 seed sample populations collected, the content of EOs among populations ranged from 3.16 to 5% (v/w). The lowest and highest EO content was determined in Ghayen (P2) and Fars (P8) populations, respectively (Table 1). Similarly, the percentage of EO in ajwain samples has been reported from Pakistan 3.5–5.2%31, India 2–4%4,32, and Iran 2–6%5,33,34,35. EO yield may vary in plants depending on species, quality (chemotype of the plant), condition (fresh or dry), the layout of plant material (e.g., leaf/stem ratio), harvest time, and also extraction method15,16,36. The EO yield is an important quality factor to bring medicinal plants to the pharmaceutical, and food industries. Seed EO constituents of the 14 ajwain populations and chromatograms are shown in Table 1 and Fig. S1. In this study, eleven constituents were identified in all 14 populations, and thymol was the major constituent ranging from 59.92 to 96.4 percent (Fig. S2). Other major constituents were p-cymene (0.55–21.15%), γ-terpinene (0.23–17.78%), and carvacrol (0.41–2.77%) among populations studied. The highest content of thymol (96.4%) and its structural isomer carvacrol (2.77%) were found in the Ghayen population (P2). Additionally, the lowest thymol content was detected in the Isfahan population (P13) (59.92%). The highest (17.78%) and lowest (0.23%) γ-terpinene content was found in the Isfahan (P13) and Ghayen (P2) populations, respectively. The Birjand population (P3) displayed the highest p-cymene content (21.15%) and (P2) showed the lowest content (0.55%).
The GC–MS spectra obtained from the Hamedan population (P7) are represented in the graphical diagram in Fig. 1. According to our results, the Ghayen population (P2) has the highest levels of thymol and carvacrol and lowest levels of p-cymene and γ-terpinene. So, a higher rate of precursors (γ-terpinene and p-cymene) to final products (thymol/carvacrol) can be converted in isolated EO35. According to the biosynthetic pathway, γ-terpinene precursor converts to thymol and carvacrol during the developmental stages37.
In this context, EO compositions of ajwain have been reported from various geographical areas. According to the chemical composition of ajwain oils, major constituents of thymol, γ-terpinene, and p-cymene11,12,33,35 carvone, limonene, and dillapiole13 and carvacrol and p-cymene14 have been documented. Up to now, the high-thymol content populations from Iran were between 34 to 55%33 48.8 to 61.435, and 65.411. However, no chemotype of the plant EO has been reported with a very high percentage of thymol (> 90%). Thymol and carvacrol percentages of seed EO of 14 populations are shown in Fig. 2. As can be seen in this figure, populations P2 and P8 have the highest thymol content (more than 90% of EO). The presence of a high percentage of thymol in the P8 and P2 can be industrially valuable. Chemotypes are named based on the main constituents in EO within single botanical species38. Normally ajwain oils on the market are those rich in thymol and/or carvacrol with strong antibacterial properties and high antioxidant potential. High purity thymol is interested in the market and will not have the subsequent purification costs. Therefore, chemotypes P2 and P8 with a high percentage of thymol 96.4. 90.57% can be significant respectively.
Estimation of phyto-constituents of extract
Significant differences were obtained among the population for total phenolic (TPC), total flavonoid (TFC), and total coumarin contents (TCC) (P ≤ 0.01) (Table 2). Natural phenolic compounds are including simple phenolics, phenolic acids, flavonoids, coumarins, tannins, stilbenes, curcuminoids, lignans, quinones, and others39. Phenolic compounds and flavonoids are major bioactive components in medicinal plants and thus can comprise an essential part of the human diet40. The present study assessed the total phenolic, flavonoid, and coumarin contents of ajwain populations, and the results are presented in Fig. 3A–C. Up to now, no studies have reported total phenol, flavonoid, and coumarin contents of Iranian ajwain populations.
Total phenol content (TPC)
The total phenolic content in the evaluated extracts varied from 26.91 (P13) in the Isfahan population to 43.20 (P2) mg GAE/g DW in the Ghayen population, Results demonstrated that TPC in the populations varied as the following the order P2 > P10 > P8 > P1 > P11 > P14 > P6, P9 > P3, P5 > P4 > P7 > P12 > P13 (Fig. 3A). In the few evaluable sources, the total phenolic content of ajwain seeds extracted with CHCl3: MeOH (1: 2) solvent was 69 mg/g DW41. In the present study, the highest phenol content (43.2 mg GAE/g DW) was recorded in the P2 population. The difference in TPC with the available report may be due to genetic diversity and differences in extraction methods. According to the presence of apolar thymol in the seed structure, a combination of polar and non-polar solvents to extract compounds may optimize the extraction performance. Various environmental conditions in different places influence the content and metabolic profile of phenolic compounds in plant populations. It seems that high temperature and high UV radiation levels, and differences in genotypes are the reasons why the Isfahan population has a high content of TPC15,16.
Total flavonoid content (TFC)
Analysis of variance showed a significant difference in TFC content at levels P ≤ 0.01. The total flavonoid contents ranged from 4.45 (P7) in the Hamedan population to 8.03 (P8) mg QE/g DW in the Fars population. P6 and P10 with 7.38 mg QE/g DW were also among the high content TFC populations (Fig. 3B). It seems that the reason for the lack of total flavonoids in Hamedan is due genetic differences and the low temperature of this region compared to other regions. Also, the reason for the high level of flavonoids in the Fars population may be due to genetic differences and high temperatures during the growing period. It has been reported that seeds and spurts of ajwain contain 0.58 and 1.15 mg/ g FW of TFC respectively42. Also, TFC of methanolic extract of Anethum graveolens L. (dill) seeds from the Apiaceae family have been reported to be 5.07 (mg QE /g)43. Flavonoid accumulation with many protective roles may be influenced by the combination of genetics (i.e., adaptation to local conditions) and environmental effects (i.e., phenotypic plasticity)44,45. Flavonoid accumulation rates among geographically different ajwain populations concerning climate can be correlated positively with temperature and UV-B radiation and negatively with precipitation (Chalker-Scott, 1999; Koski and Ashman, 2015).
Total coumarin content (TCC)
The TCC content of the T. ammi populations examined ranges from 0.079 (P12) to 0.26 (P1) mg coumarin equivalent to dry weight. The highest coumarin content was obtained from the methanolic extract of Kalat (P1) (0.260 mg CE/g DW) and the lowest value of coumarin was recorded for the population of Ardabil (Fig. 3C). Seed coumarin levels in populations can result from genetic and environmental differences. It seems that coumarin accumulation is decreased due to the coolness condition in Ardabil city during the seed maturation stage. Ajwain is a coumarin-rich source of coumarins (umbelliferone, scopoletin, xanthotoxin, bergapten) mostly found in its sprouts46. However, no literature source was found to report the amount of total coumarin in ajwain seeds. These compounds have valuable medicinal properties, including edema reduction and possible anticancer activity47 Furthermore, they are widely used as a flavoring in foods and pastries. Human exposure to coumarin from the diet has been calculated to be around 0.02 mg/kg/day and its maximum daily intake was estimated to be 0.07 mg/kg BW/day48.
Free radical scavenging effects and antioxidant activity of essential oils and extracts
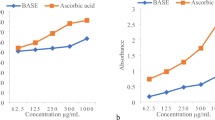
The antioxidant activities of EOs and extracts were assessed using the DPPH, FRAP free-radical scavenging, and total antioxidant capacity (TAC) assays (Fig. 4A–C).
Antioxidant activities of methanolic extracts and essential oils obtained from Trachyspermum ammi seed populations and seven antioxidant standards (A); Antioxidant activity (DPPH) IC50 (µg/ml) (B); antioxidant activity (FRAP) quantified by µmol Fe+2/g DW (C); total antioxidant capacity (TAC) quantified by mg Ascorbic acid equivalent (AAE).
In the DPPH assay, the samples were capable to decrease the DPPH free radical to evaluate their in vitro antioxidant activity. Analysis of variance on DPPH IC50 showed a significant difference in antioxidant activity of EOs and extracts among populations (P < 0.01) (Table 2). The value of DPPH (IC 50) in the essential oil varied between (1.57–2.61 µg/ml). The highest rate was related to P13 and the lowest rate was related to P2 samples. Also, the DPPH IC50 in the extract was recorded in the range of 8.06 to 31.95 respectively in P2 and P7. The antioxidant effect of Ajwain EO compared to ascorbic acid has been previously reported. According to this source, an amount of 10 µg of essential oil compared to the same concentration of vitamin C had a DPPH free radical scavenging effect of 76.4 to 97.2%49. DPPH radical scavenging activities of the methanolic extract of seeds of T. ammi in the range of 30 to 240 µg/ ml have been reported to be 65–80%. While with the same concentrations ascorbic scavenging activities of DPPH were in the range of 90 to 100%50. In the comparison of IC50 of EOs and extracts with common antioxidants; ascorbic acid (AA), BHT, PG and rutin (RU), quercetin (QU), gallic acid (GA), and thymol (TH) used in the present study, the following result was obtained from the lowest to the highest. GA < EO (P2) < Qu, PG, EO (P8) < EOs (P9 < P3 < P1 < P10, P12 < P7 < P6 < P4, P11, P14 < P13) < AA < TH < RU < BHT < EXs (P2 < P8 < P10 < P11 < P6 < P3, P5 < P1 < P13 < P9, P14 < P4 < P12 < P7) (Fig. 4A). In the present study, several antioxidants were used to better comparison with essential oils and extracts. BHT, PG, TH, and GA were used as common synthetic antioxidants. The reason for using synthetic thymol was due to comparing it with high thymol content natural studied EO and extracts. Since the plant extract contained flavonoids and phenolic acids, it was tried to use natural phenolic and flavonoid antioxidants for comparison. Ascorbic acid was used in the present study because it is a known and applicable antioxidant. Previously the antioxidant activity of some extracts from the Apiaceae family has been reported. According to these reports, IC50 of Heracleum persicum Desf., Prangos ferulacea (L.) Lindl, Chaerophyllum macropodum Boiss., Oliveria decumbens extracts were 438, 242, 623, 98.5, and 86.1 (µg/ml), respectively37,51. Also based on obtained results, ajwain seed with notable essential oil and extract can be introduced as the new promising antioxidant source from the Apiaceae family.
In the present study, FRAP was used as another method to evaluate antioxidant activity. Based on the results of the analysis of variance, a significant difference was obtained among the population’s EOs and extracts (Table 2). In the EO samples, the highest reducing power was obtained in population 2 (P2), (10.31 mM Fe+2) and the lowest was obtained in P13 (6.23 mM Fe+2) (Fig. 4B). The reducing power of the extracts was obtained in the range of 1.96 to 3.68 mM Fe+2, in which the lowest was related to P13 and the highest to P2. Also, the ferric reducing power of the samples of essential oils, extracts, and standards used in this study were as follows. GA > PG > QU, AA > BHT > TH > EO [P2 > P8) > RU > P5 > P1 > P12 > P14 ≥ P4 ≥ P3 > P9 > P6 > P7 > P13] > EX [P2 > P10 > P8 > P1, P6, P11 > P9 ≥ P14, P3 ≥ P5 > P12 > P4 > P13]. The mechanism by which extracts and EO reduce the [Fe (TPTZ)2]3+ complex to the ferrous state (Fe2+) usually involves the donation of electrons in the form of hydrogen ions and has been related to the in vitro antioxidant activity52.
The phosphomolybdenum assay is a quantitative method to evaluate the total antioxidant capacity. Significant differences were obtained in the EO samples as well as the extract samples among the populations (P < 0.01) (Table 2). The values ranged from 8.30 (P9) to 16.61 (P2) (mM AAE/mg EO) in EO samples and from 1.84 (P13) to 4.59 (P11) (µM AAE/g DW) in samples of extracts (Fig. 4C). Results demonstrated that ajwain seeds had notable total antioxidant capacity. The TAC value among antioxidant standards ranged from 11.4 to 34.08 in the following order: AA > GA > PG > TH > QU > BHT > RU. Also, this value ranged from 8.3 to 16.6 among EO samples with the highest value in P2. TCA values in extracts were recorded in the range of 1.83–4.59 with the highest value obtained in P11. Other detailed information is shown in Fig. 4C.
Antibacterial activity
The antibacterial activity of ajwain EOs was evaluated against two antibiotic resistance bacteria and their ability was compared with Cefixime as a standard. In the present study, we tried to use both gram-positive bacteria and gram-negative bacteria as samples. Staphylococcus aureus is a gram-positive pathogenic and antibiotic-resistant bacteria. It is also one of the most common causes of nosocomial infections. Also, Escherichia coli is available and inexpensive, and easily cultured in the laboratory. It is one of the most common causes of urinary tract infections. Gram-negative bacteria are also resistant to antibiotics and are an important species in the field of microbiology. One of the main problems in the field of microbiology is the resistance of microbes to antibiotics and so introducing new antibiotics is necessary53. The reasons for using Cefixime in the present study are due to its widely used, great therapeutic power, and effectiveness against a wide range of microbes.
In this study, EOs exhibited bacteriostatic activities against S. aureus (0.06–64 µg/mL) and E. coli (1–64 µg/mL) (Table 3). High thymol content EO (P2) showed high antibacterial activity (MIC = 0.06 µg/mL) against S. aureus. Also, the EO from the Isfahan population (P13) showed the lowest antibacterial activity with the highest MIC value (64 µg/mL). In the present study, the mean MIC was not significantly different on gram-negative and positive bacteria, and populations with high thymol had a high antibacterial ability, indicating the antibacterial effects of thymol. Some researchers have evaluated the antimicrobial activity of ajwain oil14,54,55. Thymol and carvacrol were found to be more effective in killing bacteria3,4,5,6,7,9. The antibacterial properties of natural products, such as essential oils and their components, are widely explored by both industrial and academic fields56. The antibacterial activity of the EOs is dependent on the composition and concentration, type, and dose of the target microorganism57. The high antibacterial potential of cumin essential oil compared to Ferula essential oil has already been identified due to the high ratio of phenolic monoterpene compounds to other monoterpenes58. It seems that the antibacterial effects of C. copticum are also mainly due to the presence of phenolic monoterpenes such as thymol, carvacrol, p-cymene, and γ-terpinene. Therefore, ajwain EO can be used as a natural agent with antibacterial properties in the food industry and the treatment of infectious diseases, especially antibiotic-resistant strains.
Hierarchical cluster analysis (HCA) of essential oil constituents
HCA was performed by using the 11 identified compounds and 14 populations (Fig. 5A). All used populations were divided into two clusters; Cluster I included P4, P6, P7, P10, P11, P12, P13, and P14 and cluster II consist of P1, P2, P5, P8, and P9 samples. In cluster I the major constituents were thymol (59.92–72.86), p-cymene (15.66–21.15), and γ-terpinene (10.22–17.78). In the second cluster thymol (80.09–96.4) and carvacrol (0.5–2.77) were the major constituents. Cluster analysis can classify studied populations into several groups, according to the chemical composition by ‘magnifying’ their similarities59. Forasmuch as, plant sources from environmentally different origins led to the emergence of new chemotypes to baring domestication and cultivation to obtain uniform chemical plants along with appropriate agricultural features60.
(A) Heat-map diagram of two-way hierarchical cluster analysis (HCA) of fourteen Trachyspermum ammi populations based on 11 essential oil constituents quantified by GC and GC–MS. Blue color with a great positive share and red color with a great negative share affects cluster formation. (B) Principal component analysis (PCA) based on EO constituents. (C) PCA is based on all studied traits. (D) PCA is based on all studied traits according to populations.
Principal component analysis (PCA)
Principal component analysis (PCA) is one of the multivariate statistical techniques used to explain differentiation between populations and to obtain more information on the variables that mainly influence the population's similarities and differences61. The PCA was performed to identify the most significant variables in the data set (Fig. 5B). The same data set (14 population × 11 components) was used in this section. The PCA showed two components with explain 83.3% of the total variance. The first principal component (PC1) had the most portion of variance (74.5%) which was given by compounds such as γ-Terpinene, α-pinene, α-Thujene, p-cymene, and limonene. The second component (PC2), explaining 8.8% of the total variance, consisted of compounds thymol, carvacrol, and 1, 8-cineol (Fig. 6). The results of PCA agreed with those of the cluster analysis the populations similarly were divided into two distinct groups including high thymol/carvacrol and high thymol/p-cymene/γ-terpinene groups (Fig. 5B). Heat map analyses were drowned to determine how constituents effect on clustering. Based on heat map analysis samples were well-classified.
Correlation between 24 traits on the studied Trachyspermum ammi populations: TPC: Total phenolic content, TFC: Total flavonoid content, TCC: Total coumarin, EO: Essential Oil yield, TSW: One thousand seed weight (g), MIC: minimum inhibitory concentration, Ec: E. coli, MIC: minimum inhibitory concentration, Sa: S. aureus, DPPH Ext.: DPPH assay Extract is expressed as IC50 index, DPPH EO: DPPH assay EO is expressed as IC50 index, FRAP Ext.: FRAP assay Extract, FRAP EO: FRAP assay Essential oil, TAC Ext: The total antioxidant capacity Extract, TAC EO: The total antioxidant capacity Essential oil.
Also, in the analysis of the principal factors (PCA) between all the evaluated traits in the populations, the first principal factor (PC1) showed 53.8% and the second principal factor (PC2) 14.7% of the variance. This analysis determined the principal component, correlation of traits, and their relationship with populations. Accordingly, traits with positive arrows show a positive correlation and two traits with non-directional arrows show a negative correlation. Accordingly, thymol and carvacrol have a high correlation with antioxidant properties and this property is correlated with populations of chemotype 1 (P1, P2, P5, P8, P9). Other relationships and details correlations are shown in Fig. 5C, D.
Correlation
Simple correlation estimated the relationship between variables. Simple correlations between 24 studied traits in the present study are shown in Fig. 6. Thymol as the major constituent of EOs showed a high positive correlation with TPC (0.71), carvacrol (0.64), FRAP EO (0.85), and FRAP ext. (0.66). Thymol also had a significant negative correlation with Mic EO (-0.74), Mic Sa (-0.69), α-Thujene (-0.84), α-Pinene (-0.77), β-Pinene (-0.75), β-Myrcene (-0.9), α-Terpinene (-0.85), p-Cymene (-0.98), Limonene (-0.89), γ-Terpinene (-0.97). TPC had a positive correlation with TFC, thymol, carvacrol, FRAP Ext., TAC Ext., and a significant negative correlation with DPPH Ext. The antioxidant methods in extracts DPPH50 vs FRAP (-0.8), DPPH50 vs TAC (-0.67) and FRAP vs TAC (0.59) were highly correlated. Similarly, in estimating the antioxidant activity of essential oil DPPH50 vs FRAP (-0.79), DPPH50 vs TAC (-0.48), and FRAP vs TAC Ext (0.55) were highly correlated. Also, the high correlation of all antioxidant methods with thymol can explain its positive effect on the antioxidant activity of the extracts and EOs. The correlations found between each of the traits can be very important in breeding programs.
Conclusion
Ajwain (T. ammi) is one of the aromatic seed spices and a rich source of specialized metabolites such as thymol. Considering that the plant is one of the main and natural sources of thymol. As natural native Iranian populations of the plant can be the primary sources for breeding and domestication of valuable thymol chemotypes, therefore the present study was conducted to determine high thymol content chemotypes. Based on the results, P2 and P8 populations with a thymol content above 90% are introduced for this aim. Fars population (P8) with high thymol, EO percent, and total flavonoid also high antibacterial and antioxidant activity is recommended for nutraceutical and pharmacological uses. Fourteen populations were classified into two chemotypes of thymol/p-cymene/γ-terpinene type and thymol/carvacrol type. A comparison of the antioxidant effects of EOs with seven synthetic and natural antioxidants used in the present study showed that the plant EOs have stronger antioxidants than these antioxidants. Therefore, EOs of the plant can be used in various industries after supplementary studies. Also, plant extracts are introduced as an antioxidant source for industrial use due to the presence of phenolic and flavonoid compounds. The antibacterial properties of EOs against two bacteria Gram-positive (S. aureus ATCC: 1431) and Gram-negative (E. coli PTCC: 1399) and their correlation with thymol indicate the importance of high thymol selected chemotypes.
Methods
Collection of plant materials
The studied plant materials (seeds of 14T. ammi populations) were collected from different regions of Iran. Ecological information, local names and usage, and the weight of one thousand seeds of populations are presented in Table 4. The plant was identified at the herbarium of Medicinal Plants and Drugs Research Institute (MPH) of Shahid Beheshti University, Tehran, Iran, and a voucher specimen (no. MPH-2938) were deposited at MPH (Fig. S3). Since the plant is not an endangered plant, the collection of samples with the permission of the university was done only for academic study by observing the necessary guidelines for collecting plants (IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora). Also, except for a few limited herbarium specimens, only a limited percentage of seeds were collected from each population. Figure 1 represented the graphical design of the present research.
Extraction of the essential oils (EOs)
To extract EOs, 50 g of each seed source was extracted by a Clevenger-type apparatus for three hours. The EO was then collected in a glass container and kept at 4 °C in the dark condition until analyzed and characterized.
GC–MS and GC analysis
The obtained EOs was analyzed by gas chromatography-flame ionization detector (GC-FID) and GC-mass spectrometry (GC–MS). The analysis was carried out using a DB-5 fused silica capillary column (length 30 m; inner diameter 0.25 mm; film thickness 0.25 μm). The injector and detector temperatures were kept at 250 and 280 °C, respectively. Helium was used as the carrier gas at a flow rate of 1.1 mL/min; the oven temperature was programmed from 60 to 250 at 5 °C/min and held for 40 min. The injection volume was 1.0 µL using a 1:10 split ratio. GC–MS analysis was carried out with a Thermoquest–Finnigan gas chromatograph equipped with DB-5 fused silica capillary column (length 60 m; inner diameter 0.25 mm; film thickness 0.25 μm) coupled with a TRACE mass spectrometer (Manchester, UK). Helium was used as the carrier gas with a flow rate of 1.1 mL/min. The MS fragmentation was performed by electronic impact (EI) at 70 eV with a scan time of 0.4 s and a mass range was 40–460 amu. The ion source and interface temperatures were 200° and 250 °C, respectively. The oven temperature was the same as above for the GC. The compounds were identified by comparison of their mass spectra with those of the internal reference mass spectra library (Adams and Wiley 7.0) and confirmed by comparison of their retention indices with authentic compounds or with those reported in the literature62,63. Retention indices were calculated using the retention times of n-alkanes (C6–C24).
Extracts preparation
500 mg of seeds were powdered and transferred to test tubes. Twenty mL of methanol (80%) was added to each sample and stirred slightly. Tubes were sonicated for 30 min (Elmasonic EASY 120 H, Germany). The samples were filtered through Whatman filter paper no. 1 and preserved in dark condition at 4 ˚C before assays.
Total phenolic content (TPC)
The TPC was estimated by the colorimetric Folin-Ciocalteu method64. 200 ml of the extracts were mixed with 1200 µL of Folin-Ciocalteu reagent (10%), 180µL of H2O, and 960µL sodium carbonate 7%; then this mixture was shaken and incubated at room temperature for 30 min in the darkness. The samples resulting blue color and their absorbance were determined at 765 nm using UV–Vis spectrophotometer (Dynamica HALO DB-20, UK). The TPC was expressed as mg GAE/g dry weight.
Determination of total flavonoid content (TFC)
The TFC was determined using aluminum chloride colorimetric assay65. The extraction solution was combined with 150 μL of 5% sodium nitrite solution after 5 min remaining, 300 μL of aluminum chloride solution (10% w/v), and 1 mL of NaOH (1 M) were added. After incubating the samples for 10 min, the mixture turned pink and the absorbance was recorded at 380 nm by spectrophotometer (Dynamica HALO DB-20, UK). The TFC was calculated as mg of quercetin equivalent (QE) per g of dry weight (DW).
Determination of total coumarin content (TCC)
The TCC was evaluated based on the Borntrager reaction66. 500 μL of the extracts were transferred to a test tube. Afterward, 2 ml of distilled water and 500 μL of lead acetate solution (5%, w/v) were added. The sample is shaken and then 7 ml of distilled water is added. Then 2 mL of prepared mixtures were transferred to a new test tube and add 8 mL of HCl solution (0.1 M, v/v). The samples remain at room temperature for 30 min and then were read at 320 nm by spectrophotometer and the TCC is expressed as milligrams of coumarin equivalents (mg CE/g DW).
Antioxidant activity assays
The antioxidant activity of the EOs and extracts of seeds was determined by using three assays of DPPH, FRAP, and TAC. The results were compared with standards including BHT (butylated hydroxyl toluene) and PG (Propyl gallate), thymol, gallic acid, ascorbic acid, rutin, and quercetin.
DPPH radical scavenging assay
The 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay was performed by the method with some modification67. DPPH scavenging activity of EOs, extracts and control antioxidants were measured. For this means DPPH (100 µM) was dissolved in methanol to prepare the fresh stock solution. The DPPH solution (2 ml) was added to the test compounds and was shaken and incubated in darkness for 30 min at room temperature. The absorbance was monitored at 517 nm against a blank using a UV–Vis spectrophotometer. The inhibition percentage of the DPPH free radical (I %) was calculated as follows:
Abscontrol, absorbance of DPPH radical; Abs sample is the absorbance of the (DPPH radical + test samples).
I% was plotted against sample concentrations to obtain the IC50 index, which was defined as the concentration of antioxidant required to decrease the initial DPPH concentration by 50% DPPH (Brand-Williams et al., 1995).
Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was determined using the reducing power technique68. The samples with antioxidant properties will reduce the ferric ion (Fe3+) to the ferrous ion (Fe2+) in an acidic medium (pH = 3.6), to form an intense blue complex (Fe2+/TPTZ). The FRAP reagent was prepared by mixing acetate buffer (300 mM, pH 3.6), a solution of 10 mM TPTZ (Tripyridyl-s-triazine) in 40 mM HCl, and 20 mM FeCl3 at a ratio of 10:1:1 (v/v/v). The FRAP reagent (2 mL) was added to test tubes and mixed thoroughly and were incubated at 37 °C in the dark for 30 min. The absorbance of the samples was taken at 593 nm in comparison to a blank. The standard curve was prepared using different concentrations of ferrous sulfate. The results were expressed in µM Fe2+/g DW.
Phosphomolybdenum assay
TAC was evaluated using phosphomolybdenum assay69. The method is based on the reduction of Mo (VI) to Mo (V) with the subsequent formation of a phosphate–Mo5+ complex. 1 ml of 0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate was added to 20 ml of distilled water and made up the volume to 50 ml adding distilled water. 50 µL of (EOs, extracts, and standards) were added to each test tube individually containing 1 ml of Molybdate reagent solution. These capped tubes were kept incubated at 95 ˚C for 90 min. The tubes were cooled at room temperature and then their absorbance was measured at 695 nm using a UV–visible spectrophotometer against blank. The antioxidant capacity was expressed as equivalents of ascorbic acid (mg AAE/g extract).
Antibacterial activity
The antibacterial potential of EOs was tested against two bacteria Gram-positive (Staphylococcus aureus ATCC: 1431) and Gram-negative (Escherichia coli PTCC: 1399). Minimum inhibitory concentration (MIC) was conducted according to the standard broth microdilution technique following the guideline of the Clinical Laboratory Standard Institute (CLSI)70 in 96 well microplates. Microdilution series of EOs were prepared in Mueller–Hinton Broth (from 0.128–06 µg/ml with a final volume of 100 µl). A suspension of fresh culture medium (18–20 h) was prepared in normal saline and turbidity was adjusted to a 0.5 McFarland tube. The suspension was diluted 1:100 with Mueller–Hinton Broth and then 100 μl of it was added to an individual well. Thus assay is performed by applying a bacterial inoculum of 0.5–1 × 105 CFU/ml to each well. With the addition of bacterial suspension, the final concentration of the test substance in each well was halved. After incubation at 37 °C for 24 h, the wells were investigated for turbidity and MIC was determined and recorded in µg/ml. Samples of EOs assessed in a concentration range of 0.03 to 64 µg/ml. Because of turbidity in some samples, the five μl (4 mg/ml) of 0.2 µm filter-assisted sterilized resazurin solution was used to distinguish how wells grow. Culture medium with bacteria suspension as positive control and culture medium as negative control were used. MIC was determined as soon as the color of the positive control well changed. The experiment was performed twice with three replications and cefixime was evaluated as a standard.
Statistical analysis
We confirmed that all methods were performed in accordance with the relevant guidelines and regulations. All experiments have been carried out in triplicate and are expressed as the mean ± standard deviation (SD). Analysis of variance (ANOVA) was applied as a statistical analysis of phytochemical data. Data analyses were carried out using SAS Version 9.4 statistical software. The comparison of the means was performed by SNK test at a 1% level. Principle component analysis (PCA), cluster analysis, Heat-map, and correlation analysis were obtained using RStudio (version 1.2.5019) URL http://www.rstudio.com/.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Dalkani, M., Hassani, A. & Darvishzadeh, R. Determination of the genetic variation in Ajowan (Carum Copticum L.) populations using multivariate statistical techniques. Rev. Cienc. Agron. 43, 698–705 (2012).
Tomar, P. & Malik, C. Botany, cultivation, chemical constituents and genetic diversity in Trachyspermum ammi. L.: An aromatic seed spice (2014).
Kazemi Oskuee, R., Behravan, J. & Ramezani, M. Chemical composition, antimicrobial activity and antiviral activity of essential oil of Carum copticum from Iran. Avicenna J. Phytomed. 1, 83–90 (2011).
Chauhan, B., Kumar, G. & Ali, M. A review on phytochemical constituents and activities of Trachyspermum ammi (L.) Sprague fruits. Am. J. PharmTech Res. 2, 329–340 (2012).
Zarshenas, M. M., Moein, M., Samani, S. M. & Petramfar, P. An overview on ajwain (Trachyspermum ammi) pharmacological effects; modern and traditional. J. Nat. Remedies 14, 98–105 (2013).
Shahrajabian, M. H., Sun, W. & Cheng, Q. Pharmaceutical Benefits and Multidimensional uses of Ajwain (Trachyspermum ammi L.). Pharmacogn. Commun. 11, 2 (2021).
Bashyal, S. & Guha, A. Evaluation of Trachyspermum ammi seeds for antimicrobial activity and phytochemical analysis. Evaluation 11, 2455–3891 (2018).
Zare, M. R. et al. Antimicrobial core–shell electrospun nanofibers containing Ajwain essential oil for accelerating infected wound healing. Int. J. Pharm. 603, 120698 (2021).
Bairwa, R., Sodha, R. & Rajawat, B. Trachyspermum ammi. Pharmacogn. Rev. 6, 56–60 (2012).
Mostafavi, H. & Pezhhanfar, S. Qualitative phytochemical analysis of ajwain (Trachyspermum ammi) from north-west Iran. Int. Res. J. Pharm. 6, 610–615 (2015).
Shojaaddini, M., Moharramipour, S. & Sahaf, B. Fumigant toxicity of essential oil from Carum copticum against Indian meal moth, Plodia interpunctella. J. Plant Prot. Res. 48, 411–419 (2008).
Moazeni, M., Saharkhiz, M. J. & Hosseini, A. A. In vitro lethal effect of ajowan (Trachyspermum ammi L.) essential oil on hydatid cyst protoscoleces. Vet. Parasitol. 187, 203–208 (2012).
Choudhury, S., Ahmed, R., Kanjilal, P. B. & Leclercq, P. A. Composition of the seed oil of Trachyspermum ammi (L.) Sprague from Northeast India. J. Essent. Oil Res. 10, 588–590 (1998).
Srivastava, M., Baby, P. & Saxena, A. GC-MS investigation and antimicrobial activity of the essential oil of Carum copticum Benth & Hook. Acta Aliment. Hung. 28, 291–295 (1999).
Kachur, K. & Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. 60, 3042–3053 (2020).
Fattahi, B., Nazeri, V., Kalantari, S., Bonfill, M. & Fattahi, M. Essential oil variation in wild-growing populations of Salvia reuterana Boiss. collected from Iran: Using GC–MS and multivariate analysis. Ind. Crop Prod. 81, 180–190 (2016).
Marrelli, M., Amodeo, V., Perri, M. R., Conforti, F. & Statti, G. Essential oils and bioactive components against arthritis: A novel perspective on their therapeutic potential. Plants 9, 1252 (2020).
Gutiérrez-del-Río, I. et al. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants 10, 1–33 (2021).
Barlow, S. & Schlatter, J. Risk assessment of carcinogens in food. Toxicol. Appl. Pharm. 243, 180–190 (2010).
Mukhopadhyay, A. K. Antioxidants-Natural and Synthetic (Amani Int’l Publishers, 2006).
Amorati, R., Foti, M. C. & Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 61, 10835–10847 (2013).
Tohidi, B., Rahimmalek, M. & Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 220, 153–161 (2017).
Rathod, N. B., Kulawik, P., Ozogul, F., Regenstein, J. M. & Ozogul, Y. Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trends Food Sci. Tech. 116, 733–748 (2021).
Anwar, S., Ahmed, N., Habibatni, S. & Abusamra, Y. in Essential Oils in Food Preservation, Flavor and Safety 181–192 (Elsevier, 2016).
Boren, K., Young, D., Woolley, C., Smith, B. & Carlson, R. Detecting essential oil adulteration. J. Environ. Anal. Chem 2, 1–3 (2015).
Chen, C.-P. & Shen, A.-Y. Synergistic antifungal activities of thymol analogues with propolis. Nat. Prod. Commun. 3, 279–282 (2008).
Ukoroije, B. R., Island, W., Island, W. & Island, W. The efficacy of Ocimum gratissimum leaf powder and ethanol extract on adult Periplanata americana under laboratory condition. Open Access Libr. J. 5, 1–17 (2018).
Escobar, A., Perez, M., Romanelli, G. & Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 13, 9243–9269 (2020).
Yashin, A., Yashin, Y., Xia, X. & Nemzer, B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants 6, 1–18 (2017).
Batiha, G. E.-S. et al. Application of natural antimicrobials in food preservation: Recent views. Food Control, 108066 (2021).
Ashraf, M. & Bhatty, M. Studies on essential oils of the Pakistan species of Trachyspermum ammi, ajwain seed oil. Pak. J. Sci. Ind. Res. 18, 232–235 (1975).
Malhotra, S. in Handbook of herbs and spices 118–137 (Elsevier, 2012).
Mirzahosseini, S. M., Noori, S. A. S., Amanzadeh, Y., Javid, M. G. & Howyzeh, M. S. Phytochemical assessment of some native ajowan (Therachyspermum ammi L.) ecotypes in Iran. Ind. Crop Prod. 105, 142–147 (2017).
Niazian, M., Sadat Noori, S. A., Tohidfar, M. & Mortazavian, S. M. M. Essential oil yield and agro-morphological traits in some Iranian ecotypes of ajowan (Carum copticum L.). J. Essent. Oil-Bear. Plants 20, 1151–1156 (2017).
Rahimmalek, M., Heidari, E. F., Ehtemam, M. H. & Mohammadi, S. Essential oil variation in Iranian Ajowan (Trachyspermum ammi (L.) Sprague) populations collected from different geographical regions in relation to climatic factors. Ind. Crop. Prod. 95, 591–598 (2017).
Sørensen, J. M. & Katsiotis, S. T. Parameters influencing the yield and composition of the essential oil from Cretan Vitex agnus-castus fruits. Planta Med. 66, 245–250 (2000).
Esmaeili, H., Karami, A. & Maggi, F. Essential oil composition, total phenolic and flavonoids contents, and antioxidant activity of Oliveria decumbens Vent. (Apiaceae) at different phenological stages. J. Clean. Prod. 198, 91–95 (2018).
Tisserand, R. & Young, R. Essential Oil Safety: A Guide for Health Care Professionals (Elsevier, 2013).
Charles, D. J. in Antioxidant Properties of Spices, Herbs and Other Sources 39–64 (Springer, 2012).
Balasundram, N., Sundram, K. & Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 99, 191–203 (2006).
Ishtiaque, S., Naz, S., Siddiqi, R., Jabeen, S. & Ahmed, J. Antioxidant activity and phenolic contents of ajwain, mustard, fenugreek and poppy seed. Recent Innov. Chem. Eng. 7, 119–127 (2014).
Hassan, A. H. et al. Exploratory assessment to evaluate seed sprouting under elevated CO2 revealed improved biomass, physiology, and nutritional value of Trachyspermum ammi. Agronomy 11, 1–16 (2021).
Kaur, N., Chahal, K. & Singh, R. Phytochemical screening and antioxidant activity of Anethum graveolens L. seed extracts. Pharma Innov. J. 7, 324–329 (2018).
Agati, G., Azzarello, E., Pollastri, S. & Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 196, 67–76 (2012).
Falcone Ferreyra, M. L., Rius, S. & Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 3, 222 (2012).
Balkhyour, M. A., Tammar, A. M., Summan, A. S. & Hassan, A. H. Enhancing biomass and productivity of coumarins and essential oil in ajwain (Trachyspermum ammi) sprouts via laser light treatment. Ind. Crop Prod. 170, 113837 (2021).
Küpeli Akkol, E., Genç, Y., Karpuz, B., Sobarzo-Sánchez, E. & Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 12, 1959 (2020).
Authority, E. F. S. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Coumarin. EFSA J. 2, 1–16 (2004).
Chatterjee, S., Goswami, N. & Kothari, N. Evaluation of antioxidant activity of essential oil from Ajwain (Trachyspermum ammi) seeds. Int. J. Green Pharm. 7, 140 (2013).
Chatterjee, S., Goswami, N. & Bhatnagar, P. Estimation of phenolic components and in vitro antioxidant activity of fennel (Foeniculum vulgare) and Ajwain (Trachyspermum ammi) seeds. Adv. Biores. 3, 109–118 (2012).
Coruh, N., Celep, A. S. & Özgökçe, F. Antioxidant properties of Prangos ferulacea (L.) Lindl., Chaerophyllum macropodum Boiss. and Heracleum persicum Desf. from Apiaceae family used as food in Eastern Anatolia and their inhibitory effects on glutathione-S-transferase. Food Chem. 100, 1237–1242 (2007).
Ribeiro, D. A. et al. Influence of seasonal variation on phenolic content and in vitro antioxidant activity of Secondatia floribunda A. DC. (Apocynaceae). Food Chem. 315, 126277 (2020).
Biswas, B., Rogers, K., McLaughlin, F., Daniels, D. & Yadav, A. Antimicrobial activities of leaf extracts of guava (Psidium guajava L.) on two gram-negative and gram-positive bacteria. Int. J. Microbiol. 2013, 1–7 (2013).
Saxena, S., Uniyal, V. & Bhatt, R. Inhibitory effect of essential oils against Trichosporon ovoides causing Piedra Hair Infection. Braz. J. Microbiol. 43, 1347–1354 (2012).
Gandomi, H., Abbaszadeh, S., JebelliJavan, A. & Sharifzadeh, A. Chemical constituents, antimicrobial and antioxidative effects of Trachyspermum ammi essential oil. J. Food Process. Preserv 38, 1690–1695 (2014).
Bakkali, F., Averbeck, S., Averbeck, D. & Idaomar, M. Biological effects of essential oils: A review. Food Chem. Toxicol. 46, 446–475 (2008).
Kaskatepe, B. et al. Chemical composition and antimicrobial activity of the commercial Origanum onites L. oil against nosocomial carbapenem resistant extended spectrum beta lactamase producer Escherichia coli isolates. Acta Biol. Hung. 68, 466–476 (2017).
Kavoosi, G., Tafsiry, A., Ebdam, A. A. & Rowshan, V. Evaluation of antioxidant and antimicrobial activities of essential oils from Carum copticum seed and Ferula assafoetida latex. J. Food Sci. 78, T356–T361 (2013).
Gong, H., Liu, W., Lv, G. & Zhou, X. Analysis of essential oils of Origanum vulgare from six production areas of China and Pakistan. Rev. Bras. Farmacogn. 24, 25–32 (2014).
Karami, A., Khoshbakht, T., Esmaeili, H. & Maggi, F. Essential oil chemical variability in Oliveria decumbens (Apiaceae) from different regions of Iran and its relationship with environmental factors. Plants 9, 680 (2020).
Šamec, D. et al. Assessment of the differences in the physical, chemical and phytochemical properties of four strawberry cultivars using principal component analysis. Food Chem. 194, 828–834 (2016).
Adams, R. P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry Vol. 456 (Allured Publishing Corporation, 2007).
Davies, N. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A 503, 1–24 (1990).
Sánchez-Rangel, J. C., Benavides, J., Heredia, J. B., Cisneros-Zevallos, L. & Jacobo-Velázquez, D. A. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 5, 5990–5999 (2013).
Kim, D.-O., Jeong, S. W. & Lee, C. Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 81, 321–326 (2003).
Osório, A. D. C. & Martins, J. L. S. Determinação de cumarina em extrato fluido e tintura de guaco por espectrofotometria derivada de primeira ordem. Rev. Cienc. Farm. Basica Apl. 40, 481–486 (2004).
Bicas, J., Neri-Numa, I., Ruiz, A., De Carvalho, J. & Pastore, G. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem. Toxicol. 49, 1610–1615 (2011).
Benzie, I. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 239, 70–76 (1996).
Prieto, P., Pineda, M. & Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 269, 337–341 (1999).
Jorgensen, J. H. Antibacterial susceptibility tests: dilution and disk diffusion methods. In Manual of Clinical Microbiology (1999).
Acknowledgements
We appreciate the Department of Horticulture of Urmia University for its comprehensive support in this research. The authors also express their appreciation to the Ministry of Science, Research and Technology of Iran (1048488), and the Research Council of Shahid Beheshti University, Tehran, Iran for their financial support.
Author information
Authors and Affiliations
Contributions
M.M. Investigation, Software, Formal analysis, Writing-Original Draft. M.F. Supervision, Visualization Conceptualization, Methodology, Data curation, Writing-Review and Editing, Project administration. M.H.M. Supervision, Data curation, Methodology, and GC-FID and GC-MS analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Modareskia, M., Fattahi, M. & Mirjalili, M.H. Thymol screening, phenolic contents, antioxidant and antibacterial activities of Iranian populations of Trachyspermum ammi (L.) Sprague (Apiaceae). Sci Rep 12, 15645 (2022). https://doi.org/10.1038/s41598-022-19594-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19594-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.