Abstract
The European eel (Anguilla anguilla) is critically endangered (according to the most recent IUCN assessment) and has suffered a 95% decline in recruitment since the 1980s, attributed in part to factors occurring during the marine phases of its life-cycle. As an adult, the European eel undertakes the longest spawning migration of all anguillid eels, a distance of 5000 to 10,000 km across the Atlantic Ocean to the Sargasso Sea. However, despite the passage of almost 100 years since Johannes Schmidt proposed the Sargasso Sea as the breeding place of European eels on the basis of larval surveys, no eggs or spawning adults have ever been sampled there to confirm this. Fundamental questions therefore remain about the oceanic migration of adult eels, including navigation mechanisms, the routes taken, timings of arrival, swimming speed and spawning locations. We attached satellite tags to 26 eels from rivers in the Azores archipelago and tracked them for periods between 40 and 366 days at speeds between 3 and 12 km day−1, and provide the first direct evidence of adult European eels reaching their presumed breeding place in the Sargasso Sea.
Similar content being viewed by others
Introduction
The extensive surveys of Johannes Schmidt throughout the Atlantic Ocean and Mediterranean Sea in the early twentieth century demonstrated a widespread distribution of eel larvae [leptocephali], with a concentration of the smallest specimens in the Sargasso Sea, far from their freshwater, estuarine and coastal growth habitats located in Europe and North Africa1. This discovery remains the single biggest step to solving the mystery of how the eels reproduce and the location of their breeding place, which has perplexed generations from Aristotle to Freud2. Schmidt concluded that ‘the spawning grounds comprise a restricted area in the western Atlantic, north-east and north of the West Indies, between 65° and 48° long, for here- and here only- are the youngest, newly hatched larvae found1. Since this time, other surveys have located leptocephali < 12 mm in length (< 20 days old) across a 2000 km wide region of the Sargasso Sea from 70 °W to 50 °W, but over a restricted latitude near temperature fronts of 22 to 24 °C within the subtropical convergence zone3,4,5. Most recently, even younger leptocephali (< 7 mm) have been found south of the northerly frontal zone, predominantly within the area bounded by the coordinates 31°N, 50°W and 24°N, 70°W6. However, even 100 years on from Schmidt’s original discovery, the inferences that can be drawn from larval data are limited, and surveys have failed to locate either adult eels or eggs. As a result, the timing and spatial extent of spawning are difficult to determine exactly7. The fact that very small larvae have been found in inter-annual surveys across a wide longitude range could be an indication that spawning is spatially and/or temporally variable between years8. Increasing knowledge on the spawning migration and the location of adult eels during the spawning period, as well as identifying the depth and oceanographic conditions for spawning, is therefore an important next step in eel research9,10,11,12,13.
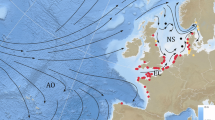
Of all the anguillid species of eel, the European eel has the longest and most complex ocean migration14,15. Attempts have been made since the 1970s to track the oceanic migrations of eels16, but significant progress has only been made in the last 10 to 15 years with the advent of pop-up satellite transmitting tags (PSAT)17. In recent studies, pop-up tags (Fig. 1A) were used to track the migrations of more than 80 eels released from five regions within Europe: the Baltic Sea, the North Sea, the Celtic Sea, the Bay of Biscay and the western Mediterranean18,19,20,21. The data from the tags were used to identify migratory routes that extended up to 5000 km from release, and which suggested routes taken by eels migrating from different countries converge when passing the Azores (Fig. 1B). However, although eels were tracked for six months or more, their migration speed was insufficient to reach the Sargasso Sea for the first presumed spawning period after migration commenced, prompting the hypothesis that the spawning migration period of eels may extend to more than 18 months20.
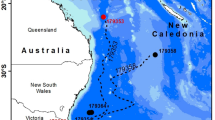
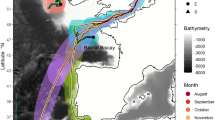
Migration of European eels from the Azores to the Sargasso Sea spawning area. (A) European eel fitted with a pop-up satellite tag; (B) historic data, showing the location of the proposed spawning area1 in relation to previous pop-up satellite tag positions (crosses) from eels released from three different locations in Europe20. Shading shows kriged minimum leptocephalus size data from ICES in a 1° grid; (C) pop-up data from European eels tagged at two Azores locations (circles) in November of 2018 (gold) or 2019 (yellow). Symbols within the crosses show if the tags detached from the eels prematurely, either due to exceeding the depth failsafe of 1400 m (downward triangle), or for other (unknown) reasons (circle). Predations are not shown. The average bearing of eels released in 2019 is shown as a yellow line, with intervals along the line marking the distance travelled at the average speed shown by eels at liberty for 120 day or less, at liberty for between 120 and 240 day, and at liberty for > 240 day. The yellow star shows the extrapolated position along the average bearing for a migratory period ending at the second peak spawning event after release (i.e. 14th February20,22, 466 day after release). White circles (at 1° grid) show where the smallest larval eels have been recorded in larval surveys conducted over the last century (large circles = larvae < 8 mm, small circles = larvae 8 mm to 12 mm), while the white dotted line shows the boundary extent of the spawning area in recent times, as presented in Miller et al.6. The hatched area shows the area defined as the Sargasso Sea Area of Collaboration23. The bathymetry used in the (B) and (C) is the GEBCO grid (GEBCO Compilation Group, 2020)24, and both figures were drawn in ESRI ArcMap 10.5, available to download at the ESRI website (https://support.esri.com/en/Products/Desktop/arcgis-desktop/arcmap/10-8-2#downloads).
To gather direct evidence on the final stage of the migration of European eels to the Sargasso Sea, (based on the assumption that European eels that spend their growth phase in the Azores would migrate to the Sargasso Sea along the same route as eels from continental Europe and North Africa), we carried out exploratory surveys for European eel in waterbodies on several Azorean islands in 2017, 2018 and 2019. Twenty-six female silver eels (mean length 911.9 mm +/- 51.8 s.d.) were captured, tagged with PSATs and released from shore in November and December 2018 and 2019 (Table 1).
Results
Twenty-three tags communicated via the ARGOS system (www.argos-system.org), of which 21 recorded substantive data before the tags detached 40 to 366 days after deployment (mean duration 187 day ± 89 s.d.). Two tags were assessed to have become detached from eels within a week of release either through attachment failure or predation. The tag pop-up locations confirm the south-westerly trajectory of their migration in the direction of the Sargasso Sea (bearings ranged from 208° to 273°), covering straight-line distances of 272 to 2275 km (mean 1179 km ± 564 s.d.) (Fig. 1C, Table 1). Five of the pop-up locations were within the Sargasso Sea boundaries25,23 and one eel was located within the presumed breeding area located between 31 °N, 50 °W and 24 °N, 70 °W6. Average migration speed ranged between 2.9 and 11.9 km day−1 (mean 6.8 km day−1 ± 2.2 s.d.).
Discussion
Despite the journey for European eels from the Azores being the ‘shortest’ migration route to the Sargasso Sea at approximately 2500 km to the eastern edge of the presumed spawning area, it is still longer than the full migration route of some tropical anguillid species14. However, the period of time that eels leaving from the Azores have to reach the spawning area before the spawning period starts is not long: larval surveys have shown that spawning each year begins in December, peaks in February/March and extends into May20,26. This provides a period of between 1 and 6 months in which eels migrating from the Azores have to complete their journey and sexual maturation process and to join other spawners. However, none of the eels tagged in this study migrated fast enough (greater than 12 km day−1) to arrive in the spawning area before the end of the recognised spawning period. Instead, their speed over ground averaged 6.5 km day−1 and the maximum speed was just over 11 km day−1. While these migration speeds are relatively slow compared to the cruising speeds of other (typically surface-dwelling) ocean migrating fishes, they are consistent with other studies of anguillid eels12,20,27,28. The data therefore support the hypothesis that, rather than make a rapid migration to spawn at the earliest opportunity, European eels may instead make a long, slow spawning migration at depth that conserves their energy and reduces mortality risk20. This timing would enable the completion of their reproductive maturation20 before they arrive at the spawning area in time for the peak of the second spawning period after the onset of their migration (Fig. 1C).
There has been a long quest to understand the migratory behaviour of anguillid eels and when Johannes Schmidt (1923)1 first identified the presumed spawning area, he stated:
Years of research rich in excitement and suspense: disappointment alternating with encouraging discoveries and periods of rapid progress with others during which the solution of the problem seemed wrapped in deeper darkness than ever
Although our study did not yet yield definitive evidence of the mechanism(s) of navigational influence such as perhaps ocean currents, olfactory cues, temperature fronts, magnetic fields or seamounts29,30,31,32,33, ours is the first direct evidence of migrating adult European eels reaching the presumed breeding place in the Sargasso Sea. This is an encouraging discovery that completes the map of the spawning migration route that has emerged over the last 10 years17,18,19,20,21 and offers some light on how to develop future work. The dramatic (95%) decline in juvenile recruitment since the 1980s of this critically endangered species34,35,36,37, highlights the importance of further research into all aspects of the life-cycle, including adult migration navigation mechanisms and spawning locations, to inform conservation measures that will lead to a sustainable recovery of the European eel population13.
Methods
Pop-up archival satellite tags (PSATs)
All eels were equipped with an X tag from Microwave Telemetry (www.microwavetelemetry.com) which is 120 mm long, with a 185 mm long antenna. The maximum diameter of the float is 33 mm. Weight in air is 45 g and net buoyancy in seawater is approximately 0.025 ± 0.006 N, corresponding to a negative weight of 2.6 g. The tag measures and stores pressure, temperature and light data every 2 min. A subset of these data are transmitted when the tags pop up at either a predetermined date or if any of the fail-safe devices are triggered, for example if a critical pressure is exceeded. The temperature measurement range is − 4° to 40 °C with a resolution of 0.23 °C. The depth range is 0 to 1300 m with a resolution varying between 0.34 and 5.4 m depending on the gain value which is automatically selected according to the depth measured at midnight each day. The time of release was programmed at 6 months (n = 10), 7 months (n = 10) or 1 year (n = 6) after deployment. The constant pressure release feature that detaches the tag if the depth reading remains within 3 m for a period of 4 days was deactivated for the first 20 days after deployment to avoid premature release with limited movements in shallow water. After a tag pops up, surface position and a time series of depth and temperature are transmitted from the tag to low Earth orbiting ARGOS satellites (http://www.argos-system.org/) from where they can be downloaded. More details on how data from the tag are coded and transmitted can be found at https://www.microwavetelemetry.com/data. The surfacing positions of tags that drifted at the surface before activating their communication with the ARGOS satellite system were back-calculated by determining the direction and velocity of surface currents observed after the tag’s first ARGOS transmission over the same period of time as the surface drift period.
Eel capture and tagging
Wild adult ‘silver’ eels were captured during their Autumn downstream migration using fyke nets set in the freshwater reaches of rivers on the Azorean Islands of San Miguel and Flores. There is no commercial eel fishery in the Azores and very little was known about the population status of European eel, hence capture sites were identified from surveys conducted in 2017, 2018 and 2019 across the archipelago. Environmental DNA testing (Nature Metrics) at the survey locations confirmed the species as A. anguilla. Three eels caught between November 14th and 22nd 2018 on San Miguel that were large enough to be tagged (865 to 990 mm; 1.4 to 2.1 kg) were transferred to a trout farm on the island and held in a raceway until tagging and release on December 9th 2018. In 2019, 23 large eels (820 to 1019 mm length, 1.3 to 2.2 kg) captured on Flores between October 16th and November 25th were transferred to the raceway of a trout farm on the same island and held for a maximum of 32 days. Tagging occurred on two occasions in 2019; 16 and 7 eels were tagged and released on November 6th and 27th, respectively, bringing the total number of eels tagged to 26 (Table 1).
All eels selected for tagging were assumed to be female based on their lengths being far greater than those typically achieved by maturing males30,38. Maturation state was quantified using Pankhurst’s ocular index (OI)39 and Fin Index38.
The PSAT attachment procedure was conducted under anaesthesia (2-phenoxyethanol, 2 ml l−1) using a three-point stainless steel wire attachment inserted dorsally under the skin40. There were no mortalities during tagging or recovery. After recovery, eels were transported to the release location in individual tanks (70 L) to prevent entanglement of the external tags. In 2018, the release occurred at a bay on the southern coast of San Miguel (37.43011 °N, 25. 28276 °W). In 2019, it was at the sheltered southern end of a bay on the west coast of Flores (39.45937 °N, 31.26213 °W).
Determining the fate of eels
All PSAT tags rise to the surface once they become detached. The data preceding the final ascent were assessed to determine the fate of the eel. These were classified as either (1) programmed release date, (2) premature detachment, causes unknown, (3) premature detachment due to exceeding tag depth limit, (4) predation identified by changes in temperature readings or behaviours consistent with ingestion by a marine mammal or surface-orientated ectotherm20, or (5) no data received.
Migration speed, distance and routes
Since the mechanism of eel migration is not known, we assumed that eels travelled at a constant speed along a constant bearing between the release position and pop-up position, and that the distance travelled was the loxodromic distance (rhumb line) between the points. Migration speed (km day−1) was calculated by dividing distance travelled by the number of days at liberty, excepting the data from eels that were predated (n = 2). To assess whether the remaining migration data (n = 21, from eels released in both 2018 and 2019) should be aggregated, the data on speed were split into three groups based on time at liberty (< 120 day, 120 to 240 day, and > 240 day) and differences in average migratory speed over the course of the migration were assessed using a Kruskal–Wallis test. The same approach was taken to assess differences in migratory bearing for eels released from Flores in 2019 (n = 19: eels released from San Miguel were excluded as the difference in release location would have a significant effect on the calculation of bearing). Although migratory speed reduced with time spent at liberty, this difference was not significant (H = 2.7, p > 0.05, df = 2). The migratory bearing did not differ significantly among groups (H = 0.17, p > 0.05, df = 2). Projected location at the time of presumed peak spawning (14th February, based on analysis of larval data reported in Righton et al. 201620) was estimated using the average migration bearing of all eels released in 2019 and the migration speed of all eels.
Larval data
Historical data on eel larvae, which were compiled by J. D. McCleave and hosted by the International Council for the Exploration of the Sea (ICES Eggs and larvae (ices.dk)) were analysed to determine the location of the smallest A. anguilla leptocephalus larvae in the Sargasso Sea. The database covers the results of eel larvae surveys from 1862 to 2007, comprising a total of 2375 hauls made using varying plankton and larval nets, and yielding a catch of more than 32,000 A. anguilla or Anguilla rostrata leptocephali. The stations were spread across the North Atlantic but concentrated in the presumed spawning area in the Sargasso Sea. The data were grouped into a 1° grid and each grid cell was classified according to the minimum size of the larvae found within it.
Ethical approval
This animal study was reviewed and approved by the Zoological Society of London Ethics Committee. Field work was covered by the appropriate Azores Government licences, namely from the Directories of Environment (Licences 989/2017/DRA, 97/2918/DRA and 33/2019/DRA) and Science and Technology (CCPI 39/2917/DRCT, 45/2018/DRCT and 20/2019/DRCT). All methods were performed in accordance with the relevant guidelines and regulations. The study is reported in accordance with ARRIVE guidelines.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
Change history
15 December 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-26117-x
References
Schmidt, J. Breeding places and migrations of the eel. Nature 111, 51–54 (1923).
Tucker, D. W. A new solution to the Atlantic eel problem. Nature 183, 495–501 (1959).
Voorhis, A. D. & Hersey, J. B. Oceanic thermal fronts in the Sargasso Sea. J. Geophys. Res. 69(18), 3809–3814 (1964).
Kleckner, R. C. & McCleave, J. D. The northern limit of spawning by Atlantic eels (Anguilla spp.) in the Sargasso Sea in relation to thermal fronts and surface water masses. J. Mar. Res. 46, 647–667 (1988).
Ullman, D. S., Cornillon, P. C. & Shan, Z. On the characteristics of subtropical fronts in the North Atlantic. J. Geophys. Res: Oceans 112, C01010 (2007).
Miller, M. J. et al. Spawning by the European eel across 2000 km of the Sargasso Sea. Biol. Lett. 15, 20180835 (2019).
Westerberg, H. et al. Larval abundance across the European eel spawning area: An analysis of recent and historic data. Fish. 19, 890–902 (2018).
Halliwell, G. R. Jr., Olson, D. B. & Peng, G. Stability of the Sargasso Sea subtropical frontal zone. J. Phys. Oceanogr. 24(6), 1166–1183 (1994).
van Ginneken, V. J. T. & Maes, G. E. The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: A literature review. Rev. Fish Biol. Fish. 15, 367–398 (2005).
Friedland, K. D., Miller, M. J. & Knights, B. Oceanic changes in the Sargasso Sea and declines in recruitment of the European eel. ICES J. Mar. Sci. 64, 519–530 (2007).
Jacoby, D. M. P. et al. Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Glob. Ecol. Conserv. 4, 321–333 (2015).
Béguer-Pon, M. et al. Tracking anguillid eels: Five decades of telemetry-based research. Mar. Freshw. Res. 69, 199 (2018).
Righton, D. et al. Important questions to progress science and sustainable management of anguillid eels. Fish 22, 762–788 (2021).
Aoyama, J. Life history and evolution of migration in catadromous eels (genus Anguilla). Aquat. Bio Sci. Monogr. 2, 1–42 (2009).
Tsukamoto, K., Aoyama, J. & Miller, M. J. Migration, speciation, and the evolution of diadromy in anguillid eels. Can. J. Fish. Aquat. Sci. 59, 1989–1998 (2002).
Tesch, F.-W. Telemetric observations on the spawning migration of the eel (Anguilla anguilla) west of the European continental shelf. Env. Biol. Fish. 3, 203–209 (1978).
Aarestrup, K. et al. Oceanic spawning migration of the European eel (Anguilla anguilla). Science 325, 1660 (2009).
Westerberg, H. et al. Behaviour of stocked and naturally recruited European eels during migration. Mar. Ecol. Prog. Ser. 496, 145–157 (2014).
Amilhat, E. et al. First evidence of European eels exiting the Mediterranean Sea during their spawning migration. Sci. Rep. 6, 21817 (2016).
Righton, D. et al. Empirical observations of the spawning migration of European eels: The long and dangerous road to the Sargasso Sea. Sci. Adv. 2, e1501694 (2016).
Verhelst, P. et al. Mapping silver eel migration routes in the North Sea. Sci Rep. 12, 318 (2022).
Kuroki, M. et al. Hatching time and larval growth of Atlantic eels in the Sargasso Sea. Mar. Biol. 164, 118. https://doi.org/10.1007/s00227-017-3150-9 (2017).
Acton, L. et al. What is the Sargasso Sea? The problem of fixing space in a fluid ocean. Polit. Geogr. 68, 86–100 (2019).
GEBCO Compilation Group. GEBCO 2020 Grid. https://doi.org/10.5285/a29c5465-b138-234d-e053-6c86abc040b9 (2020).
Miller, M. J. & Hanel, R. The Sargasso Sea Subtropical Gyre: The spawning and larval development area of both freshwater and marine eels. Sargasso Sea Alliance Science Report Series, 7, 20 pp (2011).
Munk, P. et al. Oceanic fronts in the Sargasso Sea control the early life and drift of Atlantic eels. Proc. Biol. Sci. 277, 3593–3599 (2010).
Béguer-Pon, M., Castonguay, M., Shan, S., Benchetrit, J. & Dodson, J. J. Direct observations of American eels migrating across the continental shelf to the Sargasso Sea. Nat. Commun. 6, 8705 (2015).
Westin, L. The spawning migration of European silver eel (Anguilla anguilla L.) with particular reference to stocked eel in the Baltic. Fish. Res. 38(3), 257–270 (1998).
Tesch, F.-W., Wendt, T. & Karlsson, L. Influence of geomagnetism on the activity and orientation of the eel, Anguilla anguilla (L.), as evident from laboratory experiments. Ecol. Freshw. Fish 1(1), 52–60 (1992).
Tesch, F.-W. The Eel (Blackwell Science, Oxford, UK, 2003).
Durif, C. M. F. et al. Magnetic compass orientation in the European eel. PLoS ONE 8(3), e59212 (2013).
Schabetsberger, R. et al. Hydrographic features of anguillid spawning areas: Potential signposts for migrating eels. Mar. Ecol. Prog. Ser. 554, 141–155 (2016).
Naisbett-Jones, L. C., Putman, N. F., Stephenson, J. F., Ladak, S. & Young, K. A. A magnetic map leads juvenile European eels to the Gulf stream. Curr. Biol. 27, 1236–1240 (2017).
Dekker, W. Status of the European eel stock and fisheries. In Eel Biology (eds Aida, K. et al.) 237–254 (Springer, New York, 2003).
Drouineau, H. et al. Freshwater eels: A symbol of the effects of global change. Fish Fish (Oxf) 19, 903–930 (2018).
ICES. Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL). ICES Scientific Reports. 2(85) (2020).
Pike, C., Crook, V. & Gollock, M. Anguilla anguilla. The IUCN Red List of Threatened Species 2020: e.T60344A152845178 (2020).
Durif, C., Dufour, S. & Elie, P. The silvering process of Anguilla anguilla: A new classification from the yellow resident to the silver migrating stage. J. Fish. Biol. 66, 1025–1043 (2005).
Pankhurst, N. W. Relation of visual changes to the onset of sexual maturation in the European eel Anguilla Anguilla (L.). J. Fish Biol. 21, 127–140 (1982).
Økland, F., Thorstad, E. B., Westerberg, H., Aarestrup, K. & Metcalfe, J. D. Development and testing of attachment methods for pop-up satellite archival transmitters in European eel. Anim. Biotelem. 1, 3 (2013).
Acknowledgements
The authors thank Gerbrand Michelsen for his help with the Azores project initiation and all those who assisted with the logistics and extensive fieldwork undertaken to achieve the capture and satellite tagging of these eels. Funding was provided by the UK Department for Environment, Food and Rural Affairs (Defra), Environment Agency (England) and the Oceano Azul Foundation (Eel Trek Project). Partner organisations, the Azores Government and volunteer contributions are also gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
R.M.W. conceived and led the project and all contributed to project design and delivery. G.C., S.R.R., R.M.W. and A.T.P. organised and undertook fieldwork to capture eels for tagging, with participation from A.D., R.V. and M.G. A.T.P., M.G. and K.A. satellite tagged the eels, assisted by G.C., S.R.R., R.M.W., R.V. and A.W. J.A. and R.M.W. organised licensing and permissions from the Azores Government. R.M.W., J.A., R.V. and A.W. organised funding. D.R., A.T.P. and H.W. analysed the data and D.R. prepared the figures. The paper was prepared by R.M.W. and modified by D.R., A.T.P., H.W., A.W., R.V., M.G., J.A. and A.D.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error. In the Results section, “Average migration speed ranged between 2.9 and 11.9 m day−1 (mean 6.8 km day−1 ± 2.2 s.d.).” now reads: “Average migration speed ranged between 2.9 and 11.9 km day−1 (mean 6.8 km day−1 ± 2.2 s.d.).”
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wright, R.M., Piper, A.T., Aarestrup, K. et al. First direct evidence of adult European eels migrating to their breeding place in the Sargasso Sea. Sci Rep 12, 15362 (2022). https://doi.org/10.1038/s41598-022-19248-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19248-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.