Abstract
Anemia and iron deficiency (ID) are common in patients with heart failure (HF) and intravenous (IV) administration of iron to patients hospitalized for decompensated HF with ID improves outcome. The diagnosis of ID in routine practice is based on serum ferritin and transferrin saturation (TSAT) but both have limitations; alternatives should be considered. Reticulocyte hemoglobin equivalent (Ret-He) reflects iron content in reticulocytes but its clinical utility in patients with HF remains uncertain. We prospectively enrolled 142 patients hospitalized for decompensated HF. Sixty five percent had ID as defined in current international guidelines. Ret-He was directly correlated with serum iron and ferritin concentrations and with TSAT. There was a poor relationship between quartile of Ret-He and HF hospitalization or death but increases or decreases in Ret-He between admission and discharge were associated with a worse outcome. The clinical utility of Ret-He for identifying ID and predicting response to IV iron and prognosis for patients with HF requires further investigation.
Similar content being viewed by others
Introduction
Anemia is common in patients with heart failure (HF)1,2. Iron deficiency (ID) is also frequent, occurring in 35–55% of patients with HF, regardless of the presence or absence of anemia3,4. In particular, ID is more common in advanced HF and associated with an adverse prognosis3,5. Using the gold-standard for diagnosing ID by bone marrow biopsy, the prevalence rate of iron deficiency anemia (IDA) is reported to be 73% in patients with advanced HF6.
In clinical, serum iron, ferritin, total iron binding capacity (TIBC), and transferrin saturation (TSAT) are used for the diagnosis of ID. There are two ID subtypes. One is absolute ID defined by depleted iron stores, and the other is functional ID defined by adequate iron stores but low iron availability for erythroid precursors.
In patients with HF, the most commonly used biomarkers to evaluate iron status are serum ferritin and TSAT. According to current international guidelines on HF, the cut-off values of ID are serum ferritin levels < 100 μg/L or serum ferritin levels 100–299 μg/L with TSAT < 20%7,8. However, clinical use of this evaluation is a little complicated, and the best cut-off index for ID in HF patients remains completely unclear.
Reticulocyte hemoglobin equivalent (Ret-He), which can be measured by the automated hematology analyzers, is considered to reflect iron content in reticulocytes9. Reticulocytes develop into mature erythrocytes within two days in peripheral blood. Due to the short duration of the reticulocyte stage in erythropoiesis, Ret-He is considered as a direct index of iron availability in erythropoiesis10. In addition, usefulness of Ret-He is reported as a screening marker for ID11.
However, it has been entirely unknown whether Ret-He can be used as a marker for ID in patients with HF. Therefore, in this study, we investigated the clinical utility of Ret-He in HF patients.
Results
Patients characteristics
The patient characteristics on admission are shown in Table 1. Of 142 patients, the median age of patients was 77 years and 58% of patients was men. The median left ventricular ejection fraction (LVEF) and brain natriuretic peptide (BNP) levels were 36% and 659 pg/mL. The median hemoglobin (Hb), serum iron, and TSAT were 10.6 g/dL, 40 µg/dL, and 15.8%. The median Ret-He levels was 32.0 pg on admission. According to quartile of Ret-He levels, the prevalence of hypertension and the use of loop diuretics were different among the groups.
Overall, using World Health Organization (WHO) criteria12, 82% of patients were shown anemia in this study. 78% of men and 87% of women had anemia (Fig. 1A). On the other hand, according to current guideline definition for ID, 65% patients have ID in all patients. The prevalence rate of ID was 60% in men and 72% in women (Fig. 1B). 57% of patients had IDA (men, 51% and women, 65%) (Fig. 1C).
Relationship of Ret-He levels with parameters of iron metabolism in HF patients
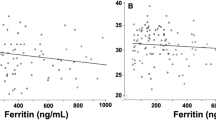
To investigate the clinical utility of Ret-He in HF patients, we next evaluated the correlation of Ret-He levels with serum iron, TSAT, ferritin, and Hb levels in HF patients. Ret-He levels were directly correlated with serum iron, TSAT, Hb, and ferritin levels in HF patients (Fig. 2A–D). In addition, to further allow interpretation of the data, we performed an additional analysis based on an expanded Y-axis in ferritin 0-500 μg/L, and a correlation between Ret-He and ferritin levels was observed (Fig. 2E).
Comparison of Ret-He levels according to iron status in HF patients
We then evaluated Ret-He levels in HF patients according to current guideline definition for ID. The median Ret-He levels were significantly lower in HF patients with ID than in those without ID (30.3 versus 34.2 pg, P < 0.0001) (Fig. 3A). We next assessed the diagnostic accuracy of Ret-He to screen for ID in HF patients by receiver-operating characteristic (ROC) analysis. The area under the curve (AUC) for Ret-He to screen for ID was 0.753 and the cut-off value for Ret-He to screen for ID was 32.4 pg (true positive fraction: 0.725, 1-false positive fraction: 0.700) (Fig. 3B). In addition, we investigated Ret-He levels in HF patients according to definition for IDA. Ret-He levels were significantly lower in HF patients with IDA than in those without IDA (30.2 versus 33.7 pg, P < 0.0001) (Fig. 3C). And, the AUC for Ret-He to screen for IDA was 0.722. Similar to ID, the cut-off value for Ret-He to screen for IDA was 32.4 pg (true positive fraction: 0.716, 1-false positive fraction: 0.617) in HF patients (Fig. 3D).
Ret-He levels in HF patients with and without iron deficiency. (A) Ret-He levels in HF patients with (n = 92) and without ID (n = 50). (B) ROC curve of Ret-He to screen for ID. (C) Ret-He levels in HF patients with (n = 82) and without (n = 60) IDA. (D) ROC curve of Ret-He to screen for IDA. ID iron deficiency, IDA iron deficiency anemia, AUC area under the curve, TPF true positive fraction, FPF false positive fraction.
Prognostic associations of Ret-He levels in HF patients
ID is associated with an adverse prognosis in HF patients3,5, we thus assessed prognostic associations of Ret-He levels in HF patients. During a median follow-up of 21.7 months, composite outcomes (all-cause death or HF readmission) occurred in 61 patients. We first investigated prognosis in HF patients according to quartile of Ret-He levels; however, there was not significant different prognosis among the groups (Fig. 4A–C).
Then, we investigated prognostic associations of Ret-He levels in HF patients, whose Ret-He levels were measured both at admission and discharge, by change in Ret-He levels between admission and discharge (ΔRet-He). The distribution of ΔRet-He is shown in Supplemental Fig. 1. As there were patients with little change in Ret-He levels between admission and discharge, we performed a prognostic analysis based on a three-group classification of change in Ret-He levels (group 1: ΔRet-He ≦ − 2 pg, group 2: − 2 pg < ΔRet-He < 2 pg, and group 3: 2 pg ≦ ΔRet-He). Of note, a statistically significant worse prognosis was observed in group 1 than in the other groups (Fig. 5A–C). The patient characteristics by a three-group classification of change in Ret-He levels are shown in Table 2. The median body mass index, TSAT, Ret-He, the prevalence of atrial fibrillation, and the use of Ca blockers were different among the groups.
Survival curves for all-cause death or heart failure readmission according to a three-group classification of change in Ret-He levels between admission and discharge in HF patients. Kaplan–Meier analysis for (A) all-cause death or HF readmission, (B) all-cause death, and (C) HF readmission in HF patients by a three-group classification of change in Ret-He levels between admission and discharge (ΔRet-He).
To investigate the prognostic impact of ΔRet-He, we finally performed univariate and multivariate Cox regression analyses for prognosis in these patients. In the univariate model, ΔRet-He ≦ − 2 pg was associated with a worse outcome, while this tended to be associated with a worse outcome in a multivariate analysis (Table 3).
Discussion
This study showed for the first time that Ret-He has the clinical utility as a simple marker for ID in HF patients. Ret-He levels were directly correlated with serum iron and ferritin levels and with TSAT. Moreover, Kaplan–Meier analysis demonstrated that increases or decreases in Ret-He between admission and discharge were associated with a worse outcome in HF patients.
The gold-standard for measurement of ID is bone marrow histology. Although there are many definitions of ID based on circulating biomarkers, it is unknown which marker is best. Serum ferritin levels are commonly used to evaluate iron status; however, as ferritin is an acute phase protein, serum ferritin levels increase in the presence of chronic inflammation. As chronic inflammation is associated with the pathogenesis of HF, it should be taken into consideration. In fact, previous studies have shown that higher serum ferritin levels are associated with an adverse prognosis in HF patients13,17. Thus, cut-off values of these markers for ID should be reconsidered for HF patients. In this regard, Ret-He can be easily obtained, it thus may be advantageous for the screening for ID in patients with HF.
Ret-He can reveal the current iron status that are clinically significant. Of note, Ret-He levels were directly correlated with standard parameters of iron metabolism in HF patients. In this study, there were a few patients whose TSAT was about 90%. These patients had liver congestion or poor nutrition due to severe HF, which resulted in low transferrin production and high TSAT. In addition, the cut-off value for Ret-He to screen for both ID and IDA as defined in current international guidelines was 32.4 pg. These results indicate that Ret-He could reflect iron status and lead more easily applicable definition to screen for ID and IDA in HF patients. Ret-He can be easily measured in the same tube of cellular blood analysis, and the costs of Ret-He measurement are also economical. Taken together, these results suggest that Ret-He has the clinical utility as a simple marker for ID in HF patients. As the reports of improved clinical symptom with intravenous (IV) iron administration focus on anemia and ID in HF patients14,15,16, the measurements of Ret-He levels is considered to have an important perspective in HF patients.
A recent study has shown that there was no association between current guideline definition for ID and mortality in HF patients17. Similarly, in this study, ID defined by current guideline was not associated with prognosis in HF patients (P = 0.472 for all-cause death or HF readmission, P = 0.926 for all-cause death, P = 0.282 for HF readmission). Then, we evaluated the prognostic impact of Ret-He levels in HF patients. However, there was not a significant difference on prognosis according to quartile of Ret-He levels. We then investigated the prognostic impact of Ret-He in HF patients, by change in Ret-He levels between admission and discharge. Of note, by a prognostic analysis based on a three-group classification of change in Ret-He levels, a worse outcome was observed in patients with ΔRet-He ≦ − 2 pg than in the other groups. Also, multivariate analysis showed that ΔRet-He ≦ − 2 pg tended to be associated with a worse outcome. These results indicate that ΔRet-He ≦ − 2 pg even after optimal HF treatment is associated with a worse outcome in HF patients. To the best of our knowledge, this study showed for the first time that the change in Ret-He levels between admission and discharge was associated with prognosis in HF patients. Treatment of ID by monitoring Ret-He levels may lead to improve prognosis in HF patients. Recent clinical studies have shown that IV iron administration is beneficial for clinical symptom in HF patients with reduced ejection fraction with ID14,15,16. However, the effects of IV iron administration on prognosis in HF patients has been entirely unknown. Iron administration may provide a benefit for prognosis particularly in HF patients with ΔRet-He ≦ − 2 pg. In conclusion, these findings support the clinical utility of Ret-He to assess ID in HF patients. However, the clinical utility of Ret-He for identifying ID and predicting response to IV iron administration and prognosis for patients with HF requires further investigation.
Study limitations
This study is a small single-centered and relatively small number study. Further validation in the future is needed.
Methods
Study protocol
We studied 207 consecutive patients who were hospitalized for acute decompensated HF according to the Framingham criteria18 between December 1, 2017 and October 31, 2018 at Hyogo Medical University Hospital. Patients with renal failure with estimated glomerular filtration rate < 10 mL/min/1.73 m2 or receiving hemodialysis (n = 18), leukemia (n = 1), autoimmune hemolytic anemia (n = 2), or active malignancy (n = 12) were excluded. In addition, we excluded 25 participants whose missing all the required iron indices and 7 participants who were lost to follow-up, which left a final analytical cohort of 142 participants (Supplementary Fig. 2). The patients’ demographic data including co-morbid conditions, clinical signs, laboratory and echocardiographic data, in-hospital treatment including oral and intravenous medication, and length of hospital stay were obtained. Echocardiography was performed by sonographer and LVEF was defined with the modified Simpson method. Hypertension was defined as systolic blood pressure ≧ 140 mmHg or diastolic blood pressure ≧ 90 mmHg or receiving anti-hypertensive drugs. Diabetes mellitus was defined as glycated hemoglobin ≧ 6.5%, casual blood glucose ≧ 200 mg/dL, or fasting blood glucose ≧ 126 mg/dL or receiving oral hypoglycemic agents and/or insulin. The presence of old myocardial infarction and cerebrovascular disease was defined based on history, clinical presentation, examinations, and medications.
Anemia was defined according to WHO criteria (Hb levels < 13 g/dL in men, Hb levels < 12 g/dL in women). ID was defined as serum ferritin levels < 100 μg/L, or serum ferritin levels 100–299 μg/L with TSAT (serum iron levels / TIBC × 100) < 20%7,14,19. IDA was defined by serum ferritin < 100 μg/L, or between 100 and 299 μg/L with TSAT < 20% and Hb levels below WHO criteria of anemia. Ret-He levels were determined by an automated hematology analyzer (XN-9100® or 1000®, Sysmex, Kobe, Japan). Blood samples were collected within 24 h from the admission. Laboratory parameters were measured at admission and discharge.
The primary outcome was defined as a composite endpoint of all-cause death or HF readmission and prospectively recorded. Follow-up was performed at discharge, and after discharge by direct contact with patients, telephone interview of patients or, if deceased, of family members, and mail, by investigators. Informed consent were written from all patients. These procedures for informed consent and enrolment were in accordance with the detailed regulations regarding informed consent described in the guidelines, and this study has been approved by the ethics committee of Hyogo Medical University (authorization numbers 3549).
Statistical analysis
Continuous variables were presented as median and interquartile range. Continuous variables were compared using 1-way analysis of variance or Kruskal–Wallis test. Continuous variables were tested for normality using the Shapiro–Wilk test. Categorical variables were made by chi-squared test or Fisher’s exact test for dichotomous variables, when appropriate. The correlations of Ret-He levels with serum iron, TSAT, ferritin, and Hb levels were assessed by Spearman correlation analysis. The cumulative incidence of a composite outcome (all-cause death or HF readmission) was estimated using Kaplan–Meier analysis. ROC analysis was performed to assess the potential capabilities of Ret-He as a marker for ID and IDA, computing the ROC curve and its AUC. The results of this analysis can be shown as the ROC curve, in which the sensitivity is plotted against the value of 1-specificity. To investigate the prognostic impact of change in Ret-He levels, covariates which were closely related to change in Ret-He levels (age, sex, body mass index, BNP, the prevalence of atrial fibrillation, and the use of Ca blockers) were chosen in this study. All tests were two tailed, and a value of P < 0.05 was considered statistically significant. All analyses were performed with R version 3.3.2. All methods were performed in accordance with relevant guidelines and regulations.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Groenveld, H. F. et al. Anemia and mortality in heart failure patients: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 52, 818–827 (2008).
Okuno, K. et al. Effective blood hemoglobin level to predict prognosis in heart failure with preserved left ventricular ejection fraction: Results of the Japanese heart failure syndrome with preserved ejection fraction registry. Heart Vessels. 34, 1168–1177 (2019).
Rocha, B. M. L., Cunha, G. J. L. & Menezes Falcão, L. F. The burden of iron deficiency in heart failure: Therapeutic approach. J. Am. Coll. Cardiol. 71, 782–798 (2018).
Klip, I. T. et al. The additive burden of iron deficiency in the cardiorenal-anaemia axis: scope of a problem and its consequences. Eur. J. Heart Fail. 16, 655–662 (2014).
Klip, I. T. et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am. Heart J. 165, 575-582.e3 (2013).
Nanas, J. N. et al. Etiology of anemia in patients with advanced heart failure. J. Am. Coll. Cardiol. 48, 2485–2489 (2006).
McDonagh, T. A. et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 42, 3599–3726 (2021).
Heidenreich, P. A. et al. (2022) AHA/ACC/HFSA guideline for the management of heart failure: A report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation 145, e895–e1032 (2022).
Joosten, E., Lioen, P., Brusselmans, C., Indevuyst, C. & Boeckx, N. Is analysis of the reticulocyte haemoglobin equivalent a useful test for the diagnosis of iron deficiency anaemia in geriatric patients?. Eur. J. Intern. Med. 24, 63–66 (2013).
Brugnara, C., Schiller, B. & Moran, J. Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron-deficient states. Clin. Lab Haematol. 28, 303–308 (2006).
Toki, Y. et al. Reticulocyte hemoglobin equivalent as a potential marker for diagnosis of iron deficiency. Int. J. Hematol. 106, 116–125 (2017).
World Health Organization Nutritional anaemias. Report of a WHO Scientific Group. WHO Tech. Rep. Ser. 405, 9–10 (1968).
Cleland, J. G. et al. Prevalence and outcomes of anemia and hematinic deficiencies in patients with chronic heart failure. JAMA Cardiol. 1, 539–547 (2016).
Anker, S. D. et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 361, 2436–2448 (2009).
van Veldhuisen, D. J. et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 136, 1374–1383 (2017).
Ponikowski, P. et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur. Heart J. 36, 657–668 (2015).
Masini, G. et al. Criteria for iron deficiency in patients with heart failure. J. Am. Coll. Cardiol. 79, 341–351 (2022).
McKee, P. A. et al. The natural history of congestive heart failure: the Framingham study. N. Engl. J. Med. 285, 1441–1446 (1971).
Ponikowski, P. et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet 396, 1895–1904 (2020).
Author information
Authors and Affiliations
Contributions
S.T., Y.N., K.O. contributed to study design and data analysis. S.Y., T.H., J.O., I.S., Y.M., E.M., K.M., K.A., K.N., K.D.M., A.G., M.A., M.I. contributed to searching databases and screening articles. S.T., Y.N., K.O. contributed to preparing tables, figures, and writing manuscript. S.Y., T.H., J.O., I.S., Y.M., E.M., K.M., K.A., K.N., K.D.M., A.G., M.A., M.I. contributed to data analysis and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tahara, S., Naito, Y., Okuno, K. et al. Clinical utility of reticulocyte hemoglobin equivalent in patients with heart failure. Sci Rep 12, 13978 (2022). https://doi.org/10.1038/s41598-022-18192-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18192-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.